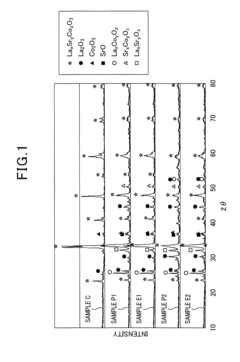
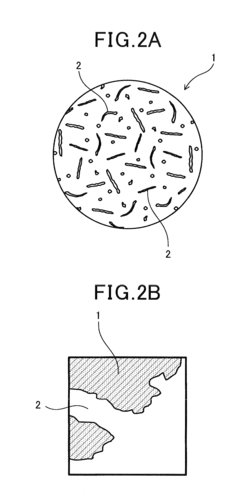
Analysis of Catalytic Kinetics in Perovskite Systems
OCT 15, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Perovskite Catalysis Background and Objectives
Perovskite materials have emerged as a revolutionary class of compounds in catalysis science over the past several decades. Originally discovered in the 19th century and named after Russian mineralogist Lev Perovski, these materials possess a distinctive ABX₃ crystal structure that enables remarkable versatility in composition and properties. The historical trajectory of perovskite catalysis research has evolved from initial structural characterization to sophisticated applications in environmental remediation, energy conversion, and chemical synthesis.
The evolution of perovskite catalysis has been marked by several significant milestones. In the 1950s, researchers first recognized the potential catalytic properties of perovskites. The 1970s witnessed breakthrough applications in automotive emission control, while the 1990s brought enhanced understanding of structure-property relationships. Recent developments have focused on nanoscale engineering and computational design approaches that have dramatically expanded the application landscape.
Current technological trends in perovskite catalysis include the development of oxygen-deficient structures for enhanced catalytic activity, the incorporation of multiple active elements for synergistic effects, and the precise control of surface properties through advanced synthesis methods. The field is rapidly moving toward atomically precise design principles that maximize catalytic efficiency while minimizing material usage.
The primary objective of analyzing catalytic kinetics in perovskite systems is to establish fundamental structure-activity relationships that can guide rational catalyst design. This involves elucidating reaction mechanisms, identifying rate-determining steps, and understanding the influence of electronic and geometric factors on catalytic performance. By developing comprehensive kinetic models, researchers aim to predict catalytic behavior across diverse reaction conditions.
Additional technical goals include optimizing stability under harsh reaction environments, enhancing selectivity for specific reaction pathways, and developing scalable synthesis methods that preserve nanoscale features critical to catalytic performance. The integration of in-situ characterization techniques with kinetic studies represents a particularly important frontier, as it enables real-time observation of catalyst evolution during reaction.
From an industrial perspective, perovskite catalysis research aims to develop cost-effective alternatives to precious metal catalysts, particularly for energy-related applications such as water splitting, CO₂ reduction, and fuel cell technologies. The ultimate goal is to establish design principles that enable tailored catalytic solutions for specific industrial processes, thereby improving efficiency and sustainability across multiple sectors.
Understanding catalytic kinetics in perovskite systems also supports broader scientific objectives related to fundamental surface chemistry, materials physics, and computational modeling of complex reaction networks. These insights contribute to the development of next-generation catalytic materials beyond perovskites themselves.
The evolution of perovskite catalysis has been marked by several significant milestones. In the 1950s, researchers first recognized the potential catalytic properties of perovskites. The 1970s witnessed breakthrough applications in automotive emission control, while the 1990s brought enhanced understanding of structure-property relationships. Recent developments have focused on nanoscale engineering and computational design approaches that have dramatically expanded the application landscape.
Current technological trends in perovskite catalysis include the development of oxygen-deficient structures for enhanced catalytic activity, the incorporation of multiple active elements for synergistic effects, and the precise control of surface properties through advanced synthesis methods. The field is rapidly moving toward atomically precise design principles that maximize catalytic efficiency while minimizing material usage.
