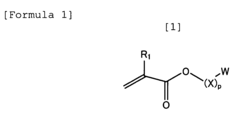
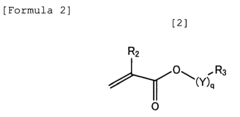
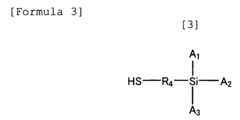
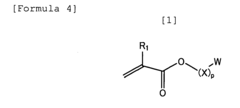
Standards and Qualifications of Biomedical Polymers in Japan
OCT 24, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Biomedical Polymer Standards Evolution in Japan
The evolution of biomedical polymer standards in Japan has followed a distinct trajectory shaped by the country's regulatory philosophy and industrial development. In the 1960s, Japan began establishing its first formal regulations for medical materials, primarily focusing on basic safety requirements rather than specific polymer standards. This period marked the foundation of Japan's approach to biomedical materials regulation, influenced by international developments but tailored to domestic healthcare priorities.
The 1970s witnessed a significant shift with the establishment of the Pharmaceutical Affairs Law (PAL), which created the first comprehensive framework for regulating medical devices and materials. During this decade, initial polymer-specific guidelines emerged, though they remained relatively broad in scope. The Japanese government recognized the growing importance of synthetic polymers in medical applications but had not yet developed the sophisticated classification systems that would come later.
A major advancement occurred in the 1980s with the formation of the Japanese Industrial Standards Committee's specialized subcommittee on biomedical polymers. This period saw the first detailed standards for biocompatibility testing and material characterization specific to polymeric materials used in medical devices. The standards began incorporating more rigorous scientific methodologies, reflecting Japan's growing expertise in polymer science and biomedical engineering.
The 1990s represented a watershed moment in Japanese biomedical polymer standardization with the implementation of the Good Manufacturing Practice (GMP) regulations specifically addressing polymer production for medical use. During this decade, Japan also began actively participating in international standardization efforts through the International Organization for Standardization (ISO), contributing significantly to global standards while harmonizing domestic requirements with international practices.
The early 2000s marked the beginning of Japan's modern regulatory approach with the revision of the PAL and establishment of the Pharmaceuticals and Medical Devices Agency (PMDA) in 2004. This period introduced more sophisticated classification systems for biomedical polymers based on risk categories and intended use, creating a tiered approach to regulation that remains influential today.
From 2010 onward, Japan has focused on developing standards that address emerging technologies such as biodegradable polymers, nanomaterials, and 3D-printed polymer structures. The Japanese regulatory framework has evolved to incorporate more predictive testing methodologies and real-world evidence in evaluating polymer performance and safety. Recent developments have emphasized sustainability considerations and alignment with global regulatory trends while maintaining Japan's distinctive emphasis on material quality and precision manufacturing.
The 1970s witnessed a significant shift with the establishment of the Pharmaceutical Affairs Law (PAL), which created the first comprehensive framework for regulating medical devices and materials. During this decade, initial polymer-specific guidelines emerged, though they remained relatively broad in scope. The Japanese government recognized the growing importance of synthetic polymers in medical applications but had not yet developed the sophisticated classification systems that would come later.
A major advancement occurred in the 1980s with the formation of the Japanese Industrial Standards Committee's specialized subcommittee on biomedical polymers. This period saw the first detailed standards for biocompatibility testing and material characterization specific to polymeric materials used in medical devices. The standards began incorporating more rigorous scientific methodologies, reflecting Japan's growing expertise in polymer science and biomedical engineering.
The 1990s represented a watershed moment in Japanese biomedical polymer standardization with the implementation of the Good Manufacturing Practice (GMP) regulations specifically addressing polymer production for medical use. During this decade, Japan also began actively participating in international standardization efforts through the International Organization for Standardization (ISO), contributing significantly to global standards while harmonizing domestic requirements with international practices.
