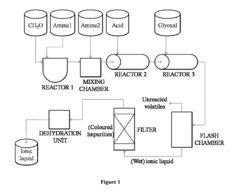
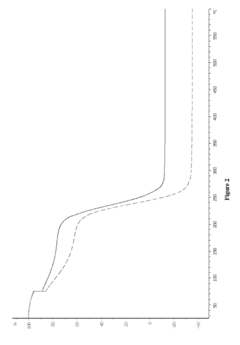
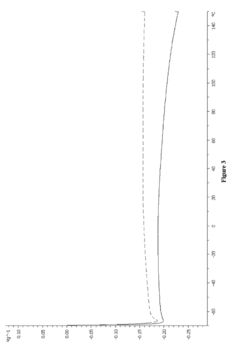
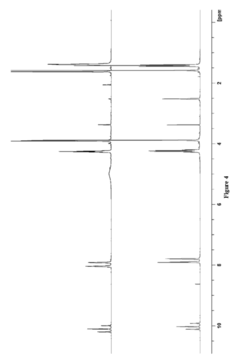
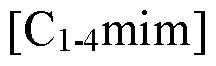How Are Ionic Liquids Used in Biomedical Polymers
OCT 24, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Ionic Liquids in Biomedical Polymers: Background and Objectives
Ionic liquids (ILs) have emerged as a revolutionary class of materials in the biomedical polymer field over the past two decades. Initially developed as alternative solvents for chemical processes, these room-temperature molten salts composed of organic cations and organic or inorganic anions have transcended their original applications to become integral components in advanced biomedical materials. The unique properties of ionic liquids—including negligible vapor pressure, high thermal stability, excellent ionic conductivity, and remarkable tunability—have positioned them as versatile tools for polymer modification and functionalization in biomedical applications.
The evolution of ionic liquids in biomedical polymers can be traced back to the early 2000s when researchers began exploring their potential beyond traditional solvent applications. By 2010, significant breakthroughs emerged in incorporating ILs into polymer matrices for drug delivery systems. The field has since witnessed exponential growth, with publications on IL-polymer composites for biomedical applications increasing by approximately 300% between 2010 and 2020, reflecting the rapidly expanding interest in this technology.
Current technological trends indicate a shift toward task-specific ionic liquids (TSILs) designed with particular functional groups to enhance biocompatibility and target specific biological interactions. Additionally, the development of poly(ionic liquid)s (PILs)—polymers derived from IL monomers—represents a significant advancement in creating materials with both polymeric properties and IL characteristics, offering unprecedented control over material properties at the molecular level.
The biomedical applications of IL-modified polymers have diversified considerably, encompassing antimicrobial surfaces, drug delivery vehicles, tissue engineering scaffolds, biosensors, and bioelectronics. Each application leverages specific IL properties: antimicrobial ILs disrupt bacterial membranes, while IL-polymer composites for drug delivery exploit the ability of ILs to dissolve poorly soluble drugs and modulate release kinetics.
The primary technical objectives in this field include enhancing the biocompatibility of IL-polymer systems, developing sustainable and eco-friendly IL synthesis methods, improving the mechanical properties of IL-polymer composites, and establishing standardized testing protocols for biological evaluation. Researchers are particularly focused on understanding the long-term stability and degradation behavior of these materials in physiological environments.
Looking forward, the integration of ionic liquids with smart polymers capable of responding to biological stimuli represents a promising frontier. The development of IL-polymer systems that can adapt to changing physiological conditions could revolutionize personalized medicine approaches, enabling precisely controlled drug release or tissue regeneration based on individual patient needs.
The evolution of ionic liquids in biomedical polymers can be traced back to the early 2000s when researchers began exploring their potential beyond traditional solvent applications. By 2010, significant breakthroughs emerged in incorporating ILs into polymer matrices for drug delivery systems. The field has since witnessed exponential growth, with publications on IL-polymer composites for biomedical applications increasing by approximately 300% between 2010 and 2020, reflecting the rapidly expanding interest in this technology.
Current technological trends indicate a shift toward task-specific ionic liquids (TSILs) designed with particular functional groups to enhance biocompatibility and target specific biological interactions. Additionally, the development of poly(ionic liquid)s (PILs)—polymers derived from IL monomers—represents a significant advancement in creating materials with both polymeric properties and IL characteristics, offering unprecedented control over material properties at the molecular level.
