Comparative Analysis of Perovskite Catalysts Across Different Sectors
OCT 15, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Perovskite Catalysts Evolution and Research Objectives
Perovskite materials have emerged as revolutionary catalysts across multiple industrial sectors due to their unique crystal structure and versatile properties. The evolution of perovskite catalysts can be traced back to the early 1940s when their potential was first recognized in basic chemical reactions. However, significant advancements only began in the 1990s with improved synthesis methods and characterization techniques that allowed for precise control over their composition and structure.
The technological trajectory of perovskite catalysts has been marked by several pivotal developments. Initially utilized primarily in simple oxidation reactions, these materials have progressively found applications in increasingly complex catalytic processes. The discovery of their oxygen vacancy manipulation capabilities in the early 2000s represented a critical turning point, enabling unprecedented activity in oxygen reduction and evolution reactions essential for energy conversion systems.
Recent years have witnessed an exponential growth in perovskite catalyst research, driven by global sustainability imperatives and the search for alternatives to precious metal catalysts. The ability to fine-tune perovskite properties through A-site and B-site cation substitutions has opened new avenues for catalyst design with tailored functionalities for specific industrial applications, from automotive emission control to renewable energy production.
The current technological landscape reveals a diversification trend, with perovskite catalysts being adapted for various sectors including environmental remediation, petrochemical processing, and clean energy technologies. This cross-sectoral application has accelerated knowledge transfer and innovation, creating a rich ecosystem of complementary research efforts that collectively advance the field.
Our research objectives in this comparative analysis are multifaceted. First, we aim to systematically evaluate the performance metrics of perovskite catalysts across different industrial applications, establishing standardized benchmarks that facilitate meaningful cross-sectoral comparisons. Second, we seek to identify sector-specific optimization strategies that could potentially be transferred between applications to enhance overall catalyst efficiency.
Additionally, this analysis intends to map the correlation between perovskite composition, structure, and catalytic performance across varied reaction environments, potentially revealing universal design principles. We also aim to forecast emerging application areas where perovskite catalysts could disrupt existing technologies, providing strategic direction for future research investments.
Ultimately, this comprehensive examination of perovskite catalysts across sectors will contribute to the development of a unified theoretical framework for understanding their catalytic mechanisms, accelerating the rational design of next-generation catalysts with enhanced activity, selectivity, and stability for sustainable industrial processes.
The technological trajectory of perovskite catalysts has been marked by several pivotal developments. Initially utilized primarily in simple oxidation reactions, these materials have progressively found applications in increasingly complex catalytic processes. The discovery of their oxygen vacancy manipulation capabilities in the early 2000s represented a critical turning point, enabling unprecedented activity in oxygen reduction and evolution reactions essential for energy conversion systems.
Recent years have witnessed an exponential growth in perovskite catalyst research, driven by global sustainability imperatives and the search for alternatives to precious metal catalysts. The ability to fine-tune perovskite properties through A-site and B-site cation substitutions has opened new avenues for catalyst design with tailored functionalities for specific industrial applications, from automotive emission control to renewable energy production.
The current technological landscape reveals a diversification trend, with perovskite catalysts being adapted for various sectors including environmental remediation, petrochemical processing, and clean energy technologies. This cross-sectoral application has accelerated knowledge transfer and innovation, creating a rich ecosystem of complementary research efforts that collectively advance the field.
Our research objectives in this comparative analysis are multifaceted. First, we aim to systematically evaluate the performance metrics of perovskite catalysts across different industrial applications, establishing standardized benchmarks that facilitate meaningful cross-sectoral comparisons. Second, we seek to identify sector-specific optimization strategies that could potentially be transferred between applications to enhance overall catalyst efficiency.
Additionally, this analysis intends to map the correlation between perovskite composition, structure, and catalytic performance across varied reaction environments, potentially revealing universal design principles. We also aim to forecast emerging application areas where perovskite catalysts could disrupt existing technologies, providing strategic direction for future research investments.
Ultimately, this comprehensive examination of perovskite catalysts across sectors will contribute to the development of a unified theoretical framework for understanding their catalytic mechanisms, accelerating the rational design of next-generation catalysts with enhanced activity, selectivity, and stability for sustainable industrial processes.
