Role of Bio-based Materials in Biomedical Polymers
OCT 24, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Bio-based Biomaterials Evolution and Objectives
Bio-based materials have emerged as a significant innovation in the field of biomedical polymers over the past several decades. The evolution of these materials can be traced back to the early 20th century when natural polymers like cellulose and rubber were first utilized in medical applications. However, it wasn't until the 1960s that systematic research into bio-based alternatives to petroleum-derived polymers began to gain momentum, driven by concerns over sustainability and biocompatibility.
The 1980s marked a pivotal shift with the development of biodegradable polymers such as polylactic acid (PLA) and polyhydroxyalkanoates (PHAs), which demonstrated promising properties for tissue engineering and drug delivery systems. This period established the foundation for modern bio-based biomaterials, emphasizing both functionality and environmental considerations.
In the 1990s and early 2000s, research expanded to include more sophisticated bio-based polymers with enhanced mechanical properties and controlled degradation profiles. The integration of natural components like chitosan, alginate, and collagen into synthetic polymer matrices created hybrid materials with improved biological interactions, addressing limitations of purely synthetic approaches.
Recent technological advancements have accelerated the development of bio-based biomaterials with unprecedented precision in structure and function. Techniques such as 3D bioprinting, electrospinning, and microfluidics have enabled the creation of complex architectures mimicking natural tissue organization. Additionally, genetic engineering approaches have facilitated the production of designer proteins and peptides with specific biological activities.
The primary objectives of bio-based biomaterials research include achieving complete biocompatibility with minimal adverse immune responses, developing materials with mechanical properties matching those of target tissues, and ensuring controlled biodegradation synchronized with tissue regeneration timelines. Furthermore, researchers aim to establish sustainable production methods that reduce environmental impact while maintaining economic viability.
Looking forward, the field is moving toward personalized biomaterials tailored to individual patient needs, smart responsive systems capable of adapting to physiological changes, and multifunctional platforms combining therapeutic, diagnostic, and regenerative capabilities. The ultimate goal remains the development of fully bio-based alternatives that can replace petroleum-derived polymers across all biomedical applications, from implantable devices to drug delivery systems and regenerative medicine scaffolds.
The 1980s marked a pivotal shift with the development of biodegradable polymers such as polylactic acid (PLA) and polyhydroxyalkanoates (PHAs), which demonstrated promising properties for tissue engineering and drug delivery systems. This period established the foundation for modern bio-based biomaterials, emphasizing both functionality and environmental considerations.
In the 1990s and early 2000s, research expanded to include more sophisticated bio-based polymers with enhanced mechanical properties and controlled degradation profiles. The integration of natural components like chitosan, alginate, and collagen into synthetic polymer matrices created hybrid materials with improved biological interactions, addressing limitations of purely synthetic approaches.
Recent technological advancements have accelerated the development of bio-based biomaterials with unprecedented precision in structure and function. Techniques such as 3D bioprinting, electrospinning, and microfluidics have enabled the creation of complex architectures mimicking natural tissue organization. Additionally, genetic engineering approaches have facilitated the production of designer proteins and peptides with specific biological activities.
The primary objectives of bio-based biomaterials research include achieving complete biocompatibility with minimal adverse immune responses, developing materials with mechanical properties matching those of target tissues, and ensuring controlled biodegradation synchronized with tissue regeneration timelines. Furthermore, researchers aim to establish sustainable production methods that reduce environmental impact while maintaining economic viability.
Looking forward, the field is moving toward personalized biomaterials tailored to individual patient needs, smart responsive systems capable of adapting to physiological changes, and multifunctional platforms combining therapeutic, diagnostic, and regenerative capabilities. The ultimate goal remains the development of fully bio-based alternatives that can replace petroleum-derived polymers across all biomedical applications, from implantable devices to drug delivery systems and regenerative medicine scaffolds.
Market Demand Analysis for Sustainable Biomedical Polymers
The global market for sustainable biomedical polymers is experiencing unprecedented growth, driven by increasing environmental concerns and regulatory pressures. Recent market analyses indicate that the biomedical polymer sector is shifting significantly toward bio-based alternatives, with sustainability becoming a key differentiator for industry players. Healthcare facilities worldwide are increasingly prioritizing eco-friendly materials in response to mounting evidence of plastic pollution's environmental impact and growing patient awareness about sustainable healthcare options.