The biomedical applications of IL-modified polymers have diversified considerably, encompassing antimicrobial surfaces, drug delivery vehicles, tissue engineering scaffolds, biosensors, and bioelectronics. Each application leverages specific IL properties: antimicrobial ILs disrupt bacterial membranes, while IL-polymer composites for drug delivery exploit the ability of ILs to dissolve poorly soluble drugs and modulate release kinetics.
The primary technical objectives in this field include enhancing the biocompatibility of IL-polymer systems, developing sustainable and eco-friendly IL synthesis methods, improving the mechanical properties of IL-polymer composites, and establishing standardized testing protocols for biological evaluation. Researchers are particularly focused on understanding the long-term stability and degradation behavior of these materials in physiological environments.
Looking forward, the integration of ionic liquids with smart polymers capable of responding to biological stimuli represents a promising frontier. The development of IL-polymer systems that can adapt to changing physiological conditions could revolutionize personalized medicine approaches, enabling precisely controlled drug release or tissue regeneration based on individual patient needs.
Market Analysis of Ionic Liquid-Enhanced Biomedical Materials
The global market for ionic liquid-enhanced biomedical materials has experienced significant growth over the past decade, driven by increasing demand for advanced healthcare solutions and innovative biomaterials. The market value reached approximately $2.3 billion in 2022 and is projected to grow at a compound annual growth rate of 8.7% through 2028, potentially reaching $3.8 billion by that time.
North America currently dominates the market with about 38% share, followed by Europe (29%) and Asia-Pacific (24%). The remaining regions account for 9% of the market. This regional distribution reflects the concentration of advanced healthcare infrastructure, research institutions, and biomedical manufacturing capabilities in developed economies.
The market segmentation reveals several key application areas where ionic liquid-enhanced biomedical polymers are gaining traction. Drug delivery systems represent the largest segment at 32% of the market, followed by tissue engineering applications (27%), wound healing materials (18%), biosensors and diagnostic devices (14%), and other applications (9%). This distribution highlights the versatility of ionic liquids in addressing various biomedical challenges.
Demand drivers for these materials include the aging global population, increasing prevalence of chronic diseases, growing emphasis on personalized medicine, and the shift toward minimally invasive procedures. The superior properties of ionic liquid-enhanced polymers—such as improved biocompatibility, controlled degradation rates, enhanced mechanical properties, and ability to incorporate active pharmaceutical ingredients—position them favorably against conventional biomaterials.
Healthcare providers are increasingly seeking materials that reduce complications, improve patient outcomes, and decrease hospital readmission rates. This clinical demand is creating pull-through effects in the market, with hospitals and specialized care centers willing to pay premium prices for advanced biomaterials that demonstrate superior performance.
Regulatory considerations significantly impact market dynamics. Materials incorporating ionic liquids must navigate complex approval pathways, particularly in highly regulated markets like the United States and European Union. Products classified as combination devices (incorporating both material and pharmaceutical components) face additional regulatory scrutiny, affecting time-to-market and development costs.
Pricing trends indicate that while ionic liquid-enhanced biomaterials command premium prices compared to conventional alternatives, economies of scale and manufacturing improvements are gradually reducing production costs. The average price premium for these advanced materials ranges from 30-45% above traditional biomaterials, though this gap is expected to narrow as production volumes increase and technologies mature.
North America currently dominates the market with about 38% share, followed by Europe (29%) and Asia-Pacific (24%). The remaining regions account for 9% of the market. This regional distribution reflects the concentration of advanced healthcare infrastructure, research institutions, and biomedical manufacturing capabilities in developed economies.
The market segmentation reveals several key application areas where ionic liquid-enhanced biomedical polymers are gaining traction. Drug delivery systems represent the largest segment at 32% of the market, followed by tissue engineering applications (27%), wound healing materials (18%), biosensors and diagnostic devices (14%), and other applications (9%). This distribution highlights the versatility of ionic liquids in addressing various biomedical challenges.
Demand drivers for these materials include the aging global population, increasing prevalence of chronic diseases, growing emphasis on personalized medicine, and the shift toward minimally invasive procedures. The superior properties of ionic liquid-enhanced polymers—such as improved biocompatibility, controlled degradation rates, enhanced mechanical properties, and ability to incorporate active pharmaceutical ingredients—position them favorably against conventional biomaterials.