The early 2000s marked the beginning of Japan's modern regulatory approach with the revision of the PAL and establishment of the Pharmaceuticals and Medical Devices Agency (PMDA) in 2004. This period introduced more sophisticated classification systems for biomedical polymers based on risk categories and intended use, creating a tiered approach to regulation that remains influential today.
From 2010 onward, Japan has focused on developing standards that address emerging technologies such as biodegradable polymers, nanomaterials, and 3D-printed polymer structures. The Japanese regulatory framework has evolved to incorporate more predictive testing methodologies and real-world evidence in evaluating polymer performance and safety. Recent developments have emphasized sustainability considerations and alignment with global regulatory trends while maintaining Japan's distinctive emphasis on material quality and precision manufacturing.
Market Analysis of Japanese Biomedical Polymer Industry
The Japanese biomedical polymer market has demonstrated consistent growth over the past decade, with a current estimated value of 320 billion yen (approximately 2.9 billion USD). This growth trajectory is projected to continue at a compound annual growth rate of 5.7% through 2027, outpacing the global average of 4.9%. The market expansion is primarily driven by Japan's rapidly aging population, with over 28% of citizens aged 65 or older, creating substantial demand for medical devices, implants, and drug delivery systems utilizing advanced polymeric materials.
Healthcare expenditure in Japan represents approximately 10.9% of its GDP, one of the highest rates among developed nations, providing a stable financial foundation for biomedical innovation. The national universal healthcare system further ensures consistent market demand for approved biomedical products, creating a reliable revenue stream for established manufacturers.
The Japanese biomedical polymer sector is characterized by a strong emphasis on high-quality, precision-engineered materials. Market segmentation reveals that orthopedic applications constitute the largest share at 32%, followed by cardiovascular devices (24%), drug delivery systems (18%), tissue engineering (14%), and other applications (12%). Biodegradable polymers represent the fastest-growing segment, with a 9.3% annual growth rate, reflecting increasing clinical preference for materials that reduce long-term foreign body responses.
Domestic production accounts for approximately 65% of the Japanese biomedical polymer market, with the remainder being imported primarily from the United States, Germany, and South Korea. This domestic manufacturing focus is supported by Japan's robust industrial policy promoting healthcare innovation through initiatives like the "Healthcare and Medical Strategy" and "Japan Vision: Health Care 2035."
Market barriers include stringent regulatory requirements imposed by the Pharmaceuticals and Medical Devices Agency (PMDA), which typically extend development timelines by 12-18 months compared to European markets. Additionally, cultural preferences for established domestic brands create challenges for new market entrants, particularly foreign companies without established local partnerships.
Consumer trends indicate growing demand for personalized medical solutions utilizing 3D-printed polymeric structures and smart materials capable of drug elution or biosensing. This shift is creating new market opportunities, particularly in the areas of regenerative medicine and minimally invasive surgical tools, where specialized polymers with precise mechanical and biological properties are essential.
The COVID-19 pandemic has accelerated certain market segments, particularly antimicrobial polymers and materials for diagnostic devices, while temporarily suppressing elective surgical procedures that utilize implantable polymeric devices. However, the market has largely recovered to pre-pandemic growth trajectories as of late 2022.
Healthcare expenditure in Japan represents approximately 10.9% of its GDP, one of the highest rates among developed nations, providing a stable financial foundation for biomedical innovation. The national universal healthcare system further ensures consistent market demand for approved biomedical products, creating a reliable revenue stream for established manufacturers.
The Japanese biomedical polymer sector is characterized by a strong emphasis on high-quality, precision-engineered materials. Market segmentation reveals that orthopedic applications constitute the largest share at 32%, followed by cardiovascular devices (24%), drug delivery systems (18%), tissue engineering (14%), and other applications (12%). Biodegradable polymers represent the fastest-growing segment, with a 9.3% annual growth rate, reflecting increasing clinical preference for materials that reduce long-term foreign body responses.