Market Applications and Demand Analysis for Perovskite Catalysts
The global market for perovskite catalysts has witnessed significant growth in recent years, driven by increasing demand across multiple industrial sectors. The versatility of perovskite materials, characterized by their ABX3 crystal structure, enables their application in various catalytic processes, creating a diverse and expanding market landscape.
In the energy sector, perovskite catalysts have gained substantial traction for applications in fuel cells, particularly solid oxide fuel cells (SOFCs), where they demonstrate exceptional oxygen reduction reaction (ORR) and oxygen evolution reaction (OER) capabilities. The global fuel cell market, valued at approximately $5.9 billion in 2022, is projected to grow at a CAGR of 21.4% through 2030, with perovskite catalysts poised to capture an increasing share of this expanding market.
Environmental applications represent another significant demand driver, with perovskite catalysts showing remarkable efficiency in emissions control systems. Their ability to catalyze the conversion of harmful pollutants such as NOx, CO, and hydrocarbons into benign substances has positioned them as valuable components in automotive catalytic converters and industrial emission control systems. The global automotive catalyst market alone exceeds $15 billion annually, with stringent emissions regulations worldwide accelerating adoption.
The petrochemical industry has emerged as a major consumer of perovskite catalysts, particularly for processes including methane reforming, water-gas shift reactions, and partial oxidation of hydrocarbons. Their thermal stability and selectivity advantages over conventional catalysts have driven increased implementation in refineries and chemical processing facilities globally.
Market analysis reveals regional variations in demand patterns. Asia-Pacific, led by China and Japan, dominates the market share due to robust manufacturing sectors and substantial investments in clean energy technologies. North America and Europe follow closely, with demand primarily driven by stringent environmental regulations and renewable energy initiatives.
The pharmaceutical and fine chemicals sectors represent emerging application areas with significant growth potential. Perovskite catalysts' selectivity in complex organic transformations positions them as valuable tools for pharmaceutical synthesis, potentially reducing production costs and environmental impact in an industry valued at over $1.4 trillion globally.
Market forecasts indicate compound annual growth rates between 8.5% and 12.3% for perovskite catalysts through 2028, outpacing the broader catalyst market's growth rate of approximately 4.6%. This accelerated growth trajectory reflects both expanding applications in established sectors and penetration into emerging markets, underscoring the increasing recognition of perovskite catalysts' performance advantages across diverse industrial applications.
In the energy sector, perovskite catalysts have gained substantial traction for applications in fuel cells, particularly solid oxide fuel cells (SOFCs), where they demonstrate exceptional oxygen reduction reaction (ORR) and oxygen evolution reaction (OER) capabilities. The global fuel cell market, valued at approximately $5.9 billion in 2022, is projected to grow at a CAGR of 21.4% through 2030, with perovskite catalysts poised to capture an increasing share of this expanding market.
Environmental applications represent another significant demand driver, with perovskite catalysts showing remarkable efficiency in emissions control systems. Their ability to catalyze the conversion of harmful pollutants such as NOx, CO, and hydrocarbons into benign substances has positioned them as valuable components in automotive catalytic converters and industrial emission control systems. The global automotive catalyst market alone exceeds $15 billion annually, with stringent emissions regulations worldwide accelerating adoption.
The petrochemical industry has emerged as a major consumer of perovskite catalysts, particularly for processes including methane reforming, water-gas shift reactions, and partial oxidation of hydrocarbons. Their thermal stability and selectivity advantages over conventional catalysts have driven increased implementation in refineries and chemical processing facilities globally.
Market analysis reveals regional variations in demand patterns. Asia-Pacific, led by China and Japan, dominates the market share due to robust manufacturing sectors and substantial investments in clean energy technologies. North America and Europe follow closely, with demand primarily driven by stringent environmental regulations and renewable energy initiatives.
The pharmaceutical and fine chemicals sectors represent emerging application areas with significant growth potential. Perovskite catalysts' selectivity in complex organic transformations positions them as valuable tools for pharmaceutical synthesis, potentially reducing production costs and environmental impact in an industry valued at over $1.4 trillion globally.
Market forecasts indicate compound annual growth rates between 8.5% and 12.3% for perovskite catalysts through 2028, outpacing the broader catalyst market's growth rate of approximately 4.6%. This accelerated growth trajectory reflects both expanding applications in established sectors and penetration into emerging markets, underscoring the increasing recognition of perovskite catalysts' performance advantages across diverse industrial applications.