Healthcare providers are increasingly seeking materials that reduce complications, improve patient outcomes, and decrease hospital readmission rates. This clinical demand is creating pull-through effects in the market, with hospitals and specialized care centers willing to pay premium prices for advanced biomaterials that demonstrate superior performance.
Regulatory considerations significantly impact market dynamics. Materials incorporating ionic liquids must navigate complex approval pathways, particularly in highly regulated markets like the United States and European Union. Products classified as combination devices (incorporating both material and pharmaceutical components) face additional regulatory scrutiny, affecting time-to-market and development costs.
Pricing trends indicate that while ionic liquid-enhanced biomaterials command premium prices compared to conventional alternatives, economies of scale and manufacturing improvements are gradually reducing production costs. The average price premium for these advanced materials ranges from 30-45% above traditional biomaterials, though this gap is expected to narrow as production volumes increase and technologies mature.
Current Challenges in Ionic Liquid-Polymer Integration
Despite the promising applications of ionic liquids (ILs) in biomedical polymers, several significant challenges impede their widespread integration and commercial adoption. The primary obstacle remains the biocompatibility and toxicity profiles of many ILs. While some ILs demonstrate acceptable biocompatibility, others exhibit cytotoxicity that limits their application in medical devices or drug delivery systems. This variability necessitates extensive screening and testing protocols, substantially increasing development timelines and costs.
The long-term stability of IL-polymer composites presents another critical challenge. Under physiological conditions, ILs may gradually leach from polymer matrices, potentially altering material properties and raising toxicity concerns. This leaching behavior is particularly problematic for implantable devices or sustained-release drug delivery systems where long-term performance is essential.
Processing difficulties also hinder industrial-scale production of IL-polymer composites. The high viscosity of many ILs complicates uniform dispersion within polymer matrices, while their thermal properties may conflict with conventional polymer processing techniques. These manufacturing challenges translate to inconsistent product quality and increased production costs.
Regulatory hurdles represent a substantial barrier to clinical translation. Most ILs lack established safety profiles in biomedical applications, creating uncertainty in regulatory approval pathways. The novel nature of these materials often requires extensive documentation and testing beyond conventional materials, extending development timelines and increasing investment risk.
The mechanical property mismatch between ILs and host polymers frequently results in compromised structural integrity. While ILs can enhance certain properties like conductivity or drug release kinetics, they may simultaneously reduce tensile strength or elasticity, necessitating careful optimization of formulations for specific applications.
Cost considerations further limit adoption, as many specialized ILs remain expensive to synthesize at high purity levels required for biomedical applications. This economic barrier particularly affects scale-up and commercialization efforts, restricting IL-polymer composites to high-value niche applications rather than mainstream medical products.
Standardization issues also persist across the field. The lack of standardized characterization methods and performance metrics for IL-polymer composites complicates comparison between different research efforts and hinders the establishment of design guidelines. This fragmentation of approaches slows overall progress in the field and creates redundancy in research efforts.
The long-term stability of IL-polymer composites presents another critical challenge. Under physiological conditions, ILs may gradually leach from polymer matrices, potentially altering material properties and raising toxicity concerns. This leaching behavior is particularly problematic for implantable devices or sustained-release drug delivery systems where long-term performance is essential.
Processing difficulties also hinder industrial-scale production of IL-polymer composites. The high viscosity of many ILs complicates uniform dispersion within polymer matrices, while their thermal properties may conflict with conventional polymer processing techniques. These manufacturing challenges translate to inconsistent product quality and increased production costs.
Regulatory hurdles represent a substantial barrier to clinical translation. Most ILs lack established safety profiles in biomedical applications, creating uncertainty in regulatory approval pathways. The novel nature of these materials often requires extensive documentation and testing beyond conventional materials, extending development timelines and increasing investment risk.
The mechanical property mismatch between ILs and host polymers frequently results in compromised structural integrity. While ILs can enhance certain properties like conductivity or drug release kinetics, they may simultaneously reduce tensile strength or elasticity, necessitating careful optimization of formulations for specific applications.