Domestic production accounts for approximately 65% of the Japanese biomedical polymer market, with the remainder being imported primarily from the United States, Germany, and South Korea. This domestic manufacturing focus is supported by Japan's robust industrial policy promoting healthcare innovation through initiatives like the "Healthcare and Medical Strategy" and "Japan Vision: Health Care 2035."
Market barriers include stringent regulatory requirements imposed by the Pharmaceuticals and Medical Devices Agency (PMDA), which typically extend development timelines by 12-18 months compared to European markets. Additionally, cultural preferences for established domestic brands create challenges for new market entrants, particularly foreign companies without established local partnerships.
Consumer trends indicate growing demand for personalized medical solutions utilizing 3D-printed polymeric structures and smart materials capable of drug elution or biosensing. This shift is creating new market opportunities, particularly in the areas of regenerative medicine and minimally invasive surgical tools, where specialized polymers with precise mechanical and biological properties are essential.
The COVID-19 pandemic has accelerated certain market segments, particularly antimicrobial polymers and materials for diagnostic devices, while temporarily suppressing elective surgical procedures that utilize implantable polymeric devices. However, the market has largely recovered to pre-pandemic growth trajectories as of late 2022.
Current Regulatory Framework and Technical Barriers
Japan's regulatory framework for biomedical polymers is primarily governed by the Pharmaceutical and Medical Device Agency (PMDA) under the Ministry of Health, Labour and Welfare (MHLW). The Japanese regulatory system follows a classification-based approach similar to other major markets but with distinct requirements that create unique challenges for manufacturers and developers. The primary legislation, the Pharmaceutical and Medical Devices Act (PMD Act), categorizes medical devices into four classes based on risk levels, with most polymer-based implantable devices falling into Class III or IV, requiring the most stringent approval processes.
The Japanese Industrial Standards (JIS) work in conjunction with international standards such as ISO 10993 series for biocompatibility testing, but often impose additional Japan-specific requirements. For biomedical polymers, the PMDA requires comprehensive documentation on manufacturing processes, raw material sourcing, and sterilization methods that exceed those of other regulatory bodies. This creates a significant technical barrier for foreign manufacturers attempting to enter the Japanese market.
A notable technical barrier is Japan's emphasis on local clinical data. Despite efforts to harmonize with global standards through the International Medical Device Regulators Forum (IMDRF), the PMDA frequently requires Japan-specific clinical evaluations even when substantial foreign clinical data exists. For novel biomedical polymers, this often necessitates additional clinical trials specifically designed for Japanese populations, significantly increasing time-to-market and development costs.
Material qualification standards in Japan present another challenge. The country maintains its own positive list of approved materials for medical applications, and polymers not previously approved in Japan face extensive testing requirements. The Japanese Pharmacopoeia (JP) and Japanese Standards for Medical Devices (JSMD) contain specific testing methodologies that sometimes differ from international standards, requiring specialized knowledge and testing capabilities.
Foreign manufacturers also encounter barriers related to quality management systems. While Japan recognizes ISO 13485 certification, the PMDA conducts its own Quality Management System audits with Japan-specific interpretations of quality standards. For biomedical polymers, this includes detailed verification of process controls, particularly for novel manufacturing techniques like 3D printing or specialized coating processes.
Translation requirements create additional complexity, as all technical documentation must be submitted in Japanese. This extends beyond simple translation to include cultural and contextual adaptation of technical specifications, particularly for novel polymer technologies where standardized terminology may not yet exist in Japanese technical language.
Recent regulatory reforms have attempted to address some of these barriers through the "Sakigake" designation system for innovative medical products and harmonization efforts with international standards. However, significant technical barriers remain, particularly for novel biomedical polymers utilizing cutting-edge technologies like nanomaterials or biodegradable composites, where Japanese standards are still evolving.
The Japanese Industrial Standards (JIS) work in conjunction with international standards such as ISO 10993 series for biocompatibility testing, but often impose additional Japan-specific requirements. For biomedical polymers, the PMDA requires comprehensive documentation on manufacturing processes, raw material sourcing, and sterilization methods that exceed those of other regulatory bodies. This creates a significant technical barrier for foreign manufacturers attempting to enter the Japanese market.