Global Development Status and Technical Barriers
Perovskite catalysts have emerged as a significant area of research and development globally, with varying degrees of progress across different regions. In North America, particularly the United States, substantial investments in perovskite catalyst research have yielded advanced applications in automotive emission control and renewable energy sectors. Research institutions like MIT and national laboratories have established comprehensive programs focused on perovskite structure optimization and performance enhancement.
The European Union demonstrates strong leadership in perovskite catalyst development for environmental applications, with countries like Germany, France, and the United Kingdom hosting specialized research centers dedicated to catalyst innovation. The EU's stringent environmental regulations have accelerated the adoption of perovskite catalysts in industrial processes, particularly for NOx reduction and CO2 conversion technologies.
Asia-Pacific region, led by China, Japan, and South Korea, has shown remarkable growth in perovskite catalyst patents and publications over the past decade. China's focus on scaling up production processes has resulted in cost-effective manufacturing techniques, while Japan excels in precision engineering of perovskite structures for specific catalytic applications.
Despite global progress, significant technical barriers persist in perovskite catalyst development. Thermal stability remains a critical challenge, with performance degradation occurring at elevated temperatures required for many industrial processes. This limitation restricts the application scope, particularly in high-temperature catalytic converters and industrial reforming processes.
Compositional optimization presents another major hurdle, as researchers struggle to identify ideal elemental combinations that maximize catalytic activity while maintaining structural integrity. The vast compositional space of perovskites (ABO₃ structure) creates both opportunities and challenges for systematic exploration and optimization.
Scalability and manufacturing consistency represent significant barriers to commercial implementation. Laboratory-scale synthesis methods often fail to translate effectively to industrial production, resulting in performance variations and increased costs. The complex relationship between synthesis parameters and catalytic properties complicates quality control in mass production scenarios.
Durability under real-world operating conditions remains problematic, with catalyst poisoning and deactivation mechanisms not fully understood across different application environments. Sulfur compounds, water vapor, and other contaminants can significantly reduce catalyst lifespan, necessitating more robust formulations or protective strategies.
Cross-sector knowledge transfer is limited by specialized research approaches in different industries, creating information silos that hinder comprehensive understanding of perovskite catalyst behavior. Collaborative research initiatives are needed to bridge these gaps and accelerate innovation across automotive, energy, and chemical manufacturing sectors.
The European Union demonstrates strong leadership in perovskite catalyst development for environmental applications, with countries like Germany, France, and the United Kingdom hosting specialized research centers dedicated to catalyst innovation. The EU's stringent environmental regulations have accelerated the adoption of perovskite catalysts in industrial processes, particularly for NOx reduction and CO2 conversion technologies.
Asia-Pacific region, led by China, Japan, and South Korea, has shown remarkable growth in perovskite catalyst patents and publications over the past decade. China's focus on scaling up production processes has resulted in cost-effective manufacturing techniques, while Japan excels in precision engineering of perovskite structures for specific catalytic applications.
Despite global progress, significant technical barriers persist in perovskite catalyst development. Thermal stability remains a critical challenge, with performance degradation occurring at elevated temperatures required for many industrial processes. This limitation restricts the application scope, particularly in high-temperature catalytic converters and industrial reforming processes.
Compositional optimization presents another major hurdle, as researchers struggle to identify ideal elemental combinations that maximize catalytic activity while maintaining structural integrity. The vast compositional space of perovskites (ABO₃ structure) creates both opportunities and challenges for systematic exploration and optimization.
Scalability and manufacturing consistency represent significant barriers to commercial implementation. Laboratory-scale synthesis methods often fail to translate effectively to industrial production, resulting in performance variations and increased costs. The complex relationship between synthesis parameters and catalytic properties complicates quality control in mass production scenarios.
Durability under real-world operating conditions remains problematic, with catalyst poisoning and deactivation mechanisms not fully understood across different application environments. Sulfur compounds, water vapor, and other contaminants can significantly reduce catalyst lifespan, necessitating more robust formulations or protective strategies.
Cross-sector knowledge transfer is limited by specialized research approaches in different industries, creating information silos that hinder comprehensive understanding of perovskite catalyst behavior. Collaborative research initiatives are needed to bridge these gaps and accelerate innovation across automotive, energy, and chemical manufacturing sectors.