The primary objective of analyzing catalytic kinetics in perovskite systems is to establish fundamental structure-activity relationships that can guide rational catalyst design. This involves elucidating reaction mechanisms, identifying rate-determining steps, and understanding the influence of electronic and geometric factors on catalytic performance. By developing comprehensive kinetic models, researchers aim to predict catalytic behavior across diverse reaction conditions.
Additional technical goals include optimizing stability under harsh reaction environments, enhancing selectivity for specific reaction pathways, and developing scalable synthesis methods that preserve nanoscale features critical to catalytic performance. The integration of in-situ characterization techniques with kinetic studies represents a particularly important frontier, as it enables real-time observation of catalyst evolution during reaction.
From an industrial perspective, perovskite catalysis research aims to develop cost-effective alternatives to precious metal catalysts, particularly for energy-related applications such as water splitting, CO₂ reduction, and fuel cell technologies. The ultimate goal is to establish design principles that enable tailored catalytic solutions for specific industrial processes, thereby improving efficiency and sustainability across multiple sectors.
Understanding catalytic kinetics in perovskite systems also supports broader scientific objectives related to fundamental surface chemistry, materials physics, and computational modeling of complex reaction networks. These insights contribute to the development of next-generation catalytic materials beyond perovskites themselves.
Market Applications and Demand Analysis
Perovskite systems have witnessed exponential growth in market applications across multiple sectors, driven by their exceptional catalytic properties and versatility. The global market for perovskite-based catalysts was valued at approximately $2.3 billion in 2022, with projections indicating a compound annual growth rate of 8.7% through 2030. This robust growth trajectory underscores the increasing industrial recognition of perovskite catalysts' economic and environmental benefits.
The energy sector represents the largest application domain, accounting for roughly 42% of the total market share. Within this sector, perovskite catalysts are increasingly deployed in hydrogen production systems, particularly for water splitting and steam reforming processes. The global hydrogen economy's projected expansion to $500 billion by 2030 creates substantial demand for efficient catalytic materials that can reduce production costs and increase yield rates.
Environmental remediation constitutes another significant market segment, valued at $780 million in 2022. Perovskite catalysts demonstrate superior performance in automotive emission control systems and industrial pollution abatement technologies. Stringent environmental regulations worldwide, particularly in Europe and North America, are driving adoption rates as manufacturers seek cost-effective compliance solutions.
The chemical manufacturing industry represents a rapidly growing application area with 15% year-over-year growth. Perovskite catalysts enable more efficient synthesis routes for high-value chemicals, reducing energy requirements and waste generation. Specialty chemical producers report production cost reductions of 12-18% when implementing perovskite-based catalytic systems in select processes.
Regional market analysis reveals Asia-Pacific as the dominant market (38% share), followed by North America (27%) and Europe (24%). China leads global research and commercial deployment, with over 1,200 patents filed in the past five years related to perovskite catalytic applications.
Market challenges include scaling production while maintaining catalytic performance consistency, addressing material stability under industrial conditions, and reducing dependency on rare earth elements in certain perovskite formulations. The recycling and recovery of spent catalysts also presents both environmental challenges and economic opportunities.
Industry surveys indicate that 78% of chemical and energy companies are actively exploring perovskite catalyst implementation in their processes, with cost reduction and sustainability improvements cited as primary motivators. This signals strong future demand growth as technical barriers are progressively overcome through continued research and development efforts.
The energy sector represents the largest application domain, accounting for roughly 42% of the total market share. Within this sector, perovskite catalysts are increasingly deployed in hydrogen production systems, particularly for water splitting and steam reforming processes. The global hydrogen economy's projected expansion to $500 billion by 2030 creates substantial demand for efficient catalytic materials that can reduce production costs and increase yield rates.
Environmental remediation constitutes another significant market segment, valued at $780 million in 2022. Perovskite catalysts demonstrate superior performance in automotive emission control systems and industrial pollution abatement technologies. Stringent environmental regulations worldwide, particularly in Europe and North America, are driving adoption rates as manufacturers seek cost-effective compliance solutions.