A notable technical barrier is Japan's emphasis on local clinical data. Despite efforts to harmonize with global standards through the International Medical Device Regulators Forum (IMDRF), the PMDA frequently requires Japan-specific clinical evaluations even when substantial foreign clinical data exists. For novel biomedical polymers, this often necessitates additional clinical trials specifically designed for Japanese populations, significantly increasing time-to-market and development costs.
Material qualification standards in Japan present another challenge. The country maintains its own positive list of approved materials for medical applications, and polymers not previously approved in Japan face extensive testing requirements. The Japanese Pharmacopoeia (JP) and Japanese Standards for Medical Devices (JSMD) contain specific testing methodologies that sometimes differ from international standards, requiring specialized knowledge and testing capabilities.
Foreign manufacturers also encounter barriers related to quality management systems. While Japan recognizes ISO 13485 certification, the PMDA conducts its own Quality Management System audits with Japan-specific interpretations of quality standards. For biomedical polymers, this includes detailed verification of process controls, particularly for novel manufacturing techniques like 3D printing or specialized coating processes.
Translation requirements create additional complexity, as all technical documentation must be submitted in Japanese. This extends beyond simple translation to include cultural and contextual adaptation of technical specifications, particularly for novel polymer technologies where standardized terminology may not yet exist in Japanese technical language.
Recent regulatory reforms have attempted to address some of these barriers through the "Sakigake" designation system for innovative medical products and harmonization efforts with international standards. However, significant technical barriers remain, particularly for novel biomedical polymers utilizing cutting-edge technologies like nanomaterials or biodegradable composites, where Japanese standards are still evolving.
Certification Processes and Testing Methodologies
01 Biodegradable polymers for medical applications
Biodegradable polymers are extensively used in biomedical applications due to their ability to break down in the body over time. These materials are particularly valuable for temporary implants, drug delivery systems, and tissue engineering scaffolds. Common biodegradable polymers include polylactic acid (PLA), polyglycolic acid (PGA), and their copolymers, which offer controlled degradation rates and biocompatibility. These materials eliminate the need for removal surgeries and reduce long-term foreign body responses.- Biodegradable polymers for medical applications: Biodegradable polymers are extensively used in biomedical applications due to their ability to break down safely in the body over time. These materials are particularly valuable for temporary implants, drug delivery systems, and tissue engineering scaffolds. Common biodegradable polymers include polylactic acid (PLA), polyglycolic acid (PGA), and their copolymers, which offer controlled degradation rates and biocompatibility. These materials eliminate the need for removal surgeries and can be engineered to release therapeutic agents as they degrade.
- Biocompatible polymers for implantable devices: Biocompatible polymers are designed to function within the body without causing adverse reactions or immune responses. These materials are crucial for long-term implantable devices such as sensors, neural interfaces, and permanent prosthetics. Key properties include resistance to protein adsorption, minimal inflammatory response, and long-term stability in physiological environments. Advanced biocompatible polymers often incorporate surface modifications or anti-fouling properties to extend device lifetime and functionality while maintaining tissue integration.
- Smart polymers with stimuli-responsive properties: Smart polymers can change their physical or chemical properties in response to environmental stimuli such as temperature, pH, light, or electrical signals. In biomedical applications, these materials enable controlled drug release, self-regulating systems, and adaptive interfaces with biological tissues. Examples include temperature-responsive polymers that undergo phase transitions at body temperature, pH-sensitive polymers that respond to different physiological environments, and electrically conductive polymers for neural interfaces and biosensing applications.