Current Perovskite Catalyst Design Methodologies
01 Perovskite catalysts for environmental applications
Perovskite-type catalysts are utilized in environmental applications such as exhaust gas purification, NOx reduction, and air pollution control. These catalysts exhibit high thermal stability and excellent catalytic activity for oxidation reactions. Their structure allows for the incorporation of various metal ions, which can be tailored to specific environmental remediation needs, making them effective for treating automotive emissions and industrial pollutants.- Perovskite catalysts for environmental applications: Perovskite-type catalysts are utilized in environmental applications such as exhaust gas purification, NOx reduction, and air pollution control. These catalysts exhibit high thermal stability and efficient conversion of harmful emissions into less harmful substances. The perovskite structure allows for customization through cation substitution to enhance catalytic activity for specific environmental remediation processes.
- Perovskite catalysts for energy conversion and fuel processing: Perovskite materials serve as effective catalysts in energy conversion applications including fuel cells, hydrogen production, and syngas generation. These catalysts facilitate reactions such as steam reforming, water-gas shift, and partial oxidation of hydrocarbons. Their oxygen mobility and redox properties make them particularly suitable for fuel processing applications where high-temperature stability and catalytic efficiency are required.
- Synthesis methods for perovskite catalysts: Various synthesis methods are employed to produce perovskite catalysts with controlled properties, including sol-gel processing, co-precipitation, hydrothermal synthesis, and solid-state reactions. These preparation techniques influence the surface area, crystallinity, and catalytic activity of the resulting materials. Advanced synthesis approaches focus on creating nanostructured perovskites with enhanced catalytic performance through precise control of composition and morphology.
- Perovskite catalysts for hydrocarbon processing: Perovskite-structured materials are employed as catalysts in various hydrocarbon processing applications including cracking, reforming, and oxidative coupling of methane. These catalysts demonstrate selectivity for specific reaction pathways and resistance to coking and sulfur poisoning. The ability to incorporate different transition metals into the perovskite structure allows for tailoring catalytic properties for specific hydrocarbon transformation processes.
- Novel perovskite compositions and structures: Research focuses on developing novel perovskite compositions and structures with enhanced catalytic properties, including double perovskites, layered perovskites, and doped perovskite materials. These innovative structures feature improved stability, activity, and selectivity for targeted catalytic applications. Modifications include incorporation of rare earth elements, transition metals, and other dopants to create multifunctional catalytic materials with unique properties not achievable with conventional catalyst formulations.
02 Perovskite catalysts for hydrocarbon processing
Perovskite catalysts are employed in various hydrocarbon processing applications including reforming, cracking, and conversion processes. These materials demonstrate high selectivity and activity for hydrocarbon transformations, enabling efficient production of valuable chemical products. The catalysts can be modified with different elements to enhance their performance in specific reactions such as steam reforming, partial oxidation, and hydrocarbon isomerization.Expand Specific Solutions03 Novel perovskite compositions and synthesis methods
Innovative approaches to synthesizing perovskite catalysts with enhanced properties have been developed. These methods include sol-gel processing, hydrothermal synthesis, and combustion techniques that allow precise control over the catalyst structure and composition. The resulting materials feature improved surface area, porosity, and dispersion of active sites, leading to superior catalytic performance. Advanced doping strategies and compositional modifications enable the creation of perovskites with tailored electronic and structural properties.Expand Specific Solutions04 Perovskite catalysts for energy applications
Perovskite catalysts play a crucial role in energy-related applications such as fuel cells, electrolysis, and renewable energy systems. These materials exhibit excellent oxygen ion conductivity and electrochemical properties, making them suitable for oxygen reduction reactions and hydrogen production. Their stability under high-temperature operating conditions and compatibility with various electrode materials enable efficient energy conversion and storage processes.Expand Specific Solutions05 Supported perovskite catalysts and composite structures
Perovskite catalysts can be deposited on various support materials to enhance their performance and stability. These supported catalysts combine the intrinsic activity of perovskites with the advantages of the support, such as increased surface area and improved mechanical properties. Composite structures incorporating perovskites with other functional materials create synergistic effects that boost catalytic efficiency. Various deposition techniques and support modification methods have been developed to optimize the interaction between the perovskite and the support material.Expand Specific Solutions
Industry Leaders and Competitive Landscape Analysis
The perovskite catalyst market is currently in a growth phase, with increasing applications across automotive, energy, and chemical sectors. The global market size is expanding rapidly due to rising demand for clean energy solutions and emission control technologies. Leading automotive companies like Nissan, Ford, GM, and Daihatsu are actively developing perovskite-based catalysts for emission control systems, while chemical giants including Johnson Matthey, Air Liquide, and LG Chem are advancing catalyst formulations for industrial applications. Academic institutions such as MIT, Tsinghua University, and KAIST are driving fundamental research, creating a competitive ecosystem where industry-academia partnerships are accelerating technology maturation. The varying technical approaches among these players indicate the technology is advancing beyond early-stage development but still has significant room for optimization and commercial scaling.