Consumer demand for sustainable biomedical products has risen dramatically over the past five years, particularly in developed markets across North America and Europe. This trend is further accelerated by government initiatives promoting circular economy principles in healthcare settings. Hospital procurement policies are increasingly incorporating sustainability metrics, creating substantial market pull for bio-based alternatives to traditional petroleum-derived polymers.
The market potential for sustainable biomedical polymers spans multiple application segments, including implantable devices, drug delivery systems, tissue engineering scaffolds, and disposable medical supplies. Among these, biodegradable implants and sustainable packaging for medical devices represent the fastest-growing segments. The aging global population and increasing prevalence of chronic diseases are further expanding the addressable market for these materials.
Financial projections for the sustainable biomedical polymers market show robust growth trajectories. Market research indicates that healthcare facilities are willing to pay premium prices for bio-based alternatives that demonstrate comparable performance to conventional polymers while offering improved environmental profiles. This price tolerance is creating attractive profit margins for innovators in this space.
Regional market analysis reveals varying adoption rates, with European markets leading in sustainability demands, followed closely by North America. Emerging economies in Asia-Pacific are showing accelerated growth rates as their healthcare infrastructure expands and environmental regulations tighten. China and India, in particular, represent significant growth opportunities due to their rapidly developing healthcare sectors and increasing domestic manufacturing capabilities for medical devices.
Supply chain considerations are becoming increasingly important in market development. The COVID-19 pandemic highlighted vulnerabilities in global medical supply chains, creating renewed interest in localized production of essential medical materials. This trend favors bio-based materials that can be sourced from regional agricultural feedstocks, potentially reducing supply chain risks while enhancing sustainability credentials.
Industry forecasts suggest that the transition toward sustainable biomedical polymers will continue to accelerate, with bio-based materials projected to capture significant market share from traditional petroleum-based polymers over the next decade. This shift represents both a challenge and an opportunity for established players and innovative startups in the biomedical materials sector.
Consumer demand for sustainable biomedical products has risen dramatically over the past five years, particularly in developed markets across North America and Europe. This trend is further accelerated by government initiatives promoting circular economy principles in healthcare settings. Hospital procurement policies are increasingly incorporating sustainability metrics, creating substantial market pull for bio-based alternatives to traditional petroleum-derived polymers.
The market potential for sustainable biomedical polymers spans multiple application segments, including implantable devices, drug delivery systems, tissue engineering scaffolds, and disposable medical supplies. Among these, biodegradable implants and sustainable packaging for medical devices represent the fastest-growing segments. The aging global population and increasing prevalence of chronic diseases are further expanding the addressable market for these materials.
Financial projections for the sustainable biomedical polymers market show robust growth trajectories. Market research indicates that healthcare facilities are willing to pay premium prices for bio-based alternatives that demonstrate comparable performance to conventional polymers while offering improved environmental profiles. This price tolerance is creating attractive profit margins for innovators in this space.
Regional market analysis reveals varying adoption rates, with European markets leading in sustainability demands, followed closely by North America. Emerging economies in Asia-Pacific are showing accelerated growth rates as their healthcare infrastructure expands and environmental regulations tighten. China and India, in particular, represent significant growth opportunities due to their rapidly developing healthcare sectors and increasing domestic manufacturing capabilities for medical devices.
Supply chain considerations are becoming increasingly important in market development. The COVID-19 pandemic highlighted vulnerabilities in global medical supply chains, creating renewed interest in localized production of essential medical materials. This trend favors bio-based materials that can be sourced from regional agricultural feedstocks, potentially reducing supply chain risks while enhancing sustainability credentials.
Industry forecasts suggest that the transition toward sustainable biomedical polymers will continue to accelerate, with bio-based materials projected to capture significant market share from traditional petroleum-based polymers over the next decade. This shift represents both a challenge and an opportunity for established players and innovative startups in the biomedical materials sector.
Current Landscape and Challenges in Bio-based Medical Materials
The global landscape of bio-based medical materials has witnessed significant growth over the past decade, driven by increasing environmental concerns and the pursuit of sustainable healthcare solutions. Currently, the market is dominated by biodegradable polymers such as polylactic acid (PLA), polyhydroxyalkanoates (PHAs), and various cellulose derivatives, which collectively represent approximately 60% of the bio-based medical materials sector.
