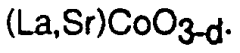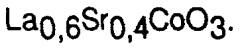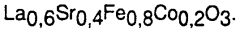Perovskite Catalyst Advancements in Chemical Processing
OCT 15, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Perovskite Catalyst Evolution and Research Objectives
Perovskite catalysts have emerged as a revolutionary class of materials in chemical processing over the past three decades. Initially discovered in the 19th century, these materials gained significant attention in the 1990s when their unique crystal structure and electronic properties were better understood. The perovskite structure, characterized by the general formula ABX₃, offers exceptional versatility through compositional engineering, allowing for precise tuning of catalytic properties by substituting different elements at the A and B sites.
The evolution of perovskite catalysts has been marked by several key milestones. Early research focused primarily on their application in solid oxide fuel cells, but by the early 2000s, researchers began exploring their potential in heterogeneous catalysis. A significant breakthrough came in 2012 when perovskites demonstrated remarkable activity for oxygen reduction and evolution reactions, sparking intense interest in their application for energy conversion processes.
Recent advancements have expanded perovskite applications into hydrocarbon activation, CO₂ conversion, and fine chemical synthesis. The period from 2018 to 2023 has witnessed particularly rapid development, with innovations in synthesis methods enabling precise control over surface properties, defect engineering, and nanostructuring. These developments have dramatically enhanced catalytic performance while reducing precious metal content.
The current technological trajectory suggests perovskites are poised to address several critical challenges in chemical processing. Their ability to catalyze multiple reaction types with high selectivity makes them promising candidates for process intensification and green chemistry applications. Additionally, their thermal stability and resistance to catalyst poisoning offer advantages in harsh industrial environments.
Our research objectives focus on three primary areas: First, developing novel perovskite compositions with enhanced activity and selectivity for targeted chemical transformations, particularly in C-H activation and CO₂ utilization. Second, establishing scalable and reproducible synthesis protocols that maintain precise control over structural and electronic properties. Third, understanding fundamental structure-property relationships through advanced characterization techniques to enable rational catalyst design.
The long-term goal is to develop perovskite-based catalytic systems that can replace current industrial catalysts, offering improved efficiency, reduced environmental impact, and economic advantages. This includes exploring hybrid systems that combine perovskites with other catalytic materials to create synergistic effects and addressing stability challenges in specific reaction environments.
The evolution of perovskite catalysts has been marked by several key milestones. Early research focused primarily on their application in solid oxide fuel cells, but by the early 2000s, researchers began exploring their potential in heterogeneous catalysis. A significant breakthrough came in 2012 when perovskites demonstrated remarkable activity for oxygen reduction and evolution reactions, sparking intense interest in their application for energy conversion processes.
Recent advancements have expanded perovskite applications into hydrocarbon activation, CO₂ conversion, and fine chemical synthesis. The period from 2018 to 2023 has witnessed particularly rapid development, with innovations in synthesis methods enabling precise control over surface properties, defect engineering, and nanostructuring. These developments have dramatically enhanced catalytic performance while reducing precious metal content.
The current technological trajectory suggests perovskites are poised to address several critical challenges in chemical processing. Their ability to catalyze multiple reaction types with high selectivity makes them promising candidates for process intensification and green chemistry applications. Additionally, their thermal stability and resistance to catalyst poisoning offer advantages in harsh industrial environments.
Our research objectives focus on three primary areas: First, developing novel perovskite compositions with enhanced activity and selectivity for targeted chemical transformations, particularly in C-H activation and CO₂ utilization. Second, establishing scalable and reproducible synthesis protocols that maintain precise control over structural and electronic properties. Third, understanding fundamental structure-property relationships through advanced characterization techniques to enable rational catalyst design.
The long-term goal is to develop perovskite-based catalytic systems that can replace current industrial catalysts, offering improved efficiency, reduced environmental impact, and economic advantages. This includes exploring hybrid systems that combine perovskites with other catalytic materials to create synergistic effects and addressing stability challenges in specific reaction environments.
Market Analysis for Advanced Catalytic Materials
The global market for advanced catalytic materials is experiencing significant growth, driven by increasing demand for efficient and sustainable chemical processing solutions. The catalytic materials market was valued at approximately $35.6 billion in 2022 and is projected to reach $47.9 billion by 2028, growing at a CAGR of 5.1%. Within this broader market, perovskite-based catalysts represent one of the fastest-growing segments, with an estimated market share of 8% that is expected to double within the next five years.