- Polymer composites for tissue engineering: Polymer composites combine different materials to create structures with enhanced mechanical, biological, or functional properties for tissue engineering applications. These composites often incorporate bioactive components such as hydroxyapatite for bone regeneration, growth factors for tissue development, or conductive elements for neural tissue engineering. The polymer matrix provides structural support while additional components promote specific cellular responses, vascularization, or tissue integration. These materials can be processed into various forms including porous scaffolds, hydrogels, or fibrous matrices.
- Polymer processing techniques for biomedical applications: Specialized processing techniques are essential for transforming biomedical polymers into functional devices and structures. These include electrospinning for creating fibrous scaffolds with controlled porosity, 3D printing for patient-specific implants and tissue constructs, solvent casting for films and coatings, and various molding techniques. Advanced processing methods often focus on preserving bioactivity of incorporated therapeutic agents, controlling micro and nanostructure, and ensuring sterility of the final product while maintaining the polymer's beneficial properties.
02 Polymers for implantable medical devices
Specialized polymers are developed for implantable medical devices that require specific mechanical properties, biocompatibility, and durability. These polymers are engineered to withstand the physiological environment while performing their intended functions. Applications include cardiovascular implants, orthopedic devices, and neural interfaces. The polymers may incorporate antimicrobial properties, anti-inflammatory agents, or surface modifications to enhance integration with surrounding tissues and prevent adverse reactions.Expand Specific Solutions03 Smart polymers with stimuli-responsive properties
Smart polymers that respond to environmental stimuli such as temperature, pH, light, or electrical signals are increasingly important in biomedical applications. These materials can change their physical or chemical properties in response to specific triggers, enabling controlled drug release, self-regulating systems, and adaptive interfaces. Applications include temperature-responsive drug delivery systems, pH-sensitive coatings for targeted release in specific body compartments, and shape-memory polymers for minimally invasive implantation procedures.Expand Specific Solutions04 Polymer-based biosensors and diagnostic devices
Polymers are utilized in the development of biosensors and diagnostic devices due to their versatility, processability, and ability to be functionalized with biorecognition elements. Conductive polymers, hydrogels, and nanocomposites enable the creation of sensitive and specific detection platforms for various biomarkers. These materials can be integrated into wearable devices, point-of-care diagnostics, and implantable sensors for continuous monitoring of physiological parameters and disease markers.Expand Specific Solutions05 Polymer-based drug delivery systems
Polymeric materials are extensively used in drug delivery systems to improve therapeutic efficacy, reduce side effects, and enable controlled release profiles. These systems include nanoparticles, microparticles, hydrogels, and implantable devices that can encapsulate and release drugs at predetermined rates or in response to specific triggers. Polymers can be engineered to target specific tissues or cells, cross biological barriers, and protect sensitive therapeutic agents from degradation, significantly enhancing treatment outcomes for various diseases.Expand Specific Solutions
Leading Japanese Companies and Research Institutions
The biomedical polymers market in Japan is in a mature growth phase, characterized by established regulatory frameworks and significant R&D investments. The market size is estimated at approximately $2-3 billion, with steady annual growth of 5-7%. Leading Japanese companies like Toray Industries, Kuraray, Toyobo, and Terumo have achieved high technical maturity in specialized biomedical polymers, particularly in areas of biocompatibility and functionality. International players such as Medtronic (via Covidien) maintain strategic positions, while domestic firms including Asahi Kasei Medical, Kaneka, and Sumitomo Bakelite leverage their materials expertise to develop innovative solutions. The market demonstrates strong integration between academic research (Tokyo Institute of Technology) and industrial applications, with increasing focus on biodegradable polymers and tissue engineering materials.
Toray Industries, Inc.
