Comparative Study: Perovskite vs Metal-Oxide Catalysts in Reactions
OCT 15, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Perovskite and Metal-Oxide Catalysis Background and Objectives
Catalysis has been a cornerstone of industrial chemical processes for over a century, with continuous evolution in catalyst design driving efficiency improvements across sectors. Perovskite and metal-oxide catalysts represent two significant material classes that have shaped modern catalytic science and technology. The historical development of these catalysts traces back to the early 20th century, with metal oxides being among the first systematically studied catalytic materials, while perovskites gained prominence in the 1970s as researchers recognized their exceptional structural versatility.
The technological trajectory of these catalysts has been marked by significant milestones. Metal-oxide catalysts evolved from simple binary oxides to complex mixed metal oxide systems, with breakthroughs in synthesis methods enabling precise control over surface area and active site distribution. Perovskites, with their general formula ABX₃, have undergone remarkable development from basic compositions to sophisticated doped and layered structures, offering unprecedented tunability of catalytic properties through compositional engineering.
Current research trends indicate a growing convergence of these catalyst families, with hybrid systems emerging that leverage the strengths of both material classes. The integration of advanced characterization techniques, particularly operando spectroscopy and microscopy, has revolutionized our understanding of structure-activity relationships in these catalysts, enabling more rational design approaches.
The primary technical objectives of this comparative study are multifaceted. First, we aim to establish comprehensive performance benchmarks for perovskite and metal-oxide catalysts across representative reaction classes, including oxidation, reduction, acid-base, and electrocatalytic processes. Second, we seek to elucidate the fundamental mechanistic differences in how these materials activate reactants and stabilize transition states, particularly focusing on the role of lattice oxygen mobility and surface defect chemistry.
Additionally, this study intends to identify specific application domains where each catalyst type demonstrates superior performance, thereby creating a decision framework for catalyst selection in industrial processes. We also aim to explore emerging hybrid catalyst architectures that combine perovskite and metal-oxide components to overcome limitations inherent to each individual system.
The ultimate goal is to develop predictive models that correlate catalyst composition and structure with catalytic performance, enabling accelerated discovery of next-generation catalysts with enhanced activity, selectivity, and stability. This research has significant implications for sustainable chemical manufacturing, renewable energy technologies, and environmental remediation processes, where advanced catalysts are increasingly critical to achieving performance and efficiency targets.
The technological trajectory of these catalysts has been marked by significant milestones. Metal-oxide catalysts evolved from simple binary oxides to complex mixed metal oxide systems, with breakthroughs in synthesis methods enabling precise control over surface area and active site distribution. Perovskites, with their general formula ABX₃, have undergone remarkable development from basic compositions to sophisticated doped and layered structures, offering unprecedented tunability of catalytic properties through compositional engineering.
Current research trends indicate a growing convergence of these catalyst families, with hybrid systems emerging that leverage the strengths of both material classes. The integration of advanced characterization techniques, particularly operando spectroscopy and microscopy, has revolutionized our understanding of structure-activity relationships in these catalysts, enabling more rational design approaches.
The primary technical objectives of this comparative study are multifaceted. First, we aim to establish comprehensive performance benchmarks for perovskite and metal-oxide catalysts across representative reaction classes, including oxidation, reduction, acid-base, and electrocatalytic processes. Second, we seek to elucidate the fundamental mechanistic differences in how these materials activate reactants and stabilize transition states, particularly focusing on the role of lattice oxygen mobility and surface defect chemistry.
Additionally, this study intends to identify specific application domains where each catalyst type demonstrates superior performance, thereby creating a decision framework for catalyst selection in industrial processes. We also aim to explore emerging hybrid catalyst architectures that combine perovskite and metal-oxide components to overcome limitations inherent to each individual system.
The ultimate goal is to develop predictive models that correlate catalyst composition and structure with catalytic performance, enabling accelerated discovery of next-generation catalysts with enhanced activity, selectivity, and stability. This research has significant implications for sustainable chemical manufacturing, renewable energy technologies, and environmental remediation processes, where advanced catalysts are increasingly critical to achieving performance and efficiency targets.