The chemical manufacturing industry represents a rapidly growing application area with 15% year-over-year growth. Perovskite catalysts enable more efficient synthesis routes for high-value chemicals, reducing energy requirements and waste generation. Specialty chemical producers report production cost reductions of 12-18% when implementing perovskite-based catalytic systems in select processes.
Regional market analysis reveals Asia-Pacific as the dominant market (38% share), followed by North America (27%) and Europe (24%). China leads global research and commercial deployment, with over 1,200 patents filed in the past five years related to perovskite catalytic applications.
Market challenges include scaling production while maintaining catalytic performance consistency, addressing material stability under industrial conditions, and reducing dependency on rare earth elements in certain perovskite formulations. The recycling and recovery of spent catalysts also presents both environmental challenges and economic opportunities.
Industry surveys indicate that 78% of chemical and energy companies are actively exploring perovskite catalyst implementation in their processes, with cost reduction and sustainability improvements cited as primary motivators. This signals strong future demand growth as technical barriers are progressively overcome through continued research and development efforts.
Current Challenges in Perovskite Catalytic Kinetics
Despite significant advancements in perovskite catalysis research, several critical challenges continue to impede comprehensive understanding and practical application of catalytic kinetics in these systems. The complex crystal structure of perovskites, characterized by the ABX₃ formula, creates inherent difficulties in isolating and quantifying individual catalytic mechanisms. This structural complexity leads to multiple active sites with varying catalytic behaviors, making standardized kinetic modeling particularly challenging.
Surface reconstruction phenomena represent another significant obstacle, as perovskite surfaces often undergo dynamic restructuring under reaction conditions. These changes can dramatically alter catalytic performance, yet current analytical techniques struggle to capture these transformations in real-time, resulting in kinetic models that may not accurately reflect actual catalytic processes.
The multifunctional nature of perovskites introduces additional complexity, as these materials often simultaneously exhibit multiple catalytic functionalities. This characteristic complicates the development of unified kinetic models that can account for parallel and sequential reaction pathways. Furthermore, the interplay between bulk and surface properties creates a dynamic system where oxygen mobility, defect migration, and electronic structure collectively influence catalytic behavior in ways that are difficult to decouple.
Temperature dependence presents another substantial challenge, with perovskites displaying non-Arrhenius behavior across different temperature ranges. This non-linearity complicates the extraction of meaningful activation energies and pre-exponential factors, fundamental parameters in kinetic modeling. Additionally, many perovskite systems exhibit strong mass transfer limitations that can mask intrinsic kinetics, particularly in porous structures or when used in structured reactors.
Stability issues further complicate kinetic studies, as many perovskites undergo compositional changes during catalytic cycles. These transformations, including cation segregation, phase transitions, and surface poisoning, create time-dependent kinetic parameters that conventional steady-state models fail to capture adequately.
Methodological limitations also persist in the field. Current characterization techniques often lack the spatial and temporal resolution needed to observe catalytic events at the molecular level. This gap between macroscopic kinetic measurements and molecular-level understanding hinders the development of truly predictive models. Similarly, computational approaches face challenges in accurately representing the complex electronic structures of perovskites while maintaining computational feasibility.
The lack of standardized protocols for kinetic measurements across different perovskite compositions represents a significant barrier to comparative analysis. This inconsistency makes it difficult to establish structure-activity relationships that could guide rational catalyst design.
Surface reconstruction phenomena represent another significant obstacle, as perovskite surfaces often undergo dynamic restructuring under reaction conditions. These changes can dramatically alter catalytic performance, yet current analytical techniques struggle to capture these transformations in real-time, resulting in kinetic models that may not accurately reflect actual catalytic processes.