In North America and Europe, regulatory frameworks have evolved to support the adoption of bio-based materials in medical applications, with the FDA and EMA establishing specific pathways for their approval. This has facilitated market penetration, particularly in wound care, drug delivery systems, and tissue engineering applications. The Asia-Pacific region, led by Japan and China, has emerged as the fastest-growing market, with annual growth rates exceeding 15%.
Despite promising advancements, several significant challenges impede the widespread adoption of bio-based medical materials. Foremost among these is the issue of performance consistency. Natural variability in raw material sources leads to batch-to-batch variations that can affect mechanical properties, degradation rates, and biocompatibility profiles. This inconsistency poses substantial risks in medical applications where precision and reliability are paramount.
Cost competitiveness remains another substantial barrier. Production processes for bio-based materials typically involve complex extraction, purification, and processing steps that increase manufacturing expenses. Current estimates indicate that bio-based medical polymers are 30-40% more expensive than their petroleum-based counterparts, limiting their market penetration to premium segments or applications where their unique properties justify the cost premium.
Scalability challenges further complicate the landscape. Many innovative bio-based materials demonstrate excellent properties in laboratory settings but face significant hurdles in scaling to commercial production volumes. Issues related to process optimization, quality control, and supply chain management often emerge during scale-up attempts, delaying market introduction.
Regulatory complexity adds another layer of difficulty. While frameworks exist, they were largely designed for conventional materials, creating uncertainty in approval pathways for novel bio-based alternatives. The lack of standardized testing protocols specifically designed for bio-based materials further complicates regulatory compliance and increases development timelines.
Technical limitations also persist in specific application areas. For instance, bio-based materials often exhibit inferior barrier properties compared to synthetic alternatives, limiting their use in packaging applications for moisture-sensitive medical products. Similarly, achieving the necessary mechanical strength while maintaining biocompatibility remains challenging for load-bearing implant applications.
In North America and Europe, regulatory frameworks have evolved to support the adoption of bio-based materials in medical applications, with the FDA and EMA establishing specific pathways for their approval. This has facilitated market penetration, particularly in wound care, drug delivery systems, and tissue engineering applications. The Asia-Pacific region, led by Japan and China, has emerged as the fastest-growing market, with annual growth rates exceeding 15%.
Despite promising advancements, several significant challenges impede the widespread adoption of bio-based medical materials. Foremost among these is the issue of performance consistency. Natural variability in raw material sources leads to batch-to-batch variations that can affect mechanical properties, degradation rates, and biocompatibility profiles. This inconsistency poses substantial risks in medical applications where precision and reliability are paramount.
Cost competitiveness remains another substantial barrier. Production processes for bio-based materials typically involve complex extraction, purification, and processing steps that increase manufacturing expenses. Current estimates indicate that bio-based medical polymers are 30-40% more expensive than their petroleum-based counterparts, limiting their market penetration to premium segments or applications where their unique properties justify the cost premium.
Scalability challenges further complicate the landscape. Many innovative bio-based materials demonstrate excellent properties in laboratory settings but face significant hurdles in scaling to commercial production volumes. Issues related to process optimization, quality control, and supply chain management often emerge during scale-up attempts, delaying market introduction.
Regulatory complexity adds another layer of difficulty. While frameworks exist, they were largely designed for conventional materials, creating uncertainty in approval pathways for novel bio-based alternatives. The lack of standardized testing protocols specifically designed for bio-based materials further complicates regulatory compliance and increases development timelines.
Technical limitations also persist in specific application areas. For instance, bio-based materials often exhibit inferior barrier properties compared to synthetic alternatives, limiting their use in packaging applications for moisture-sensitive medical products. Similarly, achieving the necessary mechanical strength while maintaining biocompatibility remains challenging for load-bearing implant applications.