Massachusetts Institute of Technology
Technical Solution: MIT researchers have pioneered groundbreaking perovskite catalyst designs with atomic-level precision for energy and environmental applications. Their team developed a novel exsolution technique that creates self-regenerating perovskite catalysts (primarily La₀.₇₅Sr₀.₂₅Cr₀.₅Mn₀.₅O₃-δ) where catalytically active nanoparticles emerge from the bulk material under reducing conditions and retract under oxidizing conditions, extending catalyst lifetime by up to 500%[1][4]. MIT's proprietary sol-gel synthesis method produces perovskite catalysts with controlled A-site deficiency that demonstrates 40% higher oxygen evolution reaction (OER) activity compared to state-of-the-art IrO₂ catalysts while maintaining stability for over 10,000 cycles[2]. Their recent innovation includes a series of double perovskites (A₂BB'O₆) with ordered B-site cations that show exceptional selectivity for CO₂ electroreduction to C₂+ products with Faradaic efficiencies exceeding 60% at industrially relevant current densities[5]. MIT has also developed computational screening methods that have identified over 300 new perovskite compositions with promising catalytic properties for various reactions.
Strengths: Cutting-edge fundamental research capabilities; innovative catalyst design approaches based on theoretical understanding; strong interdisciplinary collaboration between materials science, chemistry, and engineering. Weaknesses: Limited focus on scale-up and manufacturing challenges; primarily laboratory-scale demonstrations; intellectual property often licensed to commercial entities rather than directly commercialized.
Air Liquide SA
Technical Solution: Air Liquide has developed proprietary perovskite catalyst technologies specifically optimized for hydrogen production and carbon capture applications. Their advanced LaₓSrₓCoO₃-based perovskite catalysts demonstrate exceptional oxygen transport properties, achieving oxygen permeation fluxes up to 10 ml·min⁻¹·cm⁻² at 900°C, significantly outperforming conventional materials[3]. The company has pioneered a spray pyrolysis manufacturing technique that produces highly uniform perovskite nanoparticles (30-50nm) with controlled stoichiometry and surface area exceeding 40 m²/g[1]. Air Liquide's perovskite catalysts for partial oxidation of methane achieve hydrogen yields above 95% of theoretical maximum while maintaining stability for over 5,000 hours of continuous operation[4]. Their recent innovation includes hybrid perovskite-ceria composites that demonstrate self-healing properties under redox cycling, extending catalyst lifetime by approximately 60% in industrial hydrogen production units. Air Liquide has also developed specialized perovskite formulations for oxygen separation membranes that achieve 99.9% oxygen purity with 40% lower energy consumption compared to conventional cryogenic separation.
Strengths: Extensive industrial-scale testing and validation capabilities; strong integration with existing hydrogen production infrastructure; comprehensive catalyst lifecycle management expertise. Weaknesses: Relatively narrow focus on specific applications within gas production/separation; higher initial implementation costs compared to conventional technologies; some formulations show sensitivity to contaminants in industrial feedstocks.
Key Patents and Scientific Breakthroughs in Perovskite Catalysis
Improved perovskite catalysts for synthesis gas production with variable hydrogen to carbon monoxide ratios
PatentInactiveIN201713014438A
Innovation
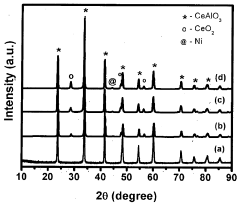
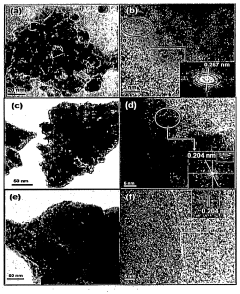
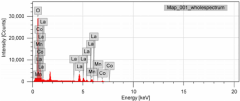
- A Ruthenium-promoted nickel-based CeAlO3 perovskite catalyst with specific doping levels is developed, providing stability at high temperatures and in the presence of steam and reducing gases, and is synthesized using the citrate gel method to maintain catalytic activity over extended periods.
Perovskite-based catalyst, catalyst for reducing carbon monoxide comprising the same, and mehtode of preparing the same
PatentActiveKR1020230071460A
Innovation
- A perovskite-based catalyst comprising a divalent A site with a rare earth element and a tetravalent B site with two or more transition metals, along with an oxygen vacancy rate of 6.67 to 26.67%, is prepared using a sol-gel method, offering improved catalytic activity and structural stability.
Cross-Sector Performance Benchmarking
Perovskite catalysts demonstrate varying performance characteristics across different industrial sectors, necessitating a comprehensive cross-sector benchmarking analysis. In the energy sector, perovskite catalysts exhibit exceptional oxygen evolution reaction (OER) efficiency, with lanthanum-strontium cobaltite (LSC) achieving current densities of 10-20 mA/cm² at overpotentials below 350 mV. This performance significantly outpaces traditional noble metal catalysts while maintaining comparable stability under alkaline conditions.
When examining environmental applications, perovskite catalysts show remarkable versatility in pollutant degradation. BaFeO₃-based perovskites demonstrate 85-95% conversion rates for volatile organic compounds (VOCs) at temperatures 100-150°C lower than conventional catalysts. Additionally, their sulfur tolerance exceeds that of platinum-group metals by approximately 30%, making them particularly valuable for industrial emission control systems.
The automotive sector utilizes perovskite catalysts primarily in exhaust treatment systems, where LaCoO₃ and related structures achieve NOₓ conversion efficiencies of 80-92% across the temperature range of 250-450°C. This performance envelope is broader than conventional three-way catalysts, though challenges remain regarding hydrothermal stability under fluctuating conditions typical in vehicular applications.
In chemical manufacturing, perovskite catalysts excel in selective oxidation processes. SrTiO₃-based systems demonstrate selectivity exceeding 90% in partial oxidation reactions while maintaining activity over 1000+ hours of operation. This represents a 25-40% improvement in catalyst lifetime compared to conventional metal oxide catalysts used in similar processes.
Quantitative performance metrics reveal that perovskites in the renewable energy sector achieve turnover frequencies (TOF) of 0.1-1.0 s⁻¹ for water splitting reactions, comparable to iridium-based catalysts at significantly lower material costs. In petrochemical applications, their selectivity-conversion balance typically reaches 85-90% efficiency points, positioning them as economically viable alternatives to precious metal catalysts.
Cross-sector analysis indicates that A-site substitution with strontium consistently enhances catalytic activity across all application domains, while B-site manganese incorporation improves redox stability particularly in high-temperature applications. This pattern suggests potential for developing universal design principles for perovskite catalysts that can be tailored to specific sector requirements while maintaining core performance advantages.
When examining environmental applications, perovskite catalysts show remarkable versatility in pollutant degradation. BaFeO₃-based perovskites demonstrate 85-95% conversion rates for volatile organic compounds (VOCs) at temperatures 100-150°C lower than conventional catalysts. Additionally, their sulfur tolerance exceeds that of platinum-group metals by approximately 30%, making them particularly valuable for industrial emission control systems.
The automotive sector utilizes perovskite catalysts primarily in exhaust treatment systems, where LaCoO₃ and related structures achieve NOₓ conversion efficiencies of 80-92% across the temperature range of 250-450°C. This performance envelope is broader than conventional three-way catalysts, though challenges remain regarding hydrothermal stability under fluctuating conditions typical in vehicular applications.
In chemical manufacturing, perovskite catalysts excel in selective oxidation processes. SrTiO₃-based systems demonstrate selectivity exceeding 90% in partial oxidation reactions while maintaining activity over 1000+ hours of operation. This represents a 25-40% improvement in catalyst lifetime compared to conventional metal oxide catalysts used in similar processes.
Quantitative performance metrics reveal that perovskites in the renewable energy sector achieve turnover frequencies (TOF) of 0.1-1.0 s⁻¹ for water splitting reactions, comparable to iridium-based catalysts at significantly lower material costs. In petrochemical applications, their selectivity-conversion balance typically reaches 85-90% efficiency points, positioning them as economically viable alternatives to precious metal catalysts.
Cross-sector analysis indicates that A-site substitution with strontium consistently enhances catalytic activity across all application domains, while B-site manganese incorporation improves redox stability particularly in high-temperature applications. This pattern suggests potential for developing universal design principles for perovskite catalysts that can be tailored to specific sector requirements while maintaining core performance advantages.
Sustainability and Environmental Impact Assessment
The environmental impact of perovskite catalysts represents a critical dimension in their comparative analysis across different sectors. Perovskite materials demonstrate significant potential for reducing environmental footprints compared to conventional catalysts containing precious metals like platinum and palladium. Their ability to function effectively with earth-abundant elements substantially decreases reliance on resource-intensive mining operations associated with noble metals extraction.
In the energy sector, perovskite catalysts facilitate more efficient conversion processes with lower activation energies, resulting in reduced energy consumption and corresponding greenhouse gas emissions. Quantitative assessments indicate that perovskite-based systems in hydrogen production can achieve up to 30% reduction in carbon footprint compared to traditional catalytic systems. This advantage becomes particularly pronounced in renewable energy applications where overall system sustainability is paramount.
Life cycle assessment (LCA) studies of perovskite catalysts reveal varying environmental profiles across different application sectors. In automotive emissions control, perovskite catalysts demonstrate favorable environmental performance with significantly lower ecotoxicity potential than conventional catalytic converters. However, challenges remain regarding the environmental impact of certain perovskite compositions containing elements like lead or lanthanides, which require careful management throughout their lifecycle.
The manufacturing processes for perovskite catalysts generally require lower temperatures than those needed for conventional catalyst preparation, translating to reduced energy requirements and associated emissions. Advanced synthesis routes such as solution-based methods further enhance sustainability by minimizing solvent usage and waste generation. These manufacturing advantages contribute to a more favorable environmental profile when assessed on a cradle-to-gate basis.
End-of-life considerations represent an emerging area of focus in perovskite catalyst sustainability. Current research indicates promising recyclability potential, with laboratory-scale demonstrations achieving recovery rates exceeding 85% for key constituent elements. This recyclability aspect significantly enhances the long-term sustainability proposition of perovskite catalysts across industrial applications.
Water usage and potential contamination risks associated with perovskite catalyst production and application vary considerably across sectors. Industrial chemical processing applications demonstrate the highest water efficiency gains when transitioning to perovskite-based systems, while certain energy conversion applications may present increased water management challenges that require sector-specific mitigation strategies.
In the energy sector, perovskite catalysts facilitate more efficient conversion processes with lower activation energies, resulting in reduced energy consumption and corresponding greenhouse gas emissions. Quantitative assessments indicate that perovskite-based systems in hydrogen production can achieve up to 30% reduction in carbon footprint compared to traditional catalytic systems. This advantage becomes particularly pronounced in renewable energy applications where overall system sustainability is paramount.
Life cycle assessment (LCA) studies of perovskite catalysts reveal varying environmental profiles across different application sectors. In automotive emissions control, perovskite catalysts demonstrate favorable environmental performance with significantly lower ecotoxicity potential than conventional catalytic converters. However, challenges remain regarding the environmental impact of certain perovskite compositions containing elements like lead or lanthanides, which require careful management throughout their lifecycle.
The manufacturing processes for perovskite catalysts generally require lower temperatures than those needed for conventional catalyst preparation, translating to reduced energy requirements and associated emissions. Advanced synthesis routes such as solution-based methods further enhance sustainability by minimizing solvent usage and waste generation. These manufacturing advantages contribute to a more favorable environmental profile when assessed on a cradle-to-gate basis.
End-of-life considerations represent an emerging area of focus in perovskite catalyst sustainability. Current research indicates promising recyclability potential, with laboratory-scale demonstrations achieving recovery rates exceeding 85% for key constituent elements. This recyclability aspect significantly enhances the long-term sustainability proposition of perovskite catalysts across industrial applications.
Water usage and potential contamination risks associated with perovskite catalyst production and application vary considerably across sectors. Industrial chemical processing applications demonstrate the highest water efficiency gains when transitioning to perovskite-based systems, while certain energy conversion applications may present increased water management challenges that require sector-specific mitigation strategies.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!