Technical Solution: Toray Industries has developed advanced biocompatible polymers that meet Japan's stringent medical device standards. Their proprietary TORAYDELEF® polyacetal resin series is specifically engineered for medical applications with superior mechanical properties and chemical resistance[1]. The company has implemented a comprehensive quality management system compliant with ISO 13485 for medical devices and follows the Japanese Pharmaceutical Affairs Law requirements. Their biomedical polymers undergo rigorous biocompatibility testing according to ISO 10993 standards, including cytotoxicity, sensitization, and hemocompatibility assessments[2]. Toray has also pioneered PMMA-based dialysis membranes that meet Japanese specifications for blood purification devices, with controlled porosity and exceptional biocompatibility profiles. Their R&D facilities in Japan work closely with regulatory authorities to ensure compliance with the PMDA (Pharmaceuticals and Medical Devices Agency) requirements for Class III and IV medical devices[3].
Strengths: Extensive experience in medical-grade polymers with established regulatory compliance pathways in Japan; strong vertical integration from raw materials to finished medical components. Weaknesses: Higher cost structure compared to competitors; longer development cycles for new materials due to comprehensive testing requirements.
Kuraray Co., Ltd.
Technical Solution: Kuraray has established itself as a leader in specialized biomedical polymers that meet Japan's strict regulatory framework. Their EVAL™ (ethylene vinyl alcohol copolymer) technology has been specifically adapted to comply with Japanese standards for implantable and blood-contacting devices[1]. The company has developed proprietary manufacturing processes that ensure ultra-high purity levels required by Japanese regulations, with contamination levels below 1 ppm for critical applications. Kuraray's biomedical polymers undergo comprehensive biocompatibility testing according to the Japanese Industrial Standards (JIS) T 0993 series, which harmonizes with ISO 10993 but includes additional Japan-specific requirements[2]. Their quality management system is certified to both ISO 13485 and Japan's JGMP (Japanese Good Manufacturing Practice) standards. Kuraray collaborates with leading Japanese medical institutions to validate their materials' performance in clinical settings, generating the necessary data for PMDA submissions[3]. Their polymers are used in various medical applications including artificial organs, drug delivery systems, and diagnostic devices approved in the Japanese market.
Strengths: Specialized expertise in high-performance barrier polymers with exceptional purity profiles; strong relationships with Japanese regulatory bodies and medical institutions. Weaknesses: Relatively narrow product portfolio compared to larger competitors; higher production costs due to specialized manufacturing processes required to meet Japanese standards.
Key Patents and Technical Documentation Analysis
High molecular compound for medical material and biochip substrate using such high molecular compound
PatentInactiveEP1882708A1
Innovation
- A polymer compound is developed, comprising ethylenically unsaturated polymerizable monomers with functional groups for fixing biologically active substances and alkylene glycol residues, which forms a stable covalent bond with the substrate, reducing nonspecific adsorption and maintaining chemical stability during washing.
Sanitary container
PatentInactiveEP0825223B1
Innovation
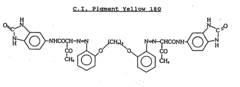
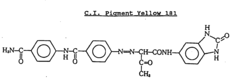
- Incorporating specific organic pigments, such as C.I. Pigment Yellow 180 or C.I. Pigment Yellow 181, into cyclic olefin polymer containers to provide effective UV shielding without compromising transparency, allowing for stable storage of sensitive materials over long periods.
International Compliance and Harmonization Efforts
Japan's biomedical polymer industry operates within a complex global regulatory landscape that necessitates significant international cooperation. The Japanese regulatory framework, primarily governed by the Pharmaceuticals and Medical Devices Agency (PMDA), has been actively participating in global harmonization initiatives to streamline approval processes and ensure consistent quality standards across borders.
The International Medical Device Regulators Forum (IMDRF), which succeeded the Global Harmonization Task Force (GHTF), represents one of the most significant platforms where Japan collaborates with other major regulatory authorities including the US FDA, European Medicines Agency, and regulatory bodies from Canada, Australia, and Brazil. Japan's PMDA has been instrumental in developing harmonized guidelines for biomedical polymer evaluation, particularly in areas of biocompatibility testing and risk assessment methodologies.