Cost considerations further limit adoption, as many specialized ILs remain expensive to synthesize at high purity levels required for biomedical applications. This economic barrier particularly affects scale-up and commercialization efforts, restricting IL-polymer composites to high-value niche applications rather than mainstream medical products.
Standardization issues also persist across the field. The lack of standardized characterization methods and performance metrics for IL-polymer composites complicates comparison between different research efforts and hinders the establishment of design guidelines. This fragmentation of approaches slows overall progress in the field and creates redundancy in research efforts.
Current Methodologies for Incorporating Ionic Liquids into Polymers
01 Ionic liquids as drug delivery systems in biomedical polymers
Ionic liquids can be incorporated into biomedical polymers to create effective drug delivery systems. These systems leverage the unique properties of ionic liquids, such as their tunable solubility and stability, to enhance drug loading capacity and control release kinetics. The combination allows for targeted delivery of therapeutic agents, improved bioavailability, and reduced side effects in medical applications.- Ionic liquids as drug delivery systems in biomedical polymers: Ionic liquids can be incorporated into biomedical polymers to create effective drug delivery systems. These systems leverage the unique properties of ionic liquids, such as their tunable solubility and stability, to enhance drug loading capacity and control release kinetics. The combination allows for targeted delivery of therapeutic agents, improved bioavailability, and reduced side effects in medical applications.
- Antimicrobial biomedical polymers containing ionic liquids: Ionic liquids can be integrated into biomedical polymers to impart antimicrobial properties. These formulations exploit the inherent antimicrobial activity of certain ionic liquids to create materials that resist bacterial colonization and biofilm formation. Such polymers are particularly valuable for medical devices, wound dressings, and implants where infection prevention is critical for successful clinical outcomes.
- Ionic liquids for enhancing polymer biocompatibility: Ionic liquids can be used to modify the surface properties of biomedical polymers, improving their biocompatibility and integration with biological tissues. By incorporating specific ionic liquids, the hydrophilicity, protein adsorption characteristics, and cell adhesion properties of polymers can be optimized. This approach enables the development of materials with reduced foreign body response and improved performance in vivo.
- Ionic liquid-polymer composites for tissue engineering: Ionic liquids can be combined with biomedical polymers to create advanced scaffolds for tissue engineering applications. These composites offer tunable mechanical properties, controlled degradation rates, and enhanced cellular interactions. The presence of ionic liquids can facilitate the incorporation of growth factors and bioactive molecules, creating microenvironments that promote tissue regeneration and functional recovery.
- Ionic liquids as processing aids for biomedical polymers: Ionic liquids serve as effective processing aids in the fabrication of biomedical polymers. They can act as solvents, plasticizers, or porogens during polymer synthesis and processing, enabling the creation of complex structures with controlled morphologies. The use of ionic liquids can improve processability, reduce the need for toxic organic solvents, and allow for milder processing conditions that preserve the bioactivity of incorporated therapeutic agents.
02 Antimicrobial biomedical polymers containing ionic liquids
Biomedical polymers can be formulated with ionic liquids to impart antimicrobial properties. These formulations exploit the inherent antimicrobial activity of certain ionic liquids to create materials that resist bacterial colonization and biofilm formation. Such polymers are particularly valuable in medical devices, wound dressings, and implants where infection prevention is critical for successful clinical outcomes.Expand Specific Solutions03 Ionic liquids for processing and modification of biomedical polymers
Ionic liquids serve as effective solvents and processing aids for biomedical polymers. They can dissolve various biopolymers like cellulose and chitin that are difficult to process with conventional solvents. Additionally, ionic liquids can be used to chemically modify polymer structures, introducing functional groups that enhance biocompatibility, biodegradability, or specific biological interactions, thereby expanding the application range of these materials in medical contexts.Expand Specific Solutions04 Ionic liquid-polymer composites for tissue engineering
Composite materials combining ionic liquids with biomedical polymers create advanced scaffolds for tissue engineering applications. These composites offer tunable mechanical properties, controlled porosity, and enhanced cell adhesion characteristics. The ionic liquid component can provide additional functionality such as electrical conductivity for neural tissue engineering or controlled degradation rates for regenerative medicine applications.Expand Specific Solutions05 Ionic liquids in electroactive biomedical polymers
Ionic liquids can be integrated with electroactive polymers to develop smart biomedical materials with responsive properties. These materials can change shape, size, or other physical characteristics in response to electrical stimuli, making them suitable for applications such as controlled drug release systems, artificial muscles, or adaptive implants. The ionic liquid component enhances ionic conductivity while maintaining biocompatibility and mechanical stability of the polymer matrix.Expand Specific Solutions
Leading Companies and Research Institutions in the Field
The ionic liquids in biomedical polymers market is currently in a growth phase, with increasing applications in drug delivery systems, tissue engineering, and antimicrobial materials. The global market size is expanding rapidly due to the unique properties of ionic liquids, including low volatility, thermal stability, and biocompatibility. From a technological maturity perspective, academic institutions like Zhejiang University, CNRS, and South China University of Technology are leading fundamental research, while companies such as Becton Dickinson, BASF, and Covestro are commercializing applications. The field shows a collaborative ecosystem between research institutions and industry players, with significant innovations coming from cross-disciplinary teams at universities. Companies like Bioniqs and Surface Innovations are developing specialized niche applications, indicating the technology's transition from research to commercial implementation.