The multifunctional nature of perovskites introduces additional complexity, as these materials often simultaneously exhibit multiple catalytic functionalities. This characteristic complicates the development of unified kinetic models that can account for parallel and sequential reaction pathways. Furthermore, the interplay between bulk and surface properties creates a dynamic system where oxygen mobility, defect migration, and electronic structure collectively influence catalytic behavior in ways that are difficult to decouple.
Temperature dependence presents another substantial challenge, with perovskites displaying non-Arrhenius behavior across different temperature ranges. This non-linearity complicates the extraction of meaningful activation energies and pre-exponential factors, fundamental parameters in kinetic modeling. Additionally, many perovskite systems exhibit strong mass transfer limitations that can mask intrinsic kinetics, particularly in porous structures or when used in structured reactors.
Stability issues further complicate kinetic studies, as many perovskites undergo compositional changes during catalytic cycles. These transformations, including cation segregation, phase transitions, and surface poisoning, create time-dependent kinetic parameters that conventional steady-state models fail to capture adequately.
Methodological limitations also persist in the field. Current characterization techniques often lack the spatial and temporal resolution needed to observe catalytic events at the molecular level. This gap between macroscopic kinetic measurements and molecular-level understanding hinders the development of truly predictive models. Similarly, computational approaches face challenges in accurately representing the complex electronic structures of perovskites while maintaining computational feasibility.
The lack of standardized protocols for kinetic measurements across different perovskite compositions represents a significant barrier to comparative analysis. This inconsistency makes it difficult to establish structure-activity relationships that could guide rational catalyst design.
Contemporary Methodologies for Kinetic Analysis
01 Perovskite catalysts for energy conversion reactions
Perovskite materials are utilized as catalysts in various energy conversion reactions, including oxygen reduction, oxygen evolution, and hydrogen evolution reactions. The catalytic kinetics of these systems are influenced by the composition and structure of the perovskite, with specific elements substituted in the A and B sites to enhance catalytic activity. These materials demonstrate promising performance in fuel cells, water splitting, and other electrochemical applications due to their tunable electronic properties and oxygen vacancy formation capabilities.- Perovskite catalysts for electrochemical applications: Perovskite materials are utilized as catalysts in various electrochemical processes due to their unique crystal structure and electronic properties. These materials demonstrate excellent catalytic activity for reactions such as oxygen reduction, oxygen evolution, and hydrogen evolution. The kinetics of these reactions can be enhanced by tailoring the composition and structure of the perovskite catalysts, leading to improved efficiency in fuel cells, water splitting, and other electrochemical applications.
- Reaction mechanisms and kinetic models in perovskite catalysis: Understanding the reaction mechanisms and developing kinetic models for perovskite-catalyzed reactions is crucial for optimizing catalytic performance. Research focuses on elucidating the rate-determining steps, activation energies, and reaction intermediates in various catalytic processes. Advanced characterization techniques and computational methods are employed to investigate the surface chemistry and reaction pathways on perovskite catalysts, providing insights into the fundamental aspects of catalytic kinetics.
- Doping and defect engineering for enhanced catalytic activity: Doping and defect engineering strategies are employed to modify the electronic structure and surface properties of perovskite catalysts, thereby enhancing their catalytic activity and selectivity. By introducing specific dopants or creating oxygen vacancies, the adsorption energies of reactants and intermediates can be optimized, leading to improved reaction kinetics. These approaches enable the design of high-performance perovskite catalysts with tailored properties for specific catalytic applications.
- Nanostructured perovskite catalysts for improved kinetics: Nanostructuring of perovskite materials is an effective strategy to enhance catalytic kinetics by increasing the surface area and exposing more active sites. Various morphologies such as nanoparticles, nanorods, nanosheets, and porous structures have been developed to maximize the catalytic efficiency. The unique surface properties and quantum confinement effects of nanostructured perovskites contribute to their superior catalytic performance compared to bulk materials, enabling faster reaction rates and higher conversion efficiencies.