Chemical processing industries, particularly petrochemicals, fine chemicals, and pharmaceuticals, are the primary consumers of advanced catalytic materials. These sectors collectively account for over 65% of the total market demand. The increasing focus on green chemistry and sustainable manufacturing processes has created a substantial market pull for novel catalytic solutions that can operate at lower temperatures, reduce energy consumption, and minimize waste production.
Regionally, Asia-Pacific dominates the market with approximately 40% share, led by China's rapidly expanding chemical manufacturing sector. North America and Europe follow with 25% and 22% market shares respectively, where stringent environmental regulations are driving adoption of advanced catalytic technologies. The Middle East is emerging as a significant market due to its petrochemical industry expansion, currently holding about 10% of the global market.
Perovskite catalysts specifically are gaining traction in high-value applications such as hydrogen production, CO2 conversion, and selective oxidation processes. The market for perovskite-based catalytic materials is estimated at $2.8 billion currently, with projections indicating growth to $5.7 billion by 2027. This accelerated growth is attributed to their superior performance characteristics including thermal stability, oxygen mobility, and tunable properties.
End-user industries are increasingly willing to pay premium prices for catalytic materials that demonstrate improved selectivity, longer lifespans, and reduced environmental impact. The average price premium for advanced perovskite catalysts over conventional alternatives ranges from 30-45%, yet the total cost of ownership analysis often favors these advanced materials due to improved process economics and reduced waste treatment costs.
Market barriers include high initial development costs, technical challenges in scaling production, and competition from established catalytic technologies. However, the potential for perovskite catalysts to enable novel chemical transformations and improve existing processes continues to drive investment in research and commercialization efforts, with venture capital funding in this sector exceeding $850 million in 2022 alone.
Chemical processing industries, particularly petrochemicals, fine chemicals, and pharmaceuticals, are the primary consumers of advanced catalytic materials. These sectors collectively account for over 65% of the total market demand. The increasing focus on green chemistry and sustainable manufacturing processes has created a substantial market pull for novel catalytic solutions that can operate at lower temperatures, reduce energy consumption, and minimize waste production.
Regionally, Asia-Pacific dominates the market with approximately 40% share, led by China's rapidly expanding chemical manufacturing sector. North America and Europe follow with 25% and 22% market shares respectively, where stringent environmental regulations are driving adoption of advanced catalytic technologies. The Middle East is emerging as a significant market due to its petrochemical industry expansion, currently holding about 10% of the global market.
Perovskite catalysts specifically are gaining traction in high-value applications such as hydrogen production, CO2 conversion, and selective oxidation processes. The market for perovskite-based catalytic materials is estimated at $2.8 billion currently, with projections indicating growth to $5.7 billion by 2027. This accelerated growth is attributed to their superior performance characteristics including thermal stability, oxygen mobility, and tunable properties.
End-user industries are increasingly willing to pay premium prices for catalytic materials that demonstrate improved selectivity, longer lifespans, and reduced environmental impact. The average price premium for advanced perovskite catalysts over conventional alternatives ranges from 30-45%, yet the total cost of ownership analysis often favors these advanced materials due to improved process economics and reduced waste treatment costs.
Market barriers include high initial development costs, technical challenges in scaling production, and competition from established catalytic technologies. However, the potential for perovskite catalysts to enable novel chemical transformations and improve existing processes continues to drive investment in research and commercialization efforts, with venture capital funding in this sector exceeding $850 million in 2022 alone.
Global Perovskite Catalyst Development Status and Barriers
Perovskite catalysts have emerged as a significant advancement in chemical processing, yet their global development faces numerous challenges. Currently, research institutions across North America, Europe, and East Asia lead development efforts, with China, the United States, and Japan contributing the most significant research output. Despite this widespread interest, several critical barriers impede broader implementation and commercialization.
Material stability represents the foremost challenge, as many perovskite catalysts exhibit degradation under industrial operating conditions. Thermal stability issues become particularly pronounced at temperatures exceeding 800°C, while chemical stability concerns arise when exposed to acidic or oxidizing environments typical in chemical processing applications. This instability significantly limits their practical deployment in continuous industrial processes.