Zhejiang University
Technical Solution: Zhejiang University has developed innovative biomedical polymer systems incorporating ionic liquids (ILs) as functional components. Their approach focuses on using ILs as both solvents and reactants in polymer synthesis, creating materials with enhanced biocompatibility and antimicrobial properties. Their research team has successfully incorporated imidazolium-based ILs into biodegradable polymers like polylactic acid (PLA) and polycaprolactone (PCL), resulting in materials with controlled drug release capabilities[1]. They've also pioneered the use of choline-based ILs as biocompatible plasticizers in hydrogel formulations, which has shown promising results for wound dressing applications with sustained antimicrobial activity[3]. Their recent work includes developing IL-modified polymer nanoparticles for targeted drug delivery systems that respond to physiological stimuli such as pH changes in tumor microenvironments.
Strengths: Superior biocompatibility of their choline-based IL formulations compared to traditional systems; excellent control over drug release kinetics; and innovative approach to creating responsive biomaterials. Weaknesses: Some of their IL-polymer systems show limited mechanical stability under physiological conditions and potential cytotoxicity concerns with certain imidazolium-based ILs at higher concentrations.
Centre National de la Recherche Scientifique
Technical Solution: The Centre National de la Recherche Scientifique (CNRS) has developed sophisticated approaches for incorporating ionic liquids into biomedical polymers. Their primary innovation lies in using task-specific ionic liquids (TSILs) as both reaction media and functional components in polymer synthesis. CNRS researchers have created cellulose-based materials dissolved in ionic liquids and regenerated into biocompatible films and fibers with enhanced mechanical properties and controlled biodegradation rates[2]. They've pioneered the use of phosphonium and ammonium-based ILs as antimicrobial agents within polymer matrices, demonstrating significant efficacy against both gram-positive and gram-negative bacteria while maintaining biocompatibility with mammalian cells[4]. Their recent breakthroughs include developing IL-polymer composites with thermoresponsive properties for controlled drug delivery applications, where drug release can be triggered by small temperature changes within physiological ranges. CNRS has also explored using ILs as electrolytes in conductive polymers for neural interfaces and biosensing applications.
Strengths: Exceptional expertise in designing task-specific ionic liquids tailored for specific biomedical applications; strong focus on green chemistry principles; and advanced characterization capabilities for IL-polymer interactions. Weaknesses: Some of their more complex IL-polymer systems face scalability challenges for industrial production, and certain formulations show limited long-term stability under storage conditions.
Key Patents and Research Breakthroughs
Coagulation of biopolymers from ionic liquid solutions using co2
PatentWO2014125438A1
Innovation
- The use of supercritical CO2 or gaseous CO2 as a coagulant to precipitate biopolymers from ionic liquid solutions, allowing for efficient recycling of ionic liquids by depressurizing the system and avoiding high-boiling solvents, thereby reducing energy consumption and costs.
Methods for dissolving polymers using mixtures of different ionic liquids and compositions comprising the mixtures
PatentInactiveUS20190040209A1
Innovation
- The use of mixtures of ionic liquids with different cations and/or anions, which can be synthesized in a one-pot process, providing a cost-effective and efficient method for dissolving polymers such as biopolymers and synthetic polymers, including cellulose, by forming ternary mixtures like 2:1:1 ratios of specific imidazolium-based ionic liquids.