Current Bio-based Solutions for Biomedical Applications
01 Biodegradable polymers for medical applications
Biodegradable polymers derived from renewable resources are increasingly used in biomedical applications due to their biocompatibility and environmentally friendly nature. These materials can be engineered to degrade at controlled rates within the body, making them ideal for temporary implants, drug delivery systems, and tissue engineering scaffolds. The natural degradation process eliminates the need for removal surgeries and reduces long-term foreign body responses.- Biodegradable polymers for medical applications: Biodegradable polymers derived from renewable resources are increasingly used in biomedical applications due to their biocompatibility and environmentally friendly nature. These bio-based materials can be engineered to degrade at controlled rates within the body, making them ideal for temporary implants, drug delivery systems, and tissue engineering scaffolds. The natural degradation process eliminates the need for removal surgeries and reduces long-term foreign body responses.
- Polysaccharide-based biomedical materials: Polysaccharides such as cellulose, chitosan, alginate, and hyaluronic acid are widely used as bio-based materials in biomedical polymers. These naturally occurring polymers offer excellent biocompatibility, biodegradability, and can be chemically modified to enhance their mechanical properties and functionality. They are particularly valuable in wound healing applications, drug delivery systems, and as scaffolds for tissue engineering due to their structural similarity to the extracellular matrix components.
- Protein-based polymers for biomedical applications: Proteins such as collagen, gelatin, silk fibroin, and elastin are being developed as bio-based polymers for various biomedical applications. These naturally occurring materials closely mimic the body's own structural proteins, offering excellent biocompatibility and cell recognition sites that promote tissue integration. They can be processed into various forms including films, fibers, hydrogels, and porous scaffolds for applications in wound healing, drug delivery, and tissue engineering.
- Bio-based composite materials for medical devices: Composite materials combining bio-based polymers with other natural or synthetic components are being developed to enhance mechanical properties and functionality for biomedical applications. These composites often incorporate bioactive ceramics, nanoparticles, or reinforcing fibers to improve strength, durability, and biological response. Such hybrid materials can be tailored for specific applications including bone implants, dental materials, and soft tissue replacements, offering improved performance compared to single-component systems.
- Diagnostic and sensing applications of bio-based polymers: Bio-based polymers are increasingly utilized in biomedical diagnostic and sensing applications. These materials can be engineered to respond to specific biological markers, pH changes, or other physiological conditions, making them valuable for biosensors, diagnostic devices, and smart drug delivery systems. Their biocompatibility allows for in vivo applications, while their renewable nature addresses sustainability concerns in healthcare technology development.
02 Polysaccharide-based biomedical materials
Polysaccharides such as cellulose, chitosan, alginate, and hyaluronic acid are being utilized as bio-based materials for biomedical polymers. These naturally occurring polymers offer excellent biocompatibility, biodegradability, and can be chemically modified to enhance their mechanical properties and functionality. They are particularly valuable in wound healing applications, drug delivery systems, and as scaffolds for tissue engineering due to their structural similarity to the extracellular matrix components.Expand Specific Solutions03 Protein-based polymers for biomedical use
Proteins such as collagen, silk fibroin, elastin, and keratin are being developed as bio-based polymers for various biomedical applications. These natural polymers possess inherent bioactivity, cell-binding domains, and can be processed into different forms including films, fibers, hydrogels, and porous scaffolds. Their biological recognition properties make them excellent candidates for tissue engineering, wound dressings, and controlled drug delivery systems.Expand Specific Solutions04 Plant oil-derived polymers in medical devices
Polymers derived from plant oils such as soybean, linseed, and castor oil are emerging as sustainable alternatives to petroleum-based materials in biomedical applications. These renewable resources can be chemically modified to produce polyesters, polyurethanes, and epoxy resins with tunable properties. The resulting biomaterials demonstrate good biocompatibility, controlled degradation rates, and mechanical properties suitable for applications ranging from soft tissue engineering to orthopedic implants.Expand Specific Solutions05 Diagnostic and sensing applications of bio-based polymers
Bio-based polymers are increasingly being utilized in biomedical diagnostic and sensing applications. These materials can be engineered to respond to specific biological stimuli, enabling their use in biosensors, diagnostic devices, and smart drug delivery systems. The inherent biocompatibility of these materials makes them particularly valuable for in vivo sensing applications, while their functional groups allow for easy conjugation with recognition elements such as antibodies, enzymes, or nucleic acids.Expand Specific Solutions
Leading Organizations in Bio-based Medical Material Development
The bio-based materials market in biomedical polymers is currently in a growth phase, with increasing adoption driven by sustainability concerns and biocompatibility advantages. The global market is expanding rapidly, estimated to reach several billion dollars by 2025, with annual growth rates exceeding 15%. Technologically, the field shows varying maturity levels across applications. Leading academic institutions (MIT, Duke University, Fudan University) are advancing fundamental research, while established companies (Boston Scientific, Medtronic Vascular) are commercializing proven applications. Emerging players like Access Vascular and SupraPolix are developing innovative niche solutions. Chinese institutions (Sichuan University, Wuhan University) are increasingly contributing to research output, while specialized firms like Bezwada Biomedical are developing proprietary polymer technologies that bridge research and commercial applications.