Scalable synthesis methods present another substantial barrier. Laboratory-scale production techniques often fail to translate effectively to industrial-scale manufacturing, creating inconsistencies in catalyst performance and properties. The precise control of stoichiometry, crystallinity, and surface properties becomes increasingly difficult at larger scales, hampering quality control and reproducibility.
Economic viability remains questionable due to the incorporation of precious metals like platinum and palladium in many high-performance perovskite formulations. These materials contribute to prohibitive production costs that make widespread adoption financially challenging compared to conventional catalysts. Additionally, the complex synthesis procedures often require specialized equipment and expertise, further increasing implementation costs.
Regulatory hurdles and environmental concerns also constrain development. Some perovskite compositions contain toxic elements like lead or rare earth metals that raise environmental and health concerns. Stringent regulations regarding the use of these materials vary significantly across regions, creating a fragmented global market and complicating international research collaboration and technology transfer.
Knowledge gaps in fundamental understanding persist despite extensive research. The precise mechanisms of catalytic activity in perovskites remain incompletely understood, particularly regarding surface interactions, reaction pathways, and the relationship between structural properties and catalytic performance. This limited mechanistic understanding hinders rational design approaches for optimizing catalyst formulations.
Standardization issues further complicate development efforts. The lack of universally accepted testing protocols and performance metrics makes comparing different perovskite catalysts challenging. This absence of standardization impedes meaningful benchmarking against conventional catalysts and slows industry adoption due to uncertainty about real-world performance benefits.
Material stability represents the foremost challenge, as many perovskite catalysts exhibit degradation under industrial operating conditions. Thermal stability issues become particularly pronounced at temperatures exceeding 800°C, while chemical stability concerns arise when exposed to acidic or oxidizing environments typical in chemical processing applications. This instability significantly limits their practical deployment in continuous industrial processes.
Scalable synthesis methods present another substantial barrier. Laboratory-scale production techniques often fail to translate effectively to industrial-scale manufacturing, creating inconsistencies in catalyst performance and properties. The precise control of stoichiometry, crystallinity, and surface properties becomes increasingly difficult at larger scales, hampering quality control and reproducibility.
Economic viability remains questionable due to the incorporation of precious metals like platinum and palladium in many high-performance perovskite formulations. These materials contribute to prohibitive production costs that make widespread adoption financially challenging compared to conventional catalysts. Additionally, the complex synthesis procedures often require specialized equipment and expertise, further increasing implementation costs.
Regulatory hurdles and environmental concerns also constrain development. Some perovskite compositions contain toxic elements like lead or rare earth metals that raise environmental and health concerns. Stringent regulations regarding the use of these materials vary significantly across regions, creating a fragmented global market and complicating international research collaboration and technology transfer.
Knowledge gaps in fundamental understanding persist despite extensive research. The precise mechanisms of catalytic activity in perovskites remain incompletely understood, particularly regarding surface interactions, reaction pathways, and the relationship between structural properties and catalytic performance. This limited mechanistic understanding hinders rational design approaches for optimizing catalyst formulations.
Standardization issues further complicate development efforts. The lack of universally accepted testing protocols and performance metrics makes comparing different perovskite catalysts challenging. This absence of standardization impedes meaningful benchmarking against conventional catalysts and slows industry adoption due to uncertainty about real-world performance benefits.
Current Perovskite Catalyst Applications in Chemical Processing
01 Perovskite catalysts for environmental applications
Perovskite-type catalysts are effective for environmental applications such as reducing emissions and pollutants. These catalysts demonstrate high activity in oxidation reactions, particularly for the removal of harmful gases like carbon monoxide, nitrogen oxides, and volatile organic compounds. Their structural stability at high temperatures makes them suitable for automotive catalytic converters and industrial emission control systems. The catalytic performance can be enhanced by controlling the composition and synthesis methods.- Perovskite catalysts for environmental applications: Perovskite-type catalysts are utilized in environmental applications such as exhaust gas purification, NOx reduction, and air pollution control. These catalysts demonstrate high efficiency in converting harmful emissions into less harmful substances through oxidation and reduction reactions. Their structural stability at high temperatures makes them particularly suitable for automotive catalytic converters and industrial emission control systems.