Biocompatibility and Safety Considerations
The biocompatibility and safety profile of ionic liquids (ILs) in biomedical polymer applications represents a critical consideration that determines their clinical viability. Despite their promising properties, the cytotoxicity of many ILs remains a significant concern, with toxicity levels varying considerably depending on their chemical structure, particularly the cation and anion combinations. Imidazolium-based ILs, while widely used, have demonstrated dose-dependent cytotoxicity in multiple cell lines, necessitating careful selection and modification for biomedical applications.
Recent research has focused on developing biocompatible ILs through strategic molecular design. The incorporation of natural components such as choline, amino acids, and carbohydrates has yielded bio-derived ILs with significantly improved safety profiles. These bio-inspired ILs demonstrate reduced cytotoxicity while maintaining the beneficial physicochemical properties that make ILs attractive for biomedical applications.
Comprehensive toxicological assessment frameworks have been established to evaluate IL safety in biomedical polymers. These protocols typically include in vitro cytotoxicity assays, hemolysis testing, inflammatory response evaluation, and genotoxicity studies. Long-term biocompatibility is assessed through subcutaneous implantation studies in animal models, monitoring for tissue reactions, degradation behavior, and systemic effects over extended periods.
The biodegradability of IL-containing polymers presents another crucial safety consideration. Non-biodegradable ILs may accumulate in tissues or the environment, creating potential long-term hazards. Research has identified several biodegradable IL structures, particularly those containing ester or amide linkages that can undergo enzymatic hydrolysis under physiological conditions, facilitating their safe elimination from biological systems.
Regulatory considerations for IL-based biomedical polymers remain complex and evolving. Currently, few ILs have received explicit regulatory approval for biomedical applications, creating challenges for clinical translation. Manufacturers must navigate comprehensive safety documentation requirements, including detailed characterization of leachables and extractables from IL-polymer systems, degradation products, and potential immunological responses.
Risk mitigation strategies for IL-polymer systems include chemical modification approaches such as covalent immobilization of ILs within polymer networks to prevent leaching, encapsulation techniques to control IL release, and surface modification to improve blood and tissue compatibility. These approaches have demonstrated significant improvements in biocompatibility while preserving the functional advantages of ILs in biomedical applications.
Future directions in biocompatibility research include the development of structure-toxicity relationship models to predict IL safety profiles, high-throughput screening methodologies for rapid assessment of novel IL structures, and advanced in vitro tissue models that better replicate in vivo conditions for more accurate safety evaluation prior to clinical testing.
Recent research has focused on developing biocompatible ILs through strategic molecular design. The incorporation of natural components such as choline, amino acids, and carbohydrates has yielded bio-derived ILs with significantly improved safety profiles. These bio-inspired ILs demonstrate reduced cytotoxicity while maintaining the beneficial physicochemical properties that make ILs attractive for biomedical applications.
Comprehensive toxicological assessment frameworks have been established to evaluate IL safety in biomedical polymers. These protocols typically include in vitro cytotoxicity assays, hemolysis testing, inflammatory response evaluation, and genotoxicity studies. Long-term biocompatibility is assessed through subcutaneous implantation studies in animal models, monitoring for tissue reactions, degradation behavior, and systemic effects over extended periods.
The biodegradability of IL-containing polymers presents another crucial safety consideration. Non-biodegradable ILs may accumulate in tissues or the environment, creating potential long-term hazards. Research has identified several biodegradable IL structures, particularly those containing ester or amide linkages that can undergo enzymatic hydrolysis under physiological conditions, facilitating their safe elimination from biological systems.
Regulatory considerations for IL-based biomedical polymers remain complex and evolving. Currently, few ILs have received explicit regulatory approval for biomedical applications, creating challenges for clinical translation. Manufacturers must navigate comprehensive safety documentation requirements, including detailed characterization of leachables and extractables from IL-polymer systems, degradation products, and potential immunological responses.
Risk mitigation strategies for IL-polymer systems include chemical modification approaches such as covalent immobilization of ILs within polymer networks to prevent leaching, encapsulation techniques to control IL release, and surface modification to improve blood and tissue compatibility. These approaches have demonstrated significant improvements in biocompatibility while preserving the functional advantages of ILs in biomedical applications.