Japan is also a signatory to the Medical Device Single Audit Program (MDSAP), which allows for a single regulatory audit to satisfy the requirements of multiple regulatory jurisdictions. This program significantly reduces the regulatory burden for manufacturers of biomedical polymers seeking multi-market approval, while maintaining rigorous quality standards across participating countries.
The harmonization of ISO 10993 standards for biological evaluation of medical devices represents another critical area where Japan has aligned its domestic requirements with international norms. Japanese manufacturers and regulatory authorities have adopted these standards while contributing to their ongoing development through active participation in ISO technical committees.
Mutual Recognition Agreements (MRAs) between Japan and other major markets have facilitated the exchange of conformity assessment results for biomedical polymers. The Japan-EU MRA, in particular, has streamlined the certification process for certain categories of medical devices incorporating polymeric materials, reducing duplicative testing requirements while maintaining safety standards.
Despite these advances, challenges remain in achieving complete regulatory convergence. Japan maintains certain country-specific requirements for biomedical polymers, particularly regarding extractables and leachables testing protocols and acceptance criteria for certain high-risk applications. These differences reflect Japan's unique approach to risk management and patient safety considerations.
The Japan Federation of Medical Devices Associations (JFMDA) works closely with international counterparts like AdvaMed and MedTech Europe to advocate for further harmonization while preserving necessary safeguards. Their collaborative efforts focus on developing common technical documents and standardized testing methodologies that can be universally accepted across major regulatory jurisdictions.
The International Medical Device Regulators Forum (IMDRF), which succeeded the Global Harmonization Task Force (GHTF), represents one of the most significant platforms where Japan collaborates with other major regulatory authorities including the US FDA, European Medicines Agency, and regulatory bodies from Canada, Australia, and Brazil. Japan's PMDA has been instrumental in developing harmonized guidelines for biomedical polymer evaluation, particularly in areas of biocompatibility testing and risk assessment methodologies.
Japan is also a signatory to the Medical Device Single Audit Program (MDSAP), which allows for a single regulatory audit to satisfy the requirements of multiple regulatory jurisdictions. This program significantly reduces the regulatory burden for manufacturers of biomedical polymers seeking multi-market approval, while maintaining rigorous quality standards across participating countries.
The harmonization of ISO 10993 standards for biological evaluation of medical devices represents another critical area where Japan has aligned its domestic requirements with international norms. Japanese manufacturers and regulatory authorities have adopted these standards while contributing to their ongoing development through active participation in ISO technical committees.
Mutual Recognition Agreements (MRAs) between Japan and other major markets have facilitated the exchange of conformity assessment results for biomedical polymers. The Japan-EU MRA, in particular, has streamlined the certification process for certain categories of medical devices incorporating polymeric materials, reducing duplicative testing requirements while maintaining safety standards.
Despite these advances, challenges remain in achieving complete regulatory convergence. Japan maintains certain country-specific requirements for biomedical polymers, particularly regarding extractables and leachables testing protocols and acceptance criteria for certain high-risk applications. These differences reflect Japan's unique approach to risk management and patient safety considerations.
The Japan Federation of Medical Devices Associations (JFMDA) works closely with international counterparts like AdvaMed and MedTech Europe to advocate for further harmonization while preserving necessary safeguards. Their collaborative efforts focus on developing common technical documents and standardized testing methodologies that can be universally accepted across major regulatory jurisdictions.
Biocompatibility and Safety Assessment Protocols
Japan has established a comprehensive framework for biocompatibility and safety assessment of biomedical polymers, which aligns with international standards while incorporating specific national requirements. The Japanese regulatory body, Pharmaceuticals and Medical Devices Agency (PMDA), enforces stringent protocols that manufacturers must follow before their polymer-based medical devices can enter the Japanese market.
The primary biocompatibility assessment framework in Japan follows the ISO 10993 series, particularly ISO 10993-1, which outlines the biological evaluation of medical devices. However, Japan has implemented additional requirements through its own standards, such as the Japanese Industrial Standards (JIS) and specific PMDA guidelines that address unique aspects of polymer safety evaluation.