- Perovskite catalysts for hydrocarbon processing: Perovskite-structured materials serve as effective catalysts in various hydrocarbon processing applications, including reforming, cracking, and hydrogenation reactions. These catalysts facilitate the conversion of crude oil fractions into valuable products and intermediates. Their unique crystal structure allows for selective catalytic activity while maintaining stability under the harsh conditions typical of petrochemical processes.
- Novel perovskite compositions and synthesis methods: Innovative approaches to synthesizing perovskite catalysts with enhanced properties have been developed. These methods include sol-gel processing, hydrothermal synthesis, and combustion techniques that allow precise control over composition, crystal structure, and surface properties. The resulting materials exhibit improved catalytic activity, selectivity, and stability compared to conventionally prepared perovskites, enabling their application in more demanding reaction environments.
- Perovskite catalysts for energy conversion and storage: Perovskite materials play a crucial role in energy conversion and storage technologies, including fuel cells, electrolyzers, and photocatalytic systems. These catalysts facilitate efficient energy conversion processes by promoting electron transfer reactions at interfaces. Their tunable electronic properties and oxygen vacancy formation capabilities make them particularly valuable for renewable energy applications and sustainable hydrogen production.
- Doped and modified perovskite catalysts: The catalytic performance of perovskite materials can be significantly enhanced through doping and modification strategies. Incorporating various metal ions into the perovskite structure or decorating the surface with active species creates synergistic effects that improve activity, selectivity, and stability. These tailored catalysts demonstrate superior performance in specific reactions, including CO oxidation, methane activation, and electrochemical oxygen reduction, making them valuable for specialized industrial applications.
02 Perovskite catalysts for energy conversion processes
Perovskite materials serve as efficient catalysts in various energy conversion processes, including fuel cells, water splitting, and hydrogen production. Their unique crystal structure allows for high oxygen mobility and electron transfer capabilities, making them particularly valuable for electrochemical applications. These catalysts can operate at intermediate to high temperatures and show promising performance in renewable energy systems. Modifications to the perovskite structure can be made to optimize conductivity and catalytic activity for specific energy conversion reactions.Expand Specific Solutions03 Synthesis methods for perovskite catalysts
Various synthesis methods have been developed to prepare perovskite catalysts with controlled properties. These include sol-gel processing, hydrothermal synthesis, co-precipitation, and solid-state reactions. The preparation method significantly influences the surface area, particle size, crystallinity, and ultimately the catalytic performance of the perovskite materials. Advanced techniques allow for the creation of nanostructured perovskites with enhanced catalytic activity due to increased surface area and active sites. Post-synthesis treatments such as calcination at specific temperatures can further optimize the catalytic properties.Expand Specific Solutions04 Doped and substituted perovskite catalysts
Doping and substitution strategies are employed to enhance the catalytic properties of perovskite materials. By partially replacing A-site or B-site cations in the ABO₃ structure with other elements, the electronic properties, oxygen vacancy concentration, and catalytic activity can be tuned. Common dopants include transition metals, rare earth elements, and alkaline earth metals. These modifications can improve stability, selectivity, and activity for specific reactions. Multi-element doping can create synergistic effects that significantly enhance catalytic performance beyond what single-element doping can achieve.Expand Specific Solutions05 Perovskite catalysts for hydrocarbon processing
Perovskite catalysts demonstrate significant potential in hydrocarbon processing applications, including reforming, cracking, and partial oxidation reactions. These materials show high activity and selectivity for converting hydrocarbons into value-added products or synthesis gas. Their resistance to carbon deposition makes them particularly suitable for long-term operation in hydrocarbon conversion processes. The redox properties of perovskites can be tailored to optimize specific hydrocarbon transformation reactions. Advanced formulations incorporate additional components to enhance stability under reducing conditions typical in hydrocarbon processing.Expand Specific Solutions
Leading Organizations in Perovskite Catalyst Research
Perovskite catalyst technology in chemical processing is currently in a growth phase, with the market expected to expand significantly due to increasing demand for more efficient and sustainable chemical processes. The global market size is projected to reach substantial value as industries seek alternatives to traditional catalysts. From a technological maturity perspective, academic institutions like Tsinghua University, KAIST, and Cornell University are leading fundamental research, while companies such as LG Chem, Honda Motor, and DENSO are focusing on commercial applications. Research collaborations between industry and academia, particularly involving Tianjin University and CSIR, are accelerating development. The competitive landscape shows a geographical concentration in East Asia, with emerging competition from North American and European research entities working on novel perovskite formulations for specialized chemical processes.