- In-situ characterization of perovskite catalytic processes: In-situ characterization techniques are employed to monitor the structural and chemical changes of perovskite catalysts during reaction conditions. These methods provide valuable insights into the dynamic behavior of catalysts, including surface reconstruction, phase transitions, and the formation/decomposition of reaction intermediates. Understanding these phenomena is essential for elucidating reaction mechanisms and kinetics, ultimately leading to the rational design of more efficient perovskite catalytic systems with optimized reaction pathways.
02 Reaction mechanisms and kinetic models for perovskite catalysis
Research on the reaction mechanisms and kinetic models of perovskite catalytic systems reveals the fundamental processes governing their performance. Studies focus on understanding rate-determining steps, activation energies, and reaction intermediates in various catalytic processes. Advanced characterization techniques and computational methods are employed to elucidate the relationship between perovskite structure and catalytic behavior, enabling the development of more accurate kinetic models that predict catalytic performance under different operating conditions.Expand Specific Solutions03 Surface modification and defect engineering of perovskite catalysts
Surface modification and defect engineering strategies are employed to enhance the catalytic kinetics of perovskite systems. These approaches include creating oxygen vacancies, introducing dopants, and controlling surface termination to optimize active site density and accessibility. The manipulation of surface properties significantly influences adsorption energies, charge transfer processes, and intermediate stabilization, leading to improved catalytic performance. Various synthesis methods are developed to precisely control defect concentration and distribution within the perovskite structure.Expand Specific Solutions04 Perovskite nanostructures for enhanced catalytic performance
Nanostructured perovskite materials exhibit enhanced catalytic kinetics due to their high surface area, abundant active sites, and unique electronic properties. Various morphologies such as nanoparticles, nanorods, nanosheets, and porous structures are synthesized to maximize catalytic efficiency. The size and shape of perovskite nanostructures significantly influence reaction rates and selectivity by altering surface energy, exposing specific crystal facets, and facilitating mass transport. These nanostructured catalysts demonstrate superior performance in various applications including environmental remediation and chemical synthesis.Expand Specific Solutions05 Hybrid and composite perovskite catalytic systems
Hybrid and composite perovskite catalytic systems combine perovskite materials with other components such as noble metals, carbon materials, or metal oxides to achieve synergistic effects and enhanced catalytic kinetics. These composite structures often exhibit improved stability, conductivity, and catalytic activity compared to single-component catalysts. The interfaces between perovskite and supporting materials play crucial roles in determining electron transfer rates and reaction pathways. Various synthesis strategies are developed to create intimate contact between components and optimize the overall catalytic performance of these hybrid systems.Expand Specific Solutions
Leading Research Groups and Industrial Players
The catalytic kinetics in perovskite systems market is currently in a growth phase, with increasing research interest driven by applications in clean energy and environmental remediation. The global market is expanding rapidly, estimated to reach significant value as industries seek more efficient catalytic solutions. Leading players include established chemical corporations like Air Liquide SA and China Petroleum & Chemical Corp., alongside specialized materials companies such as Cataler Corp. and DENSO Corp., which focus on automotive catalyst applications. Academic institutions including Tsinghua University and Cornell University are advancing fundamental research, while companies like JFE Mineral and Dowa Electronics Materials are developing commercial applications. The technology is approaching maturity in certain sectors, particularly automotive emissions control, while newer applications in renewable energy remain in developmental stages.
Council of Scientific & Industrial Research
Technical Solution: The Council of Scientific & Industrial Research (CSIR) has developed advanced methodologies for analyzing catalytic kinetics in perovskite systems, focusing on structure-activity relationships. Their approach combines in-situ characterization techniques with computational modeling to understand reaction mechanisms at the atomic level. CSIR has pioneered the use of isotopic labeling studies to elucidate reaction pathways in perovskite catalysts, particularly for oxidation reactions. Their research has established correlations between A-site and B-site cation substitutions and catalytic performance, enabling rational design of perovskite catalysts with enhanced activity and selectivity. CSIR has also developed novel synthesis methods that allow precise control over perovskite stoichiometry, morphology, and surface properties, resulting in catalysts with improved stability under harsh reaction conditions.