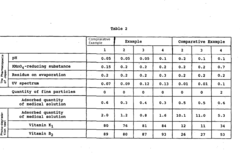
For cytotoxicity testing, Japanese protocols mandate both in vitro and in vivo assessments. The in vitro methods typically include the colony formation inhibition test, agar diffusion test, and extract dilution test using L929 mouse fibroblast cells. These tests must be conducted according to JIS T 0993-5, which provides detailed methodologies for evaluating the cytotoxic potential of biomedical polymers.
Sensitization and irritation testing protocols in Japan require the use of specific animal models, typically guinea pigs for sensitization tests and rabbits for irritation tests. The Magnusson-Kligman maximization test and closed patch test are commonly employed methods, with specific attention to Japanese environmental and genetic factors that might influence test outcomes.
Systemic toxicity assessment follows a tiered approach in Japan, beginning with acute toxicity testing and progressing to subchronic and chronic toxicity evaluations based on the intended duration of patient contact. Japanese protocols emphasize the importance of evaluating polymer degradation products and leachables, particularly for implantable devices.
Hemocompatibility testing is particularly rigorous in Japan, with specific requirements for thrombogenicity, hemolysis, and complement activation tests. The Japanese standards require that these tests be performed using both human blood and animal models, with specific attention to Japanese population genetic factors that might influence blood compatibility.
Genotoxicity and carcinogenicity assessments follow a battery approach, including Ames test, chromosomal aberration test, and micronucleus test. For long-term implantable polymers, additional carcinogenicity studies may be required according to Japanese guidelines, which often exceed international requirements in terms of test duration and observation parameters.
Implantation studies in Japan must follow specific protocols regarding animal selection, implantation sites, and histopathological evaluation methods. The Japanese standards emphasize long-term evaluation of tissue response and polymer degradation characteristics, with particular attention to local tissue reactions and potential systemic effects.
The primary biocompatibility assessment framework in Japan follows the ISO 10993 series, particularly ISO 10993-1, which outlines the biological evaluation of medical devices. However, Japan has implemented additional requirements through its own standards, such as the Japanese Industrial Standards (JIS) and specific PMDA guidelines that address unique aspects of polymer safety evaluation.
For cytotoxicity testing, Japanese protocols mandate both in vitro and in vivo assessments. The in vitro methods typically include the colony formation inhibition test, agar diffusion test, and extract dilution test using L929 mouse fibroblast cells. These tests must be conducted according to JIS T 0993-5, which provides detailed methodologies for evaluating the cytotoxic potential of biomedical polymers.
Sensitization and irritation testing protocols in Japan require the use of specific animal models, typically guinea pigs for sensitization tests and rabbits for irritation tests. The Magnusson-Kligman maximization test and closed patch test are commonly employed methods, with specific attention to Japanese environmental and genetic factors that might influence test outcomes.
Systemic toxicity assessment follows a tiered approach in Japan, beginning with acute toxicity testing and progressing to subchronic and chronic toxicity evaluations based on the intended duration of patient contact. Japanese protocols emphasize the importance of evaluating polymer degradation products and leachables, particularly for implantable devices.
Hemocompatibility testing is particularly rigorous in Japan, with specific requirements for thrombogenicity, hemolysis, and complement activation tests. The Japanese standards require that these tests be performed using both human blood and animal models, with specific attention to Japanese population genetic factors that might influence blood compatibility.
Genotoxicity and carcinogenicity assessments follow a battery approach, including Ames test, chromosomal aberration test, and micronucleus test. For long-term implantable polymers, additional carcinogenicity studies may be required according to Japanese guidelines, which often exceed international requirements in terms of test duration and observation parameters.
Implantation studies in Japan must follow specific protocols regarding animal selection, implantation sites, and histopathological evaluation methods. The Japanese standards emphasize long-term evaluation of tissue response and polymer degradation characteristics, with particular attention to local tissue reactions and potential systemic effects.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!