Tsinghua University
Technical Solution: Tsinghua University has pioneered advanced perovskite catalyst systems focused on sustainable chemical processing applications. Their research team has developed novel A-site deficient perovskite structures (A1-xBO3) that demonstrate exceptional catalytic activity for hydrocarbon conversion reactions. These catalysts exhibit remarkable thermal stability up to 900°C while maintaining structural integrity and performance. Tsinghua's approach incorporates strategic doping with transition metals to create oxygen vacancies that serve as active sites for chemical transformations. Their perovskite catalysts have shown particular efficacy in methane dry reforming processes, achieving CO2 conversion rates exceeding 90% with minimal carbon deposition over extended operation periods. The university has also developed innovative synthesis methods using sol-gel techniques combined with controlled calcination protocols that enable precise control of perovskite morphology and surface area. Recent advancements include the development of hierarchically porous perovskite structures that significantly enhance mass transfer properties while maintaining high catalytic activity.
Strengths: Exceptional thermal stability at high temperatures, remarkable resistance to carbon deposition in hydrocarbon processing, and highly tunable composition allowing for application-specific optimization. Weaknesses: Complex synthesis procedures that may challenge industrial-scale production, potential issues with mechanical stability under pressure cycling conditions, and higher material costs compared to conventional catalysts.
LG Chem Ltd.
Technical Solution: LG Chem has developed proprietary perovskite catalyst technologies specifically designed for large-scale chemical processing applications. Their approach centers on lanthanum-based perovskites (LaBO3) with carefully selected B-site cations to optimize catalytic performance for specific industrial reactions. The company has engineered perovskite catalysts with exceptional stability in sulfur-containing environments, addressing a critical challenge in petrochemical processing. LG Chem's perovskite catalysts demonstrate superior activity in oxidative coupling reactions, achieving methane conversion efficiencies up to 35% higher than conventional catalysts while operating at temperatures 100-150°C lower. Their manufacturing process incorporates advanced spray pyrolysis techniques that enable precise control of particle size distribution and surface area, critical factors for industrial catalyst performance. The company has successfully integrated these catalysts into existing chemical processing infrastructure, demonstrating compatibility with standard reactor designs while delivering significant improvements in yield and energy efficiency. Recent developments include perovskite-based membrane reactor systems that combine separation and catalytic functions for process intensification.
Strengths: Excellent scalability for industrial applications, superior resistance to sulfur poisoning, and compatibility with existing chemical processing infrastructure. Weaknesses: Higher initial investment costs compared to traditional catalysts, potential challenges with catalyst regeneration after deactivation, and intellectual property restrictions limiting customization options.
Key Patents and Breakthroughs in Perovskite Catalyst Design
Supported catalyst
PatentInactiveUS20190046961A1
Innovation
- The use of perovskite supports with specific A-site and B-site species to control the selectivity of catalytic components, allowing for the tuning of supported catalysts to favor specific reaction products by varying the B-site species without altering the catalytic component, thereby enhancing selectivity in liquid-phase reactions.
Method for dehydrogenating an organic compound using a catalyst comprising a perovskite
PatentWO1999064377A1
Innovation
- A catalyst comprising a perovskite with elements capable of exhibiting multiple oxidation states, such as manganese, iron, or cobalt, is used in a single-phase composition for dehydrogenation reactions, allowing for simpler preparation and improved catalytic performance in converting C—C bonds to C=C bonds.
Environmental Impact and Sustainability Considerations
Perovskite catalysts represent a significant advancement in chemical processing technologies, but their environmental impact and sustainability considerations must be thoroughly evaluated. The production processes for perovskite catalysts typically involve energy-intensive methods and potentially hazardous precursor materials, raising concerns about their overall environmental footprint. Recent life cycle assessments indicate that the synthesis of perovskite materials can generate substantial carbon emissions, particularly when high-temperature calcination steps are required.