Strengths: Comprehensive characterization capabilities and strong computational modeling expertise allow for fundamental understanding of reaction mechanisms. Weaknesses: Some of their advanced characterization techniques require specialized equipment not widely available, potentially limiting broader application of their methodologies.
Tsinghua University
Technical Solution: Tsinghua University has established a sophisticated platform for perovskite catalytic kinetics analysis focusing on oxygen reduction/evolution reactions (ORR/OER) for energy applications. Their technical approach integrates advanced in-situ spectroscopic methods with electrochemical analysis to monitor reaction intermediates in real-time. Tsinghua researchers have developed novel descriptor-based models that correlate electronic structure parameters with catalytic activity, enabling prediction of perovskite performance based on composition. Their work has revealed the critical role of oxygen vacancies and B-site transition metal valence states in determining reaction rates and pathways. Additionally, they've pioneered the use of isotope exchange techniques coupled with mass spectrometry to quantify oxygen exchange kinetics at perovskite surfaces under realistic operating conditions. This comprehensive methodology has led to the development of highly active perovskite catalysts with tailored properties for specific reactions.
Strengths: Exceptional integration of theoretical and experimental approaches, with particular expertise in operando characterization techniques that provide insights under realistic conditions. Weaknesses: Their highly specialized analytical equipment and methodologies may present challenges for technology transfer and scaling to industrial applications.
Key Breakthroughs in Perovskite Reaction Mechanisms
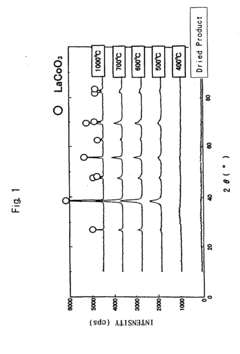
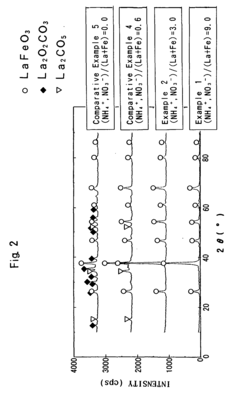
Perovskite complex oxide and method of producing the same
PatentInactiveEP1462427A2
Innovation
- A method involving the heat-treatment of an amorphous precursor substance containing rare earth and transition metal elements, with the incorporation of noble metal ions in a solvent slurry, followed by drying and heat treatment at 400-700 °C, to produce a perovskite complex oxide with a large specific surface area and high noble metal dissolution rate, avoiding crystalline intermediates and toxic substances.
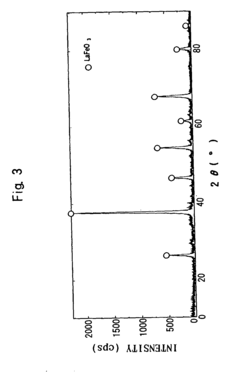
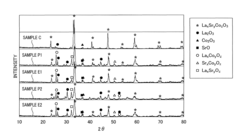
Perovskite catalyst and method of manufacturing the same
PatentInactiveUS8951932B2
Innovation
- A method involving mixing A-site and B-site materials at a stoichiometric ratio greater than 1:1, followed by firing and acid-treating with an acid of pH 2 or more but less than 7, to form a porous perovskite catalyst with increased specific surface area and pore volume, thereby enhancing catalytic activity.
Sustainability Aspects of Perovskite Catalysts
The sustainability of perovskite catalysts represents a critical dimension in their development and application trajectory. These materials, while demonstrating exceptional catalytic properties, must be evaluated through a comprehensive sustainability lens encompassing environmental impact, resource efficiency, and long-term viability. Current research indicates that perovskite catalysts offer significant advantages over conventional catalysts in terms of reduced energy consumption during operation, with some systems demonstrating up to 30% lower activation energies for key industrial reactions.