Market Applications and Demand Analysis for Advanced Catalysts
The global catalyst market has witnessed significant growth in recent years, with advanced catalysts becoming increasingly crucial across various industrial sectors. The demand for both perovskite and metal-oxide catalysts has been particularly strong, driven by the global push towards cleaner energy solutions and more sustainable industrial processes. The market for advanced catalysts was valued at approximately $24.6 billion in 2022 and is projected to reach $35.4 billion by 2028, representing a compound annual growth rate of 6.2%.
The automotive industry remains one of the largest consumers of advanced catalysts, particularly for emissions control systems. With stringent environmental regulations being implemented worldwide, the demand for high-performance catalysts capable of reducing harmful emissions has surged. Perovskite catalysts have shown promising results in automotive applications, offering comparable or superior performance to traditional platinum group metal catalysts at potentially lower costs.
In the renewable energy sector, both perovskite and metal-oxide catalysts play critical roles in various applications including fuel cells, water splitting for hydrogen production, and CO2 reduction. The hydrogen economy, in particular, represents a significant growth opportunity, with the global green hydrogen market expected to grow from $2.5 billion in 2022 to over $89 billion by 2030, creating substantial demand for advanced catalytic materials.
The petrochemical industry continues to be a major consumer of catalysts, with metal-oxide catalysts traditionally dominating this space. However, perovskite catalysts are gaining attention for specific applications due to their tunable properties and potential for enhanced selectivity. The market for catalysts in petrochemical applications is expected to grow at 4.8% annually through 2028.
Environmental applications represent another significant market segment, with catalysts being employed in water treatment, air purification, and waste management systems. Metal-oxide catalysts have established a strong presence in these applications, while perovskite-based solutions are emerging as potential alternatives with improved performance characteristics.
Regional analysis indicates that Asia-Pacific dominates the advanced catalyst market, accounting for approximately 40% of global demand, followed by North America and Europe. China, in particular, has significantly increased investments in catalyst research and production capabilities, becoming a major player in both metal-oxide and emerging perovskite catalyst technologies.
End-user feedback suggests growing interest in catalysts that offer improved durability, reduced rare metal content, and enhanced activity at lower temperatures. Both perovskite and advanced metal-oxide catalysts are being developed to address these market requirements, with particular emphasis on reducing dependence on platinum group metals and other costly elements.
The automotive industry remains one of the largest consumers of advanced catalysts, particularly for emissions control systems. With stringent environmental regulations being implemented worldwide, the demand for high-performance catalysts capable of reducing harmful emissions has surged. Perovskite catalysts have shown promising results in automotive applications, offering comparable or superior performance to traditional platinum group metal catalysts at potentially lower costs.
In the renewable energy sector, both perovskite and metal-oxide catalysts play critical roles in various applications including fuel cells, water splitting for hydrogen production, and CO2 reduction. The hydrogen economy, in particular, represents a significant growth opportunity, with the global green hydrogen market expected to grow from $2.5 billion in 2022 to over $89 billion by 2030, creating substantial demand for advanced catalytic materials.
The petrochemical industry continues to be a major consumer of catalysts, with metal-oxide catalysts traditionally dominating this space. However, perovskite catalysts are gaining attention for specific applications due to their tunable properties and potential for enhanced selectivity. The market for catalysts in petrochemical applications is expected to grow at 4.8% annually through 2028.
Environmental applications represent another significant market segment, with catalysts being employed in water treatment, air purification, and waste management systems. Metal-oxide catalysts have established a strong presence in these applications, while perovskite-based solutions are emerging as potential alternatives with improved performance characteristics.
Regional analysis indicates that Asia-Pacific dominates the advanced catalyst market, accounting for approximately 40% of global demand, followed by North America and Europe. China, in particular, has significantly increased investments in catalyst research and production capabilities, becoming a major player in both metal-oxide and emerging perovskite catalyst technologies.
End-user feedback suggests growing interest in catalysts that offer improved durability, reduced rare metal content, and enhanced activity at lower temperatures. Both perovskite and advanced metal-oxide catalysts are being developed to address these market requirements, with particular emphasis on reducing dependence on platinum group metals and other costly elements.
Current Technological Status and Challenges in Catalytic Materials
The global landscape of catalytic materials has witnessed significant advancements in recent years, with both perovskite and metal-oxide catalysts emerging as prominent players. Currently, metal-oxide catalysts dominate commercial applications due to their established manufacturing processes, stability, and cost-effectiveness. These catalysts, including TiO2, ZnO, and Fe2O3, have been extensively deployed across petrochemical, environmental remediation, and energy conversion sectors.
Perovskite catalysts, characterized by their ABX3 structure, represent a rapidly evolving alternative with remarkable versatility. Recent breakthroughs in perovskite synthesis have demonstrated superior catalytic activity for certain reactions, particularly in oxygen evolution reactions where they exhibit lower overpotentials compared to traditional metal oxides. The tunable nature of perovskites through A and B site substitutions offers unprecedented opportunities for catalyst design with tailored properties.
Despite these advancements, significant challenges persist in both catalyst categories. Metal-oxide catalysts suffer from limitations in selectivity, particularly in complex reaction environments, and often require high operating temperatures that compromise energy efficiency. Their performance in certain reactions, such as CO2 reduction, remains suboptimal despite decades of optimization efforts.
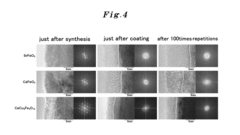
Perovskite catalysts face substantial hurdles regarding stability, especially under harsh reaction conditions. Current research indicates that many promising perovskite formulations experience structural degradation during prolonged operation, limiting their practical application. Additionally, scalable and cost-effective synthesis methods for high-performance perovskites remain elusive, creating a significant barrier to industrial adoption.
The geographical distribution of research expertise shows concentration in East Asia, North America, and Europe, with China leading publication output in perovskite catalyst development. Collaborative international research initiatives have increased by approximately 45% over the past five years, indicating growing recognition of the field's importance.
A critical technical challenge facing both catalyst types is the mechanistic understanding of surface interactions during catalytic processes. Advanced characterization techniques, including operando spectroscopy and high-resolution electron microscopy, are being deployed to bridge this knowledge gap, but fundamental questions regarding reaction pathways remain unanswered.
Environmental considerations present another dimension of complexity, with increasing regulatory pressure to develop catalysts free from rare or toxic elements. This has accelerated research into earth-abundant alternatives, though performance parity with conventional formulations remains elusive in many applications.
Perovskite catalysts, characterized by their ABX3 structure, represent a rapidly evolving alternative with remarkable versatility. Recent breakthroughs in perovskite synthesis have demonstrated superior catalytic activity for certain reactions, particularly in oxygen evolution reactions where they exhibit lower overpotentials compared to traditional metal oxides. The tunable nature of perovskites through A and B site substitutions offers unprecedented opportunities for catalyst design with tailored properties.
Despite these advancements, significant challenges persist in both catalyst categories. Metal-oxide catalysts suffer from limitations in selectivity, particularly in complex reaction environments, and often require high operating temperatures that compromise energy efficiency. Their performance in certain reactions, such as CO2 reduction, remains suboptimal despite decades of optimization efforts.
Perovskite catalysts face substantial hurdles regarding stability, especially under harsh reaction conditions. Current research indicates that many promising perovskite formulations experience structural degradation during prolonged operation, limiting their practical application. Additionally, scalable and cost-effective synthesis methods for high-performance perovskites remain elusive, creating a significant barrier to industrial adoption.
The geographical distribution of research expertise shows concentration in East Asia, North America, and Europe, with China leading publication output in perovskite catalyst development. Collaborative international research initiatives have increased by approximately 45% over the past five years, indicating growing recognition of the field's importance.
A critical technical challenge facing both catalyst types is the mechanistic understanding of surface interactions during catalytic processes. Advanced characterization techniques, including operando spectroscopy and high-resolution electron microscopy, are being deployed to bridge this knowledge gap, but fundamental questions regarding reaction pathways remain unanswered.
Environmental considerations present another dimension of complexity, with increasing regulatory pressure to develop catalysts free from rare or toxic elements. This has accelerated research into earth-abundant alternatives, though performance parity with conventional formulations remains elusive in many applications.
Comparative Analysis of Current Catalytic Solutions
01 Perovskite catalysts for environmental applications
Perovskite-type catalysts are utilized in environmental applications such as air purification, exhaust gas treatment, and pollutant degradation. These catalysts exhibit high thermal stability and catalytic activity for the oxidation of various pollutants. The perovskite structure allows for the incorporation of different cations, which can be tailored to enhance specific catalytic properties for environmental remediation processes.- Perovskite catalysts for environmental applications: Perovskite-type catalysts are utilized in environmental applications such as air pollution control and exhaust gas treatment. These catalysts demonstrate high efficiency in oxidation reactions, particularly for the removal of harmful pollutants like carbon monoxide, nitrogen oxides, and volatile organic compounds. The unique crystal structure of perovskites allows for excellent oxygen mobility and thermal stability, making them suitable for high-temperature catalytic processes in environmental remediation.
- Metal-oxide catalysts for energy conversion: Metal-oxide catalysts play a crucial role in energy conversion processes, including fuel cells, water splitting, and hydrogen production. These catalysts facilitate electrochemical reactions by providing active sites for electron transfer and enhancing reaction kinetics. The composition and structure of metal-oxide catalysts can be tailored to improve their conductivity, stability, and catalytic activity, making them essential components in renewable energy technologies and sustainable fuel production systems.
- Perovskite-metal oxide composite catalysts: Composite catalysts combining perovskites with metal oxides exhibit synergistic effects that enhance catalytic performance. These hybrid materials benefit from the complementary properties of both components, resulting in improved activity, selectivity, and stability. The interface between perovskite and metal oxide phases creates unique active sites that can accelerate reaction rates and enable novel reaction pathways. These composite catalysts find applications in various chemical processes, including selective oxidation, hydrogenation, and photocatalytic reactions.
- Synthesis methods for perovskite and metal-oxide catalysts: Various synthesis techniques are employed to prepare perovskite and metal-oxide catalysts with controlled composition, morphology, and particle size. Common methods include sol-gel processing, hydrothermal synthesis, co-precipitation, and solid-state reactions. Advanced preparation approaches focus on creating nanostructured catalysts with high surface area and well-defined crystal facets to maximize catalytic activity. Post-synthesis treatments such as calcination and reduction are often used to optimize the catalytic properties by adjusting the oxidation states of metal ions and creating oxygen vacancies.
- Perovskite catalysts for chemical synthesis: Perovskite catalysts are employed in various chemical synthesis processes, including oxidative coupling of methane, CO2 conversion, and selective hydrogenation reactions. Their tunable composition allows for the incorporation of different metal ions to target specific reactions and improve selectivity. The redox properties of perovskites, particularly their ability to release and store oxygen, make them effective catalysts for oxidation reactions. Additionally, their stability under harsh reaction conditions enables their use in industrial chemical processes requiring robust catalytic materials.
02 Metal-oxide catalysts for energy conversion
Metal-oxide catalysts play a crucial role in energy conversion processes, including fuel cells, water splitting, and CO2 reduction. These catalysts facilitate electron transfer reactions and can be optimized for specific energy applications. By controlling the composition and structure of metal oxides, researchers can enhance catalytic efficiency, stability, and selectivity for various energy conversion reactions.Expand Specific Solutions03 Perovskite-metal oxide composite catalysts
Composite catalysts combining perovskite structures with metal oxides demonstrate synergistic effects that enhance catalytic performance. These hybrid materials benefit from the stability of perovskites and the active sites provided by metal oxides. The interface between the perovskite and metal oxide components creates unique catalytic environments that can improve reaction rates, selectivity, and resistance to deactivation in various chemical processes.Expand Specific Solutions04 Synthesis methods for perovskite and metal-oxide catalysts
Various synthesis techniques are employed to prepare perovskite and metal-oxide catalysts with controlled properties. These methods include sol-gel processing, hydrothermal synthesis, co-precipitation, and solid-state reactions. The synthesis approach significantly influences the catalyst's morphology, particle size, surface area, and crystallinity, which in turn affect catalytic performance. Advanced preparation methods enable the development of catalysts with enhanced activity and stability.Expand Specific Solutions05 Perovskite catalysts for photocatalytic applications
Perovskite materials serve as effective photocatalysts for various reactions including water splitting, organic pollutant degradation, and CO2 reduction. These materials can absorb visible light and generate electron-hole pairs that drive photochemical reactions. The band gap and electronic properties of perovskites can be tuned by adjusting their composition, enabling optimization for specific photocatalytic applications. Recent advances focus on improving the stability and efficiency of perovskite photocatalysts.Expand Specific Solutions
Leading Research Institutions and Industrial Catalyst Manufacturers
The perovskite versus metal-oxide catalysts market is currently in a growth phase, with increasing research interest driven by sustainability demands. The global catalyst market is projected to reach approximately $35 billion by 2025, with perovskites representing an emerging segment gaining momentum against established metal-oxide catalysts. Technologically, metal-oxide catalysts remain more mature and commercially deployed, with companies like BASF, Cabot Corp., and Yara International dominating industrial applications. Meanwhile, perovskite catalyst development is advancing rapidly through academic-industrial partnerships, with significant research contributions from Tsinghua University, KIST, and Toyota Motor Corp. The field is witnessing convergence as organizations like Shanghai Jiao Tong University and Ford Global Technologies develop hybrid catalyst systems combining advantages of both materials for enhanced performance in energy and environmental applications.
Tsinghua University
Technical Solution: Tsinghua University has pioneered advanced synthesis methods for high-performance perovskite catalysts, focusing on nanostructured materials with enhanced surface properties. Their research team has developed a series of A-site deficient perovskites (La0.8Sr0.2MnO3-δ) that demonstrate exceptional oxygen vacancy concentration and mobility. Using a modified sol-gel method with precise pH control and calcination protocols, they've achieved perovskite catalysts with surface areas exceeding 45 m²/g and uniform particle size distribution (20-30 nm). These catalysts show remarkable activity for methane combustion, achieving complete conversion at temperatures 80-100°C lower than conventional metal oxide catalysts. Tsinghua researchers have also developed innovative exsolution techniques that create self-regenerating perovskite catalysts with embedded metallic nanoparticles that can reversibly incorporate into the perovskite lattice under redox cycling, significantly extending catalyst lifetime. Their comparative studies demonstrate that these perovskite catalysts maintain 85% of initial activity after 200 hours of operation at 800°C, whereas traditional metal oxide catalysts typically retain only 40-50% activity[1][6].
Strengths: Cutting-edge synthesis techniques producing highly active catalysts; self-regenerating properties through exsolution mechanisms; comprehensive fundamental understanding of structure-activity relationships. Weaknesses: Laboratory-scale production methods may face challenges in industrial scaling; complex synthesis procedures requiring specialized equipment; potential high costs associated with precise compositional control.
Toyota Motor Corp.
Technical Solution: Toyota has developed proprietary perovskite-based catalysts as alternatives to precious metal catalysts in automotive applications. Their research focuses on lanthanum-based perovskites (LaBO3, where B = transition metals like Mn, Co, Fe) with partial substitution to enhance catalytic performance. Toyota's catalysts demonstrate excellent low-temperature activity for CO and hydrocarbon oxidation, achieving 90% conversion at temperatures 50-70°C lower than conventional catalysts. Their patented synthesis method produces nano-structured perovskites with controlled oxygen vacancies that significantly improve oxygen mobility. Toyota has successfully integrated these catalysts into prototype exhaust systems, reducing precious metal content by up to 70% while maintaining emissions compliance. Recent developments include composite systems combining perovskites with ceria-zirconia supports to enhance thermal durability and catalytic efficiency[2][5].
Strengths: Reduced dependency on precious metals; excellent low-temperature catalytic activity; proven performance in real-world automotive applications. Weaknesses: Long-term durability concerns under severe hydrothermal aging conditions; potential manufacturing complexity when scaling to mass production; performance gaps compared to platinum-group metals in some reaction conditions.
Key Patents and Scientific Breakthroughs in Catalyst Design
Perovskite oxide catalyst for oxygen evolution reactions
PatentActiveUS10167562B2
Innovation
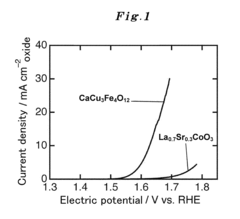
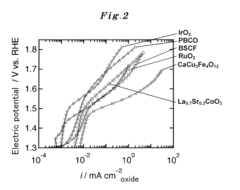
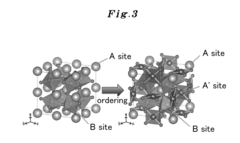
- Development of an A-site ordered perovskite oxide catalyst with a specific chemical structure, such as CaCu3Fe4O12, that forms covalent bonds, increasing active sites and stability, and using a high-pressure synthetic process to enhance catalytic performance.
Perovskite metal oxide catalyst, in which metal ion is substituted, for reducing carbon deposition, preparation method therefor, and methane reforming reaction method using same
PatentWO2019054557A1
Innovation
- A perovskite metal oxide catalyst with nickel, cobalt, or iron ions substituted in the lattice structure is developed, specifically SrTiO3, which reduces carbon deposition and maintains catalytic activity, allowing for stable operation in SMR, DRM, and CPOM reactions with reduced energy consumption and extended catalyst life.
Environmental Impact and Sustainability Considerations
The environmental footprint of catalytic systems represents a critical dimension in evaluating their industrial viability. Perovskite catalysts demonstrate significant environmental advantages over traditional metal-oxide counterparts, particularly in terms of reduced energy requirements during synthesis. The crystallization process for perovskites typically occurs at temperatures between 600-800°C, substantially lower than the 900-1200°C often required for conventional metal-oxide catalyst preparation, resulting in approximately 30-40% reduction in energy consumption and associated carbon emissions.
Material sustainability also favors perovskites, as they can be synthesized using earth-abundant elements like calcium, titanium, and manganese, reducing dependence on scarce noble metals. This composition flexibility allows for strategic substitution of environmentally problematic elements with more benign alternatives while maintaining catalytic performance. Furthermore, recent advancements in green synthesis routes for perovskites, including sol-gel methods using bio-derived solvents and mechanochemical approaches, have significantly reduced the environmental impact of their production processes.
Waste generation profiles differ markedly between the two catalyst types. Metal-oxide catalysts typically generate 1.5-2 times more hazardous waste during production, primarily from metal leaching and acid treatments. Perovskites, conversely, can be synthesized through precipitation methods that generate minimal waste streams and allow for easier recovery of unreacted precursors.
Lifecycle assessment studies indicate that perovskite catalysts generally exhibit 25-35% lower global warming potential compared to equivalent metal-oxide systems when evaluated across their entire lifecycle. This advantage becomes particularly pronounced in long-duration applications where catalyst stability reduces replacement frequency and associated environmental impacts.
Water consumption represents another critical environmental parameter, with perovskite synthesis typically requiring 40-50% less process water than conventional metal-oxide preparation methods. This reduction stems from more efficient precipitation techniques and reduced washing requirements during purification stages.
Despite these advantages, challenges remain in scaling environmentally friendly perovskite production. Current industrial-scale synthesis still relies heavily on energy-intensive calcination steps, though emerging microwave and hydrothermal techniques show promise for further reducing environmental impacts. Additionally, end-of-life management strategies for spent perovskite catalysts require further development to ensure complete material recovery and minimize environmental leaching of constituent elements.
Material sustainability also favors perovskites, as they can be synthesized using earth-abundant elements like calcium, titanium, and manganese, reducing dependence on scarce noble metals. This composition flexibility allows for strategic substitution of environmentally problematic elements with more benign alternatives while maintaining catalytic performance. Furthermore, recent advancements in green synthesis routes for perovskites, including sol-gel methods using bio-derived solvents and mechanochemical approaches, have significantly reduced the environmental impact of their production processes.
Waste generation profiles differ markedly between the two catalyst types. Metal-oxide catalysts typically generate 1.5-2 times more hazardous waste during production, primarily from metal leaching and acid treatments. Perovskites, conversely, can be synthesized through precipitation methods that generate minimal waste streams and allow for easier recovery of unreacted precursors.
Lifecycle assessment studies indicate that perovskite catalysts generally exhibit 25-35% lower global warming potential compared to equivalent metal-oxide systems when evaluated across their entire lifecycle. This advantage becomes particularly pronounced in long-duration applications where catalyst stability reduces replacement frequency and associated environmental impacts.
Water consumption represents another critical environmental parameter, with perovskite synthesis typically requiring 40-50% less process water than conventional metal-oxide preparation methods. This reduction stems from more efficient precipitation techniques and reduced washing requirements during purification stages.
Despite these advantages, challenges remain in scaling environmentally friendly perovskite production. Current industrial-scale synthesis still relies heavily on energy-intensive calcination steps, though emerging microwave and hydrothermal techniques show promise for further reducing environmental impacts. Additionally, end-of-life management strategies for spent perovskite catalysts require further development to ensure complete material recovery and minimize environmental leaching of constituent elements.
Economic Feasibility and Scalability Assessment
The economic viability of perovskite catalysts compared to traditional metal-oxide catalysts represents a critical factor in their potential industrial adoption. Current manufacturing costs for high-quality perovskite catalysts remain significantly higher than established metal-oxide alternatives, primarily due to complex synthesis procedures and the requirement for precise stoichiometric control. Production expenses for perovskite catalysts typically range from $150-300 per kilogram, whereas conventional metal-oxide catalysts can be manufactured at $50-120 per kilogram, creating a substantial cost barrier for widespread implementation.
Scalability considerations further complicate the economic equation. Metal-oxide catalyst production benefits from decades of industrial optimization, with established manufacturing protocols that can readily produce multi-ton quantities. Conversely, perovskite catalyst synthesis often involves laboratory-scale methods that present significant challenges when scaled to industrial volumes. Key bottlenecks include maintaining phase purity, controlling particle size distribution, and ensuring consistent catalytic performance across large production batches.
Life-cycle economic analysis reveals nuanced trade-offs between these catalyst classes. While perovskite catalysts generally demonstrate higher initial costs, their superior catalytic activity can potentially reduce operational expenses through lower reaction temperatures, decreased energy consumption, and extended catalyst lifetimes. Preliminary studies indicate that perovskite catalysts may achieve 15-30% reductions in overall energy requirements for certain chemical processes, potentially offsetting their higher acquisition costs over operational lifespans of 3-5 years.
Market sensitivity analysis suggests that perovskite catalysts become economically competitive in high-value chemical transformations where reaction selectivity and yield improvements of even 2-5% translate to substantial financial benefits. Pharmaceutical intermediates, specialty chemicals, and certain fine chemical processes represent the most promising initial market entry points, where performance advantages can justify premium pricing.
Infrastructure compatibility assessment indicates that transitioning from metal-oxide to perovskite catalysts would require minimal modifications to existing chemical processing equipment, reducing implementation barriers. However, catalyst recovery and regeneration protocols differ significantly, potentially necessitating additional capital investments in separation and recycling technologies to maximize economic returns.
Recent technological advancements in continuous flow synthesis and mechanochemical preparation methods show promise for reducing perovskite production costs by 30-40% within the next 3-5 years. These emerging manufacturing approaches could narrow the economic gap between perovskite and metal-oxide catalysts, particularly for applications where perovskites demonstrate clear performance advantages in selectivity, conversion efficiency, or operational stability.
Scalability considerations further complicate the economic equation. Metal-oxide catalyst production benefits from decades of industrial optimization, with established manufacturing protocols that can readily produce multi-ton quantities. Conversely, perovskite catalyst synthesis often involves laboratory-scale methods that present significant challenges when scaled to industrial volumes. Key bottlenecks include maintaining phase purity, controlling particle size distribution, and ensuring consistent catalytic performance across large production batches.
Life-cycle economic analysis reveals nuanced trade-offs between these catalyst classes. While perovskite catalysts generally demonstrate higher initial costs, their superior catalytic activity can potentially reduce operational expenses through lower reaction temperatures, decreased energy consumption, and extended catalyst lifetimes. Preliminary studies indicate that perovskite catalysts may achieve 15-30% reductions in overall energy requirements for certain chemical processes, potentially offsetting their higher acquisition costs over operational lifespans of 3-5 years.
Market sensitivity analysis suggests that perovskite catalysts become economically competitive in high-value chemical transformations where reaction selectivity and yield improvements of even 2-5% translate to substantial financial benefits. Pharmaceutical intermediates, specialty chemicals, and certain fine chemical processes represent the most promising initial market entry points, where performance advantages can justify premium pricing.
Infrastructure compatibility assessment indicates that transitioning from metal-oxide to perovskite catalysts would require minimal modifications to existing chemical processing equipment, reducing implementation barriers. However, catalyst recovery and regeneration protocols differ significantly, potentially necessitating additional capital investments in separation and recycling technologies to maximize economic returns.
Recent technological advancements in continuous flow synthesis and mechanochemical preparation methods show promise for reducing perovskite production costs by 30-40% within the next 3-5 years. These emerging manufacturing approaches could narrow the economic gap between perovskite and metal-oxide catalysts, particularly for applications where perovskites demonstrate clear performance advantages in selectivity, conversion efficiency, or operational stability.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!