Water consumption and potential contamination present another critical environmental consideration. Traditional perovskite synthesis often utilizes solvents that may pose risks to aquatic ecosystems if improperly managed. However, emerging green synthesis routes are demonstrating promising reductions in water usage and hazardous waste generation, with some innovative approaches achieving up to 80% decrease in environmental impact compared to conventional methods.
The durability and stability of perovskite catalysts directly influence their sustainability profile. Catalysts with extended operational lifespans reduce replacement frequency and associated resource consumption. Recent advancements in perovskite structure stabilization have yielded materials maintaining over 90% activity after 1000 hours of operation under industrial conditions, representing a significant improvement over earlier generations that degraded rapidly.
Resource efficiency considerations are paramount, particularly regarding the use of rare or critical elements in perovskite formulations. Several research groups have successfully developed perovskite catalysts that substitute scarce elements with earth-abundant alternatives while maintaining comparable catalytic performance. These developments align with circular economy principles and reduce dependency on geopolitically sensitive material supply chains.
End-of-life management strategies for spent perovskite catalysts are evolving rapidly. Recovery and recycling technologies specifically designed for perovskite materials have demonstrated recovery rates exceeding 85% for valuable components. These processes not only mitigate environmental impacts but also improve the economic viability of perovskite catalyst implementation across various chemical processing applications.
Regulatory frameworks worldwide are increasingly emphasizing sustainable catalyst technologies. Perovskite catalysts that demonstrate reduced energy requirements, lower emissions, and minimal waste generation are positioned advantageously as industries face stricter environmental compliance standards. Companies investing in environmentally optimized perovskite catalyst technologies may benefit from regulatory incentives and improved corporate sustainability metrics.
The integration of perovskite catalysts into existing chemical processing infrastructure presents opportunities for significant environmental benefits through process intensification. By enabling reactions at lower temperatures or pressures, these advanced catalysts can reduce overall energy consumption by 15-30% in certain applications, contributing substantially to industrial decarbonization efforts.
Water consumption and potential contamination present another critical environmental consideration. Traditional perovskite synthesis often utilizes solvents that may pose risks to aquatic ecosystems if improperly managed. However, emerging green synthesis routes are demonstrating promising reductions in water usage and hazardous waste generation, with some innovative approaches achieving up to 80% decrease in environmental impact compared to conventional methods.
The durability and stability of perovskite catalysts directly influence their sustainability profile. Catalysts with extended operational lifespans reduce replacement frequency and associated resource consumption. Recent advancements in perovskite structure stabilization have yielded materials maintaining over 90% activity after 1000 hours of operation under industrial conditions, representing a significant improvement over earlier generations that degraded rapidly.
Resource efficiency considerations are paramount, particularly regarding the use of rare or critical elements in perovskite formulations. Several research groups have successfully developed perovskite catalysts that substitute scarce elements with earth-abundant alternatives while maintaining comparable catalytic performance. These developments align with circular economy principles and reduce dependency on geopolitically sensitive material supply chains.
End-of-life management strategies for spent perovskite catalysts are evolving rapidly. Recovery and recycling technologies specifically designed for perovskite materials have demonstrated recovery rates exceeding 85% for valuable components. These processes not only mitigate environmental impacts but also improve the economic viability of perovskite catalyst implementation across various chemical processing applications.
Regulatory frameworks worldwide are increasingly emphasizing sustainable catalyst technologies. Perovskite catalysts that demonstrate reduced energy requirements, lower emissions, and minimal waste generation are positioned advantageously as industries face stricter environmental compliance standards. Companies investing in environmentally optimized perovskite catalyst technologies may benefit from regulatory incentives and improved corporate sustainability metrics.
The integration of perovskite catalysts into existing chemical processing infrastructure presents opportunities for significant environmental benefits through process intensification. By enabling reactions at lower temperatures or pressures, these advanced catalysts can reduce overall energy consumption by 15-30% in certain applications, contributing substantially to industrial decarbonization efforts.
Scalability and Industrial Implementation Challenges
Despite the promising laboratory results of perovskite catalysts in chemical processing, significant challenges remain in scaling these technologies to industrial levels. The transition from bench-scale to commercial implementation faces several critical barriers that must be addressed systematically to realize the full potential of these advanced materials.