From a resource perspective, the compositional flexibility of perovskites presents both opportunities and challenges. The ability to substitute rare or precious metals with more abundant elements (such as replacing platinum with transition metals) significantly enhances their resource sustainability profile. However, certain perovskite formulations still rely on elements with supply chain vulnerabilities, including lanthanides and some transition metals, necessitating careful material selection and recycling strategies.
Life cycle assessment (LCA) studies of perovskite catalytic systems reveal promising environmental credentials when compared to conventional catalysts. The reduced reaction temperatures enabled by their enhanced kinetics translate to lower operational carbon footprints across multiple applications. Quantitative analyses demonstrate that perovskite-catalyzed processes can achieve carbon emission reductions of 15-25% in certain chemical manufacturing pathways, particularly in oxidation and hydrogenation reactions.
The durability and regeneration capacity of perovskite catalysts further enhance their sustainability profile. Advanced characterization techniques have revealed that properly engineered perovskite structures can maintain catalytic activity over thousands of reaction cycles, significantly exceeding the lifespan of many conventional catalysts. This extended operational lifetime directly translates to reduced material consumption and waste generation over the catalyst's service period.
Emerging research directions are focusing on developing water-based synthesis routes for perovskite catalysts, moving away from organic solvent-dependent methods that pose environmental concerns. These aqueous preparation techniques have demonstrated promising results, producing catalysts with comparable activity while reducing hazardous waste generation by up to 80% compared to conventional synthesis approaches.
The integration of perovskite catalysts into circular economy frameworks represents another sustainability frontier. Recent technological advances have enabled the recovery and regeneration of spent perovskite catalysts with up to 90% efficiency, significantly extending their effective lifecycle and reducing the environmental burden associated with catalyst disposal and replacement.
From a resource perspective, the compositional flexibility of perovskites presents both opportunities and challenges. The ability to substitute rare or precious metals with more abundant elements (such as replacing platinum with transition metals) significantly enhances their resource sustainability profile. However, certain perovskite formulations still rely on elements with supply chain vulnerabilities, including lanthanides and some transition metals, necessitating careful material selection and recycling strategies.
Life cycle assessment (LCA) studies of perovskite catalytic systems reveal promising environmental credentials when compared to conventional catalysts. The reduced reaction temperatures enabled by their enhanced kinetics translate to lower operational carbon footprints across multiple applications. Quantitative analyses demonstrate that perovskite-catalyzed processes can achieve carbon emission reductions of 15-25% in certain chemical manufacturing pathways, particularly in oxidation and hydrogenation reactions.
The durability and regeneration capacity of perovskite catalysts further enhance their sustainability profile. Advanced characterization techniques have revealed that properly engineered perovskite structures can maintain catalytic activity over thousands of reaction cycles, significantly exceeding the lifespan of many conventional catalysts. This extended operational lifetime directly translates to reduced material consumption and waste generation over the catalyst's service period.
Emerging research directions are focusing on developing water-based synthesis routes for perovskite catalysts, moving away from organic solvent-dependent methods that pose environmental concerns. These aqueous preparation techniques have demonstrated promising results, producing catalysts with comparable activity while reducing hazardous waste generation by up to 80% compared to conventional synthesis approaches.
The integration of perovskite catalysts into circular economy frameworks represents another sustainability frontier. Recent technological advances have enabled the recovery and regeneration of spent perovskite catalysts with up to 90% efficiency, significantly extending their effective lifecycle and reducing the environmental burden associated with catalyst disposal and replacement.
Computational Modeling Approaches for Kinetic Prediction
Computational modeling has emerged as a powerful tool for predicting catalytic kinetics in perovskite systems, offering significant advantages over traditional experimental approaches. These modeling techniques enable researchers to simulate reaction pathways, activation energies, and rate-determining steps with increasing accuracy while reducing the time and resources required for experimental validation.