Bezwada Biomedical LLC
Technical Solution: Bezwada Biomedical has developed a proprietary platform of bio-based polymers specifically engineered for medical applications. Their technology centers on amino acid-derived polyesteramides that combine the biodegradability of polyesters with the functionality and biocompatibility of polyamides. These materials are synthesized from naturally occurring compounds including phenols, fatty acids, and amino acids to create polymers with controlled degradation profiles. The company's patented "TyroSoft" technology incorporates tyrosine-derived diphenols into polymer backbones, creating materials with excellent mechanical properties and controlled drug release capabilities. Their bio-based polymers feature pendant functional groups that allow for post-polymerization modification, enabling attachment of bioactive molecules, drugs, or cell-recognition motifs. Bezwada has also pioneered absorbable polymers derived from natural fatty acids that demonstrate superior tissue integration and minimal inflammatory response for applications in soft tissue repair and regeneration.
Strengths: Highly customizable degradation profiles tailored to specific medical applications; reduced toxicity concerns compared to synthetic polymers; ability to incorporate bioactive components directly into polymer structure. Weaknesses: Limited long-term clinical data compared to established synthetic materials; higher production costs; potential regulatory hurdles for novel biomaterial approval.
Massachusetts Institute of Technology
Technical Solution: MIT has pioneered innovative bio-based polymeric materials for biomedical applications through their advanced biomaterials research platform. Their technology focuses on developing biodegradable polymers derived from renewable resources such as polysaccharides, proteins, and plant-derived monomers. MIT researchers have created novel hydrogel systems incorporating alginate, hyaluronic acid, and cellulose derivatives with tunable mechanical properties and degradation profiles specifically designed for tissue engineering applications. Their platform includes a proprietary crosslinking methodology that enhances the mechanical stability of these bio-based materials while maintaining biocompatibility. MIT has also developed bio-based shape memory polymers that respond to physiological stimuli, enabling minimally invasive implantation procedures for various medical devices. Their technology incorporates antimicrobial peptides derived from natural sources to create infection-resistant biomaterials for wound healing applications.
Strengths: Superior biocompatibility with reduced foreign body response compared to synthetic alternatives; precisely controlled degradation rates matching tissue regeneration timelines; sustainable sourcing reducing environmental impact. Weaknesses: Higher production costs compared to petroleum-based polymers; batch-to-batch variability of natural materials requiring extensive quality control; potential challenges in scaling manufacturing processes.
Key Innovations in Bio-based Polymer Technology
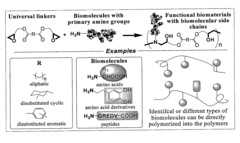
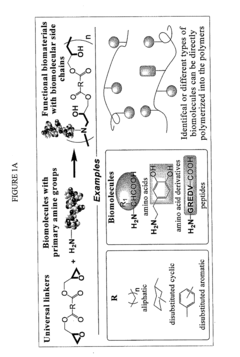
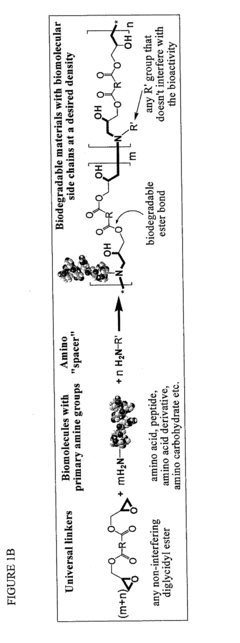
Bio-based monomers and polymers
PatentActiveUS20150259464A1
Innovation
- Development of a novel class of bio-based monomers and polymers derived from compounds like butane diol, succinic acid, L-lactic acid, and vegetable oil-based materials, which can be polymerized to form biodegradable, absorbable, and non-absorbable polymers like polyamides, polyesters, polyureas, and polyurethanes with controlled degradation profiles.
Biomimetic polymers and uses thereof
PatentInactiveUS20090297607A1
Innovation
- Development of biodegradable polymers incorporating biomolecules, such as neurotransmitters and nucleic acids, which are polymerized with diglycidyl esters to create biomimetic polymers that can stimulate neurite growth, deliver therapeutic agents, and promote wound healing by forming matrices with bioactive factors.
Regulatory Framework for Bio-based Medical Polymers
The regulatory landscape for bio-based medical polymers is complex and evolving, reflecting the intersection of environmental sustainability goals with stringent biomedical safety requirements. At the global level, organizations such as the International Organization for Standardization (ISO) have established standards like ISO 10993 for biological evaluation of medical devices, which increasingly incorporate considerations for bio-based materials. These standards ensure that regardless of origin, all materials meet consistent safety and performance benchmarks.
In the United States, the Food and Drug Administration (FDA) oversees the approval process for medical devices and materials through various regulatory pathways including 510(k) clearance and Premarket Approval (PMA). The FDA has recently shown increased interest in sustainable materials, establishing the Sustainable Materials Management program that provides guidance for manufacturers incorporating bio-based polymers into medical applications.
The European Union implements more progressive regulations through the Medical Device Regulation (MDR) and In Vitro Diagnostic Regulation (IVDR), which explicitly encourage the use of sustainable materials when possible without compromising safety. Additionally, the EU's REACH (Registration, Evaluation, Authorization and Restriction of Chemicals) regulation impacts bio-based polymers by requiring thorough documentation of their environmental and health impacts.
Japan's Pharmaceuticals and Medical Devices Agency (PMDA) has developed specific guidelines for biodegradable materials in medical applications, recognizing their unique characteristics and potential benefits. Similarly, China's National Medical Products Administration (NMPA) has established accelerated review processes for innovative medical materials, including certain categories of bio-based polymers.
Regulatory challenges specific to bio-based medical polymers include batch-to-batch consistency, given the natural variability of biological raw materials. Manufacturers must implement robust quality control systems to ensure consistent performance across production lots. Long-term stability testing protocols are also more demanding for bio-based materials, requiring extended shelf-life validation studies.
Certification systems like the USDA BioPreferred program provide additional frameworks for verifying the bio-based content of materials, though these are voluntary and separate from medical device regulations. Industry stakeholders are actively working with regulatory bodies to develop more specific guidance documents for bio-based medical polymers, addressing their unique characteristics while maintaining the paramount focus on patient safety.
In the United States, the Food and Drug Administration (FDA) oversees the approval process for medical devices and materials through various regulatory pathways including 510(k) clearance and Premarket Approval (PMA). The FDA has recently shown increased interest in sustainable materials, establishing the Sustainable Materials Management program that provides guidance for manufacturers incorporating bio-based polymers into medical applications.
The European Union implements more progressive regulations through the Medical Device Regulation (MDR) and In Vitro Diagnostic Regulation (IVDR), which explicitly encourage the use of sustainable materials when possible without compromising safety. Additionally, the EU's REACH (Registration, Evaluation, Authorization and Restriction of Chemicals) regulation impacts bio-based polymers by requiring thorough documentation of their environmental and health impacts.
Japan's Pharmaceuticals and Medical Devices Agency (PMDA) has developed specific guidelines for biodegradable materials in medical applications, recognizing their unique characteristics and potential benefits. Similarly, China's National Medical Products Administration (NMPA) has established accelerated review processes for innovative medical materials, including certain categories of bio-based polymers.
Regulatory challenges specific to bio-based medical polymers include batch-to-batch consistency, given the natural variability of biological raw materials. Manufacturers must implement robust quality control systems to ensure consistent performance across production lots. Long-term stability testing protocols are also more demanding for bio-based materials, requiring extended shelf-life validation studies.
Certification systems like the USDA BioPreferred program provide additional frameworks for verifying the bio-based content of materials, though these are voluntary and separate from medical device regulations. Industry stakeholders are actively working with regulatory bodies to develop more specific guidance documents for bio-based medical polymers, addressing their unique characteristics while maintaining the paramount focus on patient safety.
Sustainability Impact Assessment of Bio-based Medical Materials
The sustainability impact of bio-based medical materials represents a critical dimension in evaluating their overall value proposition for healthcare applications. When assessing these materials through a sustainability lens, multiple environmental parameters must be considered throughout their entire lifecycle, from raw material extraction to end-of-life management.
Bio-based medical polymers demonstrate significant advantages in carbon footprint reduction compared to their petroleum-derived counterparts. Research indicates that materials derived from renewable resources such as polylactic acid (PLA), polyhydroxyalkanoates (PHAs), and cellulose-based polymers can reduce greenhouse gas emissions by 30-70% depending on production methods and feedstock sources. This reduction stems primarily from the carbon sequestration that occurs during biomass growth.
Water consumption patterns also differ markedly between conventional and bio-based medical materials. While bio-based materials often require substantial water inputs during agricultural production phases, they typically demand less water during processing stages. Comprehensive water footprint analyses reveal that regional factors significantly influence the sustainability profile, with materials derived from drought-resistant crops or agricultural waste streams offering optimal water efficiency.
Energy efficiency metrics present a complex picture. Current bio-based material production often requires more energy during conversion processes than established petroleum-based manufacturing. However, this gap continues to narrow as biorefinery technologies advance. The renewable nature of the energy that can be used in bio-based production further enhances their sustainability credentials when integrated with green energy infrastructure.
Waste management considerations reveal perhaps the most compelling sustainability advantage. Many bio-based medical materials offer enhanced biodegradability or compostability under appropriate conditions, potentially reducing medical waste volumes that traditionally require specialized disposal. This characteristic becomes particularly valuable for single-use medical devices and packaging, which constitute a significant portion of healthcare waste streams.
Toxicity profiles of bio-based materials generally show reduced environmental impact during production and disposal phases. The absence of potentially harmful additives like phthalates and bisphenols, commonly found in conventional medical plastics, represents a significant ecological advantage and aligns with increasing regulatory pressure toward greener chemistry in healthcare.
Land use implications remain a challenging aspect of sustainability assessment. The agricultural production required for bio-based materials raises questions about potential competition with food crops and impacts on biodiversity. However, next-generation feedstocks utilizing agricultural waste, algae, and non-food crops grown on marginal lands offer promising pathways to mitigate these concerns.
Bio-based medical polymers demonstrate significant advantages in carbon footprint reduction compared to their petroleum-derived counterparts. Research indicates that materials derived from renewable resources such as polylactic acid (PLA), polyhydroxyalkanoates (PHAs), and cellulose-based polymers can reduce greenhouse gas emissions by 30-70% depending on production methods and feedstock sources. This reduction stems primarily from the carbon sequestration that occurs during biomass growth.
Water consumption patterns also differ markedly between conventional and bio-based medical materials. While bio-based materials often require substantial water inputs during agricultural production phases, they typically demand less water during processing stages. Comprehensive water footprint analyses reveal that regional factors significantly influence the sustainability profile, with materials derived from drought-resistant crops or agricultural waste streams offering optimal water efficiency.
Energy efficiency metrics present a complex picture. Current bio-based material production often requires more energy during conversion processes than established petroleum-based manufacturing. However, this gap continues to narrow as biorefinery technologies advance. The renewable nature of the energy that can be used in bio-based production further enhances their sustainability credentials when integrated with green energy infrastructure.
Waste management considerations reveal perhaps the most compelling sustainability advantage. Many bio-based medical materials offer enhanced biodegradability or compostability under appropriate conditions, potentially reducing medical waste volumes that traditionally require specialized disposal. This characteristic becomes particularly valuable for single-use medical devices and packaging, which constitute a significant portion of healthcare waste streams.
Toxicity profiles of bio-based materials generally show reduced environmental impact during production and disposal phases. The absence of potentially harmful additives like phthalates and bisphenols, commonly found in conventional medical plastics, represents a significant ecological advantage and aligns with increasing regulatory pressure toward greener chemistry in healthcare.
Land use implications remain a challenging aspect of sustainability assessment. The agricultural production required for bio-based materials raises questions about potential competition with food crops and impacts on biodiversity. However, next-generation feedstocks utilizing agricultural waste, algae, and non-food crops grown on marginal lands offer promising pathways to mitigate these concerns.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!