Production scalability represents the foremost challenge, as current synthesis methods for high-quality perovskite catalysts often involve complex procedures with precise control requirements. Batch-to-batch consistency becomes increasingly difficult to maintain when scaling up production volumes, potentially compromising catalyst performance and reliability in industrial settings. The development of continuous flow manufacturing processes could potentially address these issues but requires substantial engineering innovation.
Cost considerations further complicate industrial implementation. While perovskite catalysts demonstrate superior performance metrics, their production costs currently exceed those of conventional catalysts by a significant margin. This cost differential stems from expensive precursor materials, energy-intensive synthesis procedures, and specialized equipment requirements. Economic viability demands either cost reduction strategies or performance improvements substantial enough to justify the premium pricing.
Stability under industrial conditions presents another major hurdle. Commercial chemical processes typically operate under harsh environments with high temperatures, pressures, and exposure to various chemical species. Perovskite catalysts must maintain their structural integrity and catalytic activity under these demanding conditions for extended periods to be commercially viable. Current formulations often show degradation rates incompatible with industrial service lifetimes.
Regulatory compliance and environmental considerations also impact implementation timelines. Novel materials face rigorous safety assessments before industrial adoption, particularly in sensitive applications like food processing or pharmaceutical manufacturing. Additionally, the environmental footprint of perovskite catalyst production, including potential toxicity of certain precursors and waste streams, requires careful evaluation and mitigation.
Integration with existing industrial infrastructure represents a practical challenge that is often overlooked. Most chemical plants have established processes optimized around conventional catalysts. Retrofitting these facilities to accommodate perovskite catalysts may require significant capital investment and process redesign. Developing drop-in replacement solutions that minimize disruption to existing operations would accelerate adoption but presents additional technical challenges.
Addressing these implementation barriers requires collaborative efforts between academic researchers, industrial engineers, and regulatory bodies. Strategic research investments should focus on developing scalable synthesis methods, enhancing catalyst stability, and reducing production costs while maintaining performance advantages. Pilot-scale demonstrations in relevant industrial environments will be crucial to building confidence and generating the operational data needed for full commercial implementation.
Production scalability represents the foremost challenge, as current synthesis methods for high-quality perovskite catalysts often involve complex procedures with precise control requirements. Batch-to-batch consistency becomes increasingly difficult to maintain when scaling up production volumes, potentially compromising catalyst performance and reliability in industrial settings. The development of continuous flow manufacturing processes could potentially address these issues but requires substantial engineering innovation.
Cost considerations further complicate industrial implementation. While perovskite catalysts demonstrate superior performance metrics, their production costs currently exceed those of conventional catalysts by a significant margin. This cost differential stems from expensive precursor materials, energy-intensive synthesis procedures, and specialized equipment requirements. Economic viability demands either cost reduction strategies or performance improvements substantial enough to justify the premium pricing.
Stability under industrial conditions presents another major hurdle. Commercial chemical processes typically operate under harsh environments with high temperatures, pressures, and exposure to various chemical species. Perovskite catalysts must maintain their structural integrity and catalytic activity under these demanding conditions for extended periods to be commercially viable. Current formulations often show degradation rates incompatible with industrial service lifetimes.
Regulatory compliance and environmental considerations also impact implementation timelines. Novel materials face rigorous safety assessments before industrial adoption, particularly in sensitive applications like food processing or pharmaceutical manufacturing. Additionally, the environmental footprint of perovskite catalyst production, including potential toxicity of certain precursors and waste streams, requires careful evaluation and mitigation.
Integration with existing industrial infrastructure represents a practical challenge that is often overlooked. Most chemical plants have established processes optimized around conventional catalysts. Retrofitting these facilities to accommodate perovskite catalysts may require significant capital investment and process redesign. Developing drop-in replacement solutions that minimize disruption to existing operations would accelerate adoption but presents additional technical challenges.
Addressing these implementation barriers requires collaborative efforts between academic researchers, industrial engineers, and regulatory bodies. Strategic research investments should focus on developing scalable synthesis methods, enhancing catalyst stability, and reducing production costs while maintaining performance advantages. Pilot-scale demonstrations in relevant industrial environments will be crucial to building confidence and generating the operational data needed for full commercial implementation.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!