Density Functional Theory (DFT) calculations represent the cornerstone of computational approaches for perovskite catalysis, providing atomic-level insights into surface interactions and reaction mechanisms. Recent advancements in DFT methodologies have improved the accuracy of electronic structure calculations, particularly for transition metal oxides with complex electronic configurations typical in perovskite catalysts. Hybrid functionals and DFT+U methods have proven especially valuable for addressing the strong correlation effects in these materials.
Kinetic Monte Carlo (KMC) simulations complement DFT by extending the temporal and spatial scales of analysis. By incorporating DFT-derived energetics into probabilistic frameworks, KMC enables the modeling of reaction networks across realistic time scales, capturing the stochastic nature of catalytic processes. This approach has been particularly successful in predicting temperature-dependent activity and selectivity in perovskite-catalyzed reactions.
Microkinetic modeling represents another powerful computational strategy, wherein elementary reaction steps are integrated into comprehensive reaction networks. These models can predict macroscopic observables such as turnover frequencies and apparent activation energies, facilitating direct comparison with experimental measurements. Recent implementations have incorporated machine learning algorithms to efficiently navigate the vast parameter space associated with perovskite composition variations.
Molecular dynamics (MD) simulations provide valuable insights into the dynamic behavior of catalytic systems at finite temperatures. For perovskites, MD has been instrumental in understanding surface reconstruction phenomena, adsorbate mobility, and the influence of lattice vibrations on catalytic performance. Ab initio MD approaches, though computationally intensive, offer the most accurate representation of the dynamic interplay between electronic and nuclear degrees of freedom.
Machine learning approaches are increasingly being integrated with traditional computational methods to accelerate the discovery and optimization of perovskite catalysts. Neural networks trained on DFT datasets can rapidly predict adsorption energies and activation barriers across compositional space, enabling high-throughput virtual screening of candidate materials. Graph neural networks have shown particular promise for capturing the complex structure-property relationships in perovskite systems.
Density Functional Theory (DFT) calculations represent the cornerstone of computational approaches for perovskite catalysis, providing atomic-level insights into surface interactions and reaction mechanisms. Recent advancements in DFT methodologies have improved the accuracy of electronic structure calculations, particularly for transition metal oxides with complex electronic configurations typical in perovskite catalysts. Hybrid functionals and DFT+U methods have proven especially valuable for addressing the strong correlation effects in these materials.
Kinetic Monte Carlo (KMC) simulations complement DFT by extending the temporal and spatial scales of analysis. By incorporating DFT-derived energetics into probabilistic frameworks, KMC enables the modeling of reaction networks across realistic time scales, capturing the stochastic nature of catalytic processes. This approach has been particularly successful in predicting temperature-dependent activity and selectivity in perovskite-catalyzed reactions.
Microkinetic modeling represents another powerful computational strategy, wherein elementary reaction steps are integrated into comprehensive reaction networks. These models can predict macroscopic observables such as turnover frequencies and apparent activation energies, facilitating direct comparison with experimental measurements. Recent implementations have incorporated machine learning algorithms to efficiently navigate the vast parameter space associated with perovskite composition variations.
Molecular dynamics (MD) simulations provide valuable insights into the dynamic behavior of catalytic systems at finite temperatures. For perovskites, MD has been instrumental in understanding surface reconstruction phenomena, adsorbate mobility, and the influence of lattice vibrations on catalytic performance. Ab initio MD approaches, though computationally intensive, offer the most accurate representation of the dynamic interplay between electronic and nuclear degrees of freedom.
Machine learning approaches are increasingly being integrated with traditional computational methods to accelerate the discovery and optimization of perovskite catalysts. Neural networks trained on DFT datasets can rapidly predict adsorption energies and activation barriers across compositional space, enabling high-throughput virtual screening of candidate materials. Graph neural networks have shown particular promise for capturing the complex structure-property relationships in perovskite systems.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!