Future directions in biocompatibility research include the development of structure-toxicity relationship models to predict IL safety profiles, high-throughput screening methodologies for rapid assessment of novel IL structures, and advanced in vitro tissue models that better replicate in vivo conditions for more accurate safety evaluation prior to clinical testing.
Regulatory Framework for Ionic Liquid-Based Medical Devices
The regulatory landscape for ionic liquid-based medical devices presents a complex framework that manufacturers and researchers must navigate. In the United States, the Food and Drug Administration (FDA) classifies these devices based on risk levels, with ionic liquid-containing polymers typically falling under Class II or III, requiring either 510(k) clearance or premarket approval (PMA). The novel nature of ionic liquids in biomedical applications often necessitates additional safety and efficacy data beyond standard requirements.
The European Union's regulatory approach centers on the Medical Device Regulation (MDR) and In Vitro Diagnostic Regulation (IVDR), which replaced the previous Medical Device Directive in 2021. These regulations impose stricter requirements for clinical evidence, post-market surveillance, and risk management for ionic liquid-polymer composites. Notably, the MDR specifically addresses nanomaterials and novel material combinations, directly impacting ionic liquid applications in medical polymers.
In Asia, Japan's Pharmaceuticals and Medical Devices Agency (PMDA) and China's National Medical Products Administration (NMPA) have established their own regulatory pathways for innovative materials in medical devices. These frameworks generally emphasize biocompatibility testing and leachable/extractable assessments specific to ionic liquid components, reflecting growing concerns about long-term safety profiles.
International standards organizations play a crucial role in harmonizing requirements across jurisdictions. ISO 10993 series for biocompatibility testing has been updated to address novel materials including ionic liquids, while ASTM F3163 provides guidance on characterizing and testing polymeric biomaterials. The International Conference on Harmonisation (ICH) guidelines further inform toxicity testing approaches for these innovative materials.
Regulatory challenges specific to ionic liquid-polymer composites include the lack of established safety databases, uncertainty regarding long-term stability in biological environments, and potential for unexpected interactions with biological systems. Regulatory bodies increasingly require manufacturers to implement comprehensive extractable and leachable testing protocols to identify potential migration of ionic liquid components from the polymer matrix.
Recent regulatory trends indicate movement toward adaptive licensing pathways for innovative materials, allowing conditional approvals with enhanced post-market surveillance requirements. This approach aims to balance innovation with patient safety for technologies like ionic liquid-functionalized polymers where long-term clinical data may be limited but potential therapeutic benefits are significant.
The European Union's regulatory approach centers on the Medical Device Regulation (MDR) and In Vitro Diagnostic Regulation (IVDR), which replaced the previous Medical Device Directive in 2021. These regulations impose stricter requirements for clinical evidence, post-market surveillance, and risk management for ionic liquid-polymer composites. Notably, the MDR specifically addresses nanomaterials and novel material combinations, directly impacting ionic liquid applications in medical polymers.
In Asia, Japan's Pharmaceuticals and Medical Devices Agency (PMDA) and China's National Medical Products Administration (NMPA) have established their own regulatory pathways for innovative materials in medical devices. These frameworks generally emphasize biocompatibility testing and leachable/extractable assessments specific to ionic liquid components, reflecting growing concerns about long-term safety profiles.
International standards organizations play a crucial role in harmonizing requirements across jurisdictions. ISO 10993 series for biocompatibility testing has been updated to address novel materials including ionic liquids, while ASTM F3163 provides guidance on characterizing and testing polymeric biomaterials. The International Conference on Harmonisation (ICH) guidelines further inform toxicity testing approaches for these innovative materials.
Regulatory challenges specific to ionic liquid-polymer composites include the lack of established safety databases, uncertainty regarding long-term stability in biological environments, and potential for unexpected interactions with biological systems. Regulatory bodies increasingly require manufacturers to implement comprehensive extractable and leachable testing protocols to identify potential migration of ionic liquid components from the polymer matrix.
Recent regulatory trends indicate movement toward adaptive licensing pathways for innovative materials, allowing conditional approvals with enhanced post-market surveillance requirements. This approach aims to balance innovation with patient safety for technologies like ionic liquid-functionalized polymers where long-term clinical data may be limited but potential therapeutic benefits are significant.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!