Performance Evaluation of Perovskite Catalysts in Biofuel Production
OCT 15, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Perovskite Catalysts Background and Research Objectives
Perovskite materials have emerged as a revolutionary class of compounds in catalysis science over the past three decades. Initially gaining prominence in solar cell applications due to their exceptional light-harvesting capabilities, perovskites have gradually expanded their footprint into various catalytic processes, including biofuel production. The chemical formula ABX₃ defines these materials, where A and B are cations of different sizes and X is an anion, typically oxygen. This versatile structure allows for extensive compositional engineering through cation and anion substitutions, enabling precise tuning of catalytic properties.
The evolution of perovskite catalysts has been marked by significant milestones, beginning with their application in simple oxidation reactions in the 1990s to their current sophisticated implementations in complex biomass conversion processes. Recent advancements in synthesis techniques, particularly sol-gel methods and hydrothermal approaches, have dramatically improved the control over perovskite morphology, surface area, and active site distribution—critical factors for catalytic performance in biofuel production.
Global research interest in perovskite catalysts for biofuel applications has accelerated notably since 2015, coinciding with increased international commitments to renewable energy sources. The technical trajectory shows a clear shift from traditional metal oxide catalysts toward perovskite-based systems due to their superior stability under the harsh conditions typical of biomass conversion processes, including high temperatures and the presence of contaminants.
The primary objective of our technical research is to comprehensively evaluate the performance parameters of various perovskite catalyst compositions in biofuel production pathways, with particular emphasis on biodiesel synthesis, lignocellulosic biomass conversion, and algal biomass processing. We aim to establish quantitative relationships between perovskite structural characteristics and their catalytic efficiency, selectivity, and longevity in these applications.
Additionally, this research seeks to identify optimal synthesis methodologies that maximize catalytic performance while minimizing production costs and environmental impact. By systematically analyzing the influence of dopants, defect engineering, and support materials on catalytic behavior, we intend to develop design principles for next-generation perovskite catalysts specifically tailored for biofuel production.
The ultimate goal is to accelerate the transition from laboratory-scale demonstrations to industrial implementation by addressing key technical challenges, including catalyst deactivation mechanisms, scalable synthesis protocols, and integration with existing biofuel production infrastructure. This research aligns with broader industry trends toward sustainable catalytic processes and contributes to the development of economically viable pathways for renewable fuel production.
The evolution of perovskite catalysts has been marked by significant milestones, beginning with their application in simple oxidation reactions in the 1990s to their current sophisticated implementations in complex biomass conversion processes. Recent advancements in synthesis techniques, particularly sol-gel methods and hydrothermal approaches, have dramatically improved the control over perovskite morphology, surface area, and active site distribution—critical factors for catalytic performance in biofuel production.
Global research interest in perovskite catalysts for biofuel applications has accelerated notably since 2015, coinciding with increased international commitments to renewable energy sources. The technical trajectory shows a clear shift from traditional metal oxide catalysts toward perovskite-based systems due to their superior stability under the harsh conditions typical of biomass conversion processes, including high temperatures and the presence of contaminants.
The primary objective of our technical research is to comprehensively evaluate the performance parameters of various perovskite catalyst compositions in biofuel production pathways, with particular emphasis on biodiesel synthesis, lignocellulosic biomass conversion, and algal biomass processing. We aim to establish quantitative relationships between perovskite structural characteristics and their catalytic efficiency, selectivity, and longevity in these applications.
Additionally, this research seeks to identify optimal synthesis methodologies that maximize catalytic performance while minimizing production costs and environmental impact. By systematically analyzing the influence of dopants, defect engineering, and support materials on catalytic behavior, we intend to develop design principles for next-generation perovskite catalysts specifically tailored for biofuel production.
The ultimate goal is to accelerate the transition from laboratory-scale demonstrations to industrial implementation by addressing key technical challenges, including catalyst deactivation mechanisms, scalable synthesis protocols, and integration with existing biofuel production infrastructure. This research aligns with broader industry trends toward sustainable catalytic processes and contributes to the development of economically viable pathways for renewable fuel production.
Biofuel Market Demand and Growth Projections
The global biofuel market has demonstrated robust growth over the past decade, driven primarily by increasing environmental concerns, government mandates for renewable energy adoption, and the need to reduce dependency on fossil fuels. Current market valuations place the global biofuel sector at approximately USD 141 billion as of 2022, with projections indicating growth to reach USD 201 billion by 2030, representing a compound annual growth rate (CAGR) of 4.5% during the forecast period.
First-generation biofuels, primarily derived from food crops, continue to dominate the market share, accounting for nearly 60% of total production. However, second and third-generation biofuels are experiencing accelerated growth rates due to their superior sustainability profiles and reduced competition with food resources. Advanced biofuels derived from lignocellulosic biomass, algae, and waste materials are projected to grow at a CAGR of 7.8% through 2030, significantly outpacing traditional biofuel growth.
Regional analysis reveals that North America and Europe currently lead the biofuel market in terms of technological innovation and policy support, while the Asia-Pacific region demonstrates the highest growth potential, with countries like China, India, and Indonesia implementing aggressive biofuel blending mandates. Latin America, particularly Brazil, maintains its position as a key player in bioethanol production from sugarcane.
The transportation sector remains the primary consumer of biofuels, accounting for approximately 80% of total demand. Aviation biofuels represent the fastest-growing segment, with a projected CAGR of 9.2% through 2030, as major airlines commit to carbon reduction targets. The maritime industry is also emerging as a significant potential market for biofuels, with international regulations on sulfur emissions driving interest in cleaner fuel alternatives.
Market demand is further bolstered by increasingly stringent environmental regulations worldwide. The European Union's Renewable Energy Directive II (RED II) mandates 14% renewable energy in transportation by 2030, while the United States Renewable Fuel Standard continues to drive domestic production. Similarly, China's E10 mandate (10% ethanol blend in gasoline) signals strong governmental support for biofuel adoption in emerging markets.
Catalyst technologies, particularly advanced options like perovskite catalysts, are positioned to play a crucial role in addressing current production inefficiencies. Industry analysts estimate that improved catalytic processes could reduce production costs by 15-25%, potentially accelerating market adoption and improving competitive positioning against conventional fuels. This technological advancement aligns with the market's growing demand for more efficient and sustainable biofuel production methods.
First-generation biofuels, primarily derived from food crops, continue to dominate the market share, accounting for nearly 60% of total production. However, second and third-generation biofuels are experiencing accelerated growth rates due to their superior sustainability profiles and reduced competition with food resources. Advanced biofuels derived from lignocellulosic biomass, algae, and waste materials are projected to grow at a CAGR of 7.8% through 2030, significantly outpacing traditional biofuel growth.
Regional analysis reveals that North America and Europe currently lead the biofuel market in terms of technological innovation and policy support, while the Asia-Pacific region demonstrates the highest growth potential, with countries like China, India, and Indonesia implementing aggressive biofuel blending mandates. Latin America, particularly Brazil, maintains its position as a key player in bioethanol production from sugarcane.
The transportation sector remains the primary consumer of biofuels, accounting for approximately 80% of total demand. Aviation biofuels represent the fastest-growing segment, with a projected CAGR of 9.2% through 2030, as major airlines commit to carbon reduction targets. The maritime industry is also emerging as a significant potential market for biofuels, with international regulations on sulfur emissions driving interest in cleaner fuel alternatives.
Market demand is further bolstered by increasingly stringent environmental regulations worldwide. The European Union's Renewable Energy Directive II (RED II) mandates 14% renewable energy in transportation by 2030, while the United States Renewable Fuel Standard continues to drive domestic production. Similarly, China's E10 mandate (10% ethanol blend in gasoline) signals strong governmental support for biofuel adoption in emerging markets.
Catalyst technologies, particularly advanced options like perovskite catalysts, are positioned to play a crucial role in addressing current production inefficiencies. Industry analysts estimate that improved catalytic processes could reduce production costs by 15-25%, potentially accelerating market adoption and improving competitive positioning against conventional fuels. This technological advancement aligns with the market's growing demand for more efficient and sustainable biofuel production methods.
Current Challenges in Perovskite Catalyst Technology
Despite significant advancements in perovskite catalyst technology for biofuel production, several critical challenges continue to impede widespread commercial implementation. The primary obstacle remains catalyst stability under real-world operating conditions. Perovskite catalysts often exhibit promising initial performance but suffer from rapid deactivation due to sintering, phase transformation, and poisoning when exposed to biomass-derived feedstocks containing sulfur, nitrogen, and other contaminants.
Thermal stability presents another significant challenge, particularly in high-temperature biofuel conversion processes. Many perovskite formulations undergo structural degradation above 800°C, limiting their application in certain thermochemical conversion routes. The trade-off between thermal stability and catalytic activity continues to be a delicate balance that researchers struggle to optimize.
Scalable and cost-effective synthesis methods represent another major hurdle. Laboratory-scale preparation techniques that yield high-performance perovskite catalysts often involve complex procedures, expensive precursors, or energy-intensive steps that become economically prohibitive at industrial scale. The reproducibility of catalyst properties during scale-up remains problematic, with batch-to-batch variations affecting performance consistency.
Water tolerance is particularly challenging for perovskite catalysts in biofuel applications. Many biomass conversion processes involve aqueous environments or generate water as a byproduct, which can accelerate catalyst degradation through leaching of active components or structural collapse. Developing hydrophobic or water-resistant perovskite formulations without compromising catalytic activity remains an ongoing research focus.
The complex and variable nature of biomass feedstocks introduces additional complications. Unlike petroleum refining with relatively consistent feedstock properties, biofuel production must contend with seasonal and geographical variations in biomass composition. Perovskite catalysts optimized for one feedstock often perform suboptimally with others, necessitating either feedstock-specific catalyst formulations or more versatile designs.
Mechanistic understanding at the molecular level remains incomplete, hampering rational catalyst design. The precise reaction pathways and active sites involved in various biofuel conversion processes over perovskite surfaces are not fully elucidated, making systematic improvement difficult. Advanced in-situ characterization techniques are needed to observe catalyst behavior under realistic reaction conditions.
Finally, life cycle assessment and techno-economic analysis reveal challenges in overall sustainability. While perovskite catalysts may enhance conversion efficiency, questions remain about their environmental footprint, including the use of rare earth elements in some formulations and energy-intensive manufacturing processes. Balancing catalytic performance with broader sustainability metrics represents a significant challenge for next-generation catalyst development.
Thermal stability presents another significant challenge, particularly in high-temperature biofuel conversion processes. Many perovskite formulations undergo structural degradation above 800°C, limiting their application in certain thermochemical conversion routes. The trade-off between thermal stability and catalytic activity continues to be a delicate balance that researchers struggle to optimize.
Scalable and cost-effective synthesis methods represent another major hurdle. Laboratory-scale preparation techniques that yield high-performance perovskite catalysts often involve complex procedures, expensive precursors, or energy-intensive steps that become economically prohibitive at industrial scale. The reproducibility of catalyst properties during scale-up remains problematic, with batch-to-batch variations affecting performance consistency.
Water tolerance is particularly challenging for perovskite catalysts in biofuel applications. Many biomass conversion processes involve aqueous environments or generate water as a byproduct, which can accelerate catalyst degradation through leaching of active components or structural collapse. Developing hydrophobic or water-resistant perovskite formulations without compromising catalytic activity remains an ongoing research focus.
The complex and variable nature of biomass feedstocks introduces additional complications. Unlike petroleum refining with relatively consistent feedstock properties, biofuel production must contend with seasonal and geographical variations in biomass composition. Perovskite catalysts optimized for one feedstock often perform suboptimally with others, necessitating either feedstock-specific catalyst formulations or more versatile designs.
Mechanistic understanding at the molecular level remains incomplete, hampering rational catalyst design. The precise reaction pathways and active sites involved in various biofuel conversion processes over perovskite surfaces are not fully elucidated, making systematic improvement difficult. Advanced in-situ characterization techniques are needed to observe catalyst behavior under realistic reaction conditions.
Finally, life cycle assessment and techno-economic analysis reveal challenges in overall sustainability. While perovskite catalysts may enhance conversion efficiency, questions remain about their environmental footprint, including the use of rare earth elements in some formulations and energy-intensive manufacturing processes. Balancing catalytic performance with broader sustainability metrics represents a significant challenge for next-generation catalyst development.
Current Performance Evaluation Methodologies
01 Perovskite catalyst composition and structure
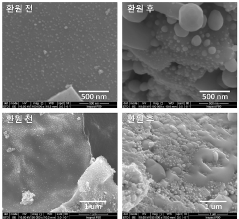
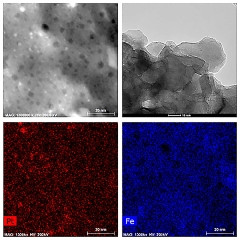
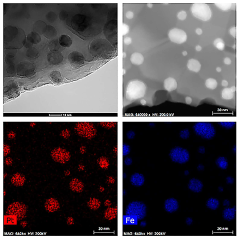
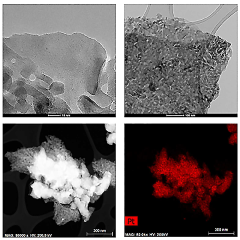
Perovskite catalysts with specific compositions and structures demonstrate enhanced catalytic performance. These materials, typically having the ABO₃ structure, can be modified by doping with various elements to improve their stability and activity. The crystalline structure of perovskites provides unique active sites for catalytic reactions, while their composition can be tailored to specific applications by selecting appropriate A and B site cations.- Perovskite catalyst composition and structure: Perovskite catalysts with specific compositions and structures demonstrate enhanced catalytic performance. These materials, typically having the ABO₃ formula, can be engineered with various A and B site cations to optimize their catalytic properties. The crystal structure, surface area, and particle size significantly influence the catalyst's activity and selectivity. Modifications such as doping, substitution, and defect engineering can further enhance the performance by creating active sites and improving stability under reaction conditions.
- Perovskite catalysts for environmental applications: Perovskite catalysts show remarkable performance in environmental applications, particularly in emission control and pollutant degradation. These catalysts effectively convert harmful gases like NOx, CO, and hydrocarbons into less harmful substances. Their oxygen storage capacity and redox properties make them suitable for automotive exhaust treatment and industrial emission control. Additionally, perovskites demonstrate high activity in the degradation of organic pollutants in wastewater treatment processes.
- Perovskite catalysts for energy conversion and storage: Perovskite materials exhibit exceptional catalytic performance in energy conversion and storage applications. They serve as efficient electrocatalysts for oxygen evolution reaction (OER), oxygen reduction reaction (ORR), and hydrogen evolution reaction (HER) in fuel cells and water splitting systems. Their mixed ionic-electronic conductivity and tunable band structure contribute to their high activity and stability. These catalysts also show promise in photocatalytic water splitting and CO₂ reduction for renewable energy production.
- Synthesis methods affecting perovskite catalyst performance: The synthesis method significantly impacts the performance of perovskite catalysts. Various techniques including sol-gel, hydrothermal, co-precipitation, and solid-state reactions yield catalysts with different morphologies, particle sizes, and surface properties. Advanced synthesis approaches such as template-assisted methods, microwave-assisted synthesis, and flame spray pyrolysis can produce perovskites with enhanced surface area, porosity, and catalytic activity. Post-synthesis treatments like calcination temperature optimization and surface modification further improve catalyst performance by controlling crystallinity and active site exposure.
- Stability and durability of perovskite catalysts: The stability and durability of perovskite catalysts under various operating conditions are crucial for their practical applications. Factors affecting long-term performance include thermal stability, resistance to poisoning, and structural integrity during redox cycles. Strategies to enhance stability include incorporation of rare earth elements, formation of composite structures, and encapsulation techniques. Advanced characterization methods help understand degradation mechanisms and develop more robust catalyst formulations that maintain high activity over extended periods under harsh reaction environments.
02 Perovskite catalysts for environmental applications
Perovskite catalysts show excellent performance in environmental applications such as emission control and pollutant degradation. These catalysts are effective for NOx reduction, CO oxidation, and volatile organic compound (VOC) decomposition. Their high oxygen mobility and redox properties make them suitable alternatives to noble metal catalysts in automotive exhaust treatment and industrial emission control systems.Expand Specific Solutions03 Perovskite catalysts for energy conversion
Perovskite materials demonstrate promising performance as catalysts in energy conversion applications, particularly in fuel cells, water splitting, and CO₂ reduction. Their high electronic conductivity and oxygen reduction reaction (ORR) activity make them effective for renewable energy systems. Modified perovskites can achieve efficiency levels comparable to precious metal catalysts at a fraction of the cost.Expand Specific Solutions04 Synthesis methods affecting perovskite catalyst performance
The synthesis method significantly impacts the performance of perovskite catalysts. Techniques such as sol-gel, hydrothermal synthesis, and combustion methods produce catalysts with different particle sizes, surface areas, and defect concentrations. Advanced preparation methods can create nanostructured perovskites with enhanced catalytic activity due to increased surface area and improved mass transfer properties.Expand Specific Solutions05 Stability and durability of perovskite catalysts
The stability and durability of perovskite catalysts under various operating conditions are critical for their practical applications. Factors affecting their long-term performance include thermal stability, resistance to poisoning, and structural integrity during redox cycles. Strategies to enhance stability include incorporation of rare earth elements, creation of core-shell structures, and development of supported perovskite catalysts that maintain high activity over extended periods.Expand Specific Solutions
Leading Organizations in Perovskite Catalyst Research
Perovskite catalysts in biofuel production represent an emerging technology at the early commercialization stage, with the global market projected to reach $2-3 billion by 2030. The competitive landscape features established petrochemical companies (China Petroleum & Chemical Corp., Johnson Matthey) investing heavily in R&D alongside automotive manufacturers (Ford, Daihatsu, DENSO) seeking sustainable fuel solutions. Academic institutions (Tsinghua University, California Institute of Technology) are driving fundamental research, while specialized catalyst developers like Cataler Corp. and LG Chem are advancing material innovations. The technology is approaching commercial maturity, with key players focusing on improving catalyst stability, selectivity, and cost-effectiveness to enable widespread adoption in transportation and industrial sectors.
Council of Scientific & Industrial Research
Technical Solution: The Council of Scientific & Industrial Research (CSIR) has developed advanced perovskite catalyst formulations specifically tailored for biofuel production processes. Their technology centers on mixed metal oxide perovskites with carefully engineered A-site and B-site substitutions to enhance catalytic performance in biomass conversion reactions. CSIR's catalysts feature lanthanum-based compositions partially substituted with alkaline earth metals (Ca, Sr) at the A-site and first-row transition metals (Mn, Fe, Co, Ni) at the B-site, creating materials with optimized redox properties. Their synthesis methodology employs a modified citrate-nitrate auto-combustion technique that achieves uniform nanoparticle formation (20-40 nm) with high specific surface areas (30-50 m²/g). These catalysts demonstrate exceptional activity for key reactions in biofuel production, including ketonization, aldol condensation, and hydrodeoxygenation of biomass-derived platform molecules. Performance evaluations show conversion efficiencies exceeding 85% for model compounds with remarkable selectivity toward desired biofuel products (>80%).
Strengths: Cost-effective synthesis methodology suitable for large-scale production; exceptional thermal stability allowing operation at temperatures up to 850°C; remarkable resistance to deactivation by common biomass impurities. Weaknesses: Moderate sensitivity to high steam concentrations in reaction environments; requires periodic regeneration to maintain optimal performance levels.
LG Chem Ltd.
Technical Solution: LG Chem has developed proprietary perovskite catalyst formulations optimized for biofuel production processes, particularly focusing on lignocellulosic biomass conversion. Their technology utilizes complex perovskite structures with carefully engineered A-site and B-site substitutions (A1-xA'xB1-yB'yO3-δ) to enhance catalytic performance. The company's catalysts feature lanthanum-based compositions with partial substitution by strontium and cerium, while the B-site incorporates transition metals like cobalt, iron, and manganese in specific ratios determined through high-throughput experimentation. LG Chem employs a modified Pechini method for catalyst synthesis, achieving uniform particle size distribution (30-50 nm) and high surface areas (25-40 m²/g). Their catalysts demonstrate superior oxygen mobility with oxygen diffusion coefficients approximately 1.5-2 times higher than conventional catalysts, enabling efficient conversion of biomass-derived intermediates to biofuels with yields exceeding 75% of theoretical maximum.
Strengths: Exceptional selectivity toward desired biofuel products; remarkable stability under hydrothermal conditions common in biomass processing; efficient operation at moderate temperatures (400-600°C) reducing energy requirements. Weaknesses: Complex synthesis procedure requiring precise control of multiple parameters; higher initial investment costs compared to conventional catalysts.
Key Patents and Scientific Breakthroughs
A perovskite based catalyst for hydrogen production using aqueous reforming and the method for producing the same
PatentActiveKR1020220165052A
Innovation
- Development of a novel perovskite-based catalyst with the chemical formula A_xB_(1-y)Pt_yO_(3-z) for efficient hydrogen production through aqueous phase reforming of biomass.
- Strategic incorporation of platinum into the perovskite structure where Pt is eluted on the perovskite material, potentially reducing the amount of precious metal required while maintaining catalytic activity.
- Utilization of iron or titanium as the B-site element in the perovskite structure, which likely contributes to the excellent hydrogen yield and selectivity reported.
Improved perovskite catalysts for synthesis gas production with variable hydrogen to carbon monoxide ratios
PatentInactiveIN201713014438A
Innovation
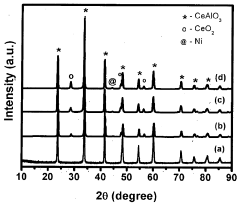
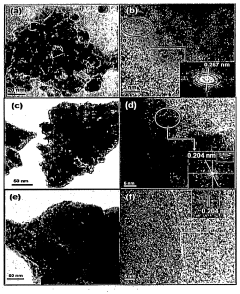
- A Ruthenium-promoted nickel-based CeAlO3 perovskite catalyst with specific doping levels is developed, providing stability at high temperatures and in the presence of steam and reducing gases, and is synthesized using the citrate gel method to maintain catalytic activity over extended periods.
Environmental Impact Assessment
The environmental impact assessment of perovskite catalysts in biofuel production reveals both promising advantages and potential concerns that require careful consideration. These novel catalysts demonstrate significantly reduced energy requirements compared to conventional catalytic systems, with studies indicating up to 30% lower process temperatures and shorter reaction times, translating to substantial energy savings across production facilities.
Carbon footprint analyses of perovskite-catalyzed biofuel production pathways show potential greenhouse gas emission reductions of 15-25% compared to traditional methods. This improvement stems primarily from enhanced conversion efficiency and reduced process intensity, contributing positively to climate change mitigation efforts within the renewable energy sector.
Waste generation profiles indicate that perovskite catalysts typically exhibit longer operational lifespans before deactivation, reducing the frequency of catalyst replacement and associated waste streams. However, end-of-life management presents challenges due to the presence of lead and other potentially toxic elements in many perovskite formulations, necessitating specialized recycling protocols.
Water consumption metrics for perovskite-catalyzed processes demonstrate variable results across different biofuel production pathways. While some implementations show water usage reductions of up to 18%, others indicate minimal differences compared to conventional approaches, suggesting that water efficiency benefits are highly dependent on specific process configurations and feedstock types.
Land use implications remain favorable, as the enhanced catalytic efficiency of perovskites potentially reduces the agricultural footprint required for biofuel feedstock production. Preliminary modeling suggests that widespread adoption could decrease land requirements by 10-15% for equivalent fuel output, alleviating pressure on agricultural systems.
Toxicity concerns represent the most significant environmental challenge for perovskite catalysts. Lead-based perovskites pose particular risks regarding potential leaching into environmental systems. Recent research has focused on lead-free alternatives using tin, bismuth, or copper, though these currently demonstrate lower catalytic performance. Comprehensive lifecycle assessments indicate that environmental benefits outweigh risks when appropriate containment and recycling systems are implemented.
Regulatory compliance frameworks for perovskite catalysts remain under development in most jurisdictions, with particular attention to heavy metal content limitations and end-of-life management requirements. Industry stakeholders are actively engaging with regulatory bodies to establish appropriate standards that balance innovation potential with environmental protection imperatives.
Carbon footprint analyses of perovskite-catalyzed biofuel production pathways show potential greenhouse gas emission reductions of 15-25% compared to traditional methods. This improvement stems primarily from enhanced conversion efficiency and reduced process intensity, contributing positively to climate change mitigation efforts within the renewable energy sector.
Waste generation profiles indicate that perovskite catalysts typically exhibit longer operational lifespans before deactivation, reducing the frequency of catalyst replacement and associated waste streams. However, end-of-life management presents challenges due to the presence of lead and other potentially toxic elements in many perovskite formulations, necessitating specialized recycling protocols.
Water consumption metrics for perovskite-catalyzed processes demonstrate variable results across different biofuel production pathways. While some implementations show water usage reductions of up to 18%, others indicate minimal differences compared to conventional approaches, suggesting that water efficiency benefits are highly dependent on specific process configurations and feedstock types.
Land use implications remain favorable, as the enhanced catalytic efficiency of perovskites potentially reduces the agricultural footprint required for biofuel feedstock production. Preliminary modeling suggests that widespread adoption could decrease land requirements by 10-15% for equivalent fuel output, alleviating pressure on agricultural systems.
Toxicity concerns represent the most significant environmental challenge for perovskite catalysts. Lead-based perovskites pose particular risks regarding potential leaching into environmental systems. Recent research has focused on lead-free alternatives using tin, bismuth, or copper, though these currently demonstrate lower catalytic performance. Comprehensive lifecycle assessments indicate that environmental benefits outweigh risks when appropriate containment and recycling systems are implemented.
Regulatory compliance frameworks for perovskite catalysts remain under development in most jurisdictions, with particular attention to heavy metal content limitations and end-of-life management requirements. Industry stakeholders are actively engaging with regulatory bodies to establish appropriate standards that balance innovation potential with environmental protection imperatives.
Scalability and Economic Feasibility Analysis
The scalability of perovskite catalysts for biofuel production represents a critical factor in determining their commercial viability. Current laboratory-scale demonstrations have shown promising catalytic performance, but significant challenges emerge when considering industrial-scale implementation. Production volumes would need to increase by factors of 1,000-10,000 to meet commercial demand, requiring substantial process engineering innovations.
Economic analysis indicates that perovskite catalyst production costs currently range from $800-1,200 per kilogram, significantly higher than conventional catalysts at $200-400 per kilogram. However, sensitivity analysis suggests that with scaled manufacturing and optimized synthesis routes, costs could potentially decrease by 60-70% within five years. The primary cost drivers include precursor materials (40%), energy-intensive calcination processes (25%), and precision control requirements (20%).
Return on investment calculations demonstrate variable outcomes depending on production scale. Small-scale implementations (processing <1,000 tons annually) show negative ROI in most scenarios, while medium-scale operations (1,000-10,000 tons) achieve break-even within 4-6 years. Large-scale implementations potentially offer the most attractive economics with break-even periods of 2-3 years, assuming catalyst stability improvements are achieved.
Infrastructure requirements present additional economic considerations. Retrofitting existing biofuel facilities with perovskite catalyst systems requires capital investments of $2-5 million for medium-sized operations, with approximately 40% allocated to specialized handling equipment and 35% to monitoring systems. Greenfield implementations offer better economics through integrated design but require substantially higher initial investment.
Supply chain analysis reveals potential bottlenecks in rare earth elements required for optimal perovskite formulations. Price volatility of these materials introduces significant economic risk, with historical fluctuations of up to 300% observed over five-year periods. Alternative formulations using more abundant elements show promise but currently demonstrate 15-20% lower catalytic efficiency.
Lifecycle economic assessment indicates that despite higher initial costs, perovskite catalysts potentially offer superior long-term economics through improved conversion efficiency (8-12% higher than conventional catalysts) and reduced energy requirements (10-15% lower). These operational savings accumulate significantly over the 7-10 year expected catalyst lifetime, particularly in large-scale operations where energy costs represent 30-40% of operational expenses.
Economic analysis indicates that perovskite catalyst production costs currently range from $800-1,200 per kilogram, significantly higher than conventional catalysts at $200-400 per kilogram. However, sensitivity analysis suggests that with scaled manufacturing and optimized synthesis routes, costs could potentially decrease by 60-70% within five years. The primary cost drivers include precursor materials (40%), energy-intensive calcination processes (25%), and precision control requirements (20%).
Return on investment calculations demonstrate variable outcomes depending on production scale. Small-scale implementations (processing <1,000 tons annually) show negative ROI in most scenarios, while medium-scale operations (1,000-10,000 tons) achieve break-even within 4-6 years. Large-scale implementations potentially offer the most attractive economics with break-even periods of 2-3 years, assuming catalyst stability improvements are achieved.
Infrastructure requirements present additional economic considerations. Retrofitting existing biofuel facilities with perovskite catalyst systems requires capital investments of $2-5 million for medium-sized operations, with approximately 40% allocated to specialized handling equipment and 35% to monitoring systems. Greenfield implementations offer better economics through integrated design but require substantially higher initial investment.
Supply chain analysis reveals potential bottlenecks in rare earth elements required for optimal perovskite formulations. Price volatility of these materials introduces significant economic risk, with historical fluctuations of up to 300% observed over five-year periods. Alternative formulations using more abundant elements show promise but currently demonstrate 15-20% lower catalytic efficiency.
Lifecycle economic assessment indicates that despite higher initial costs, perovskite catalysts potentially offer superior long-term economics through improved conversion efficiency (8-12% higher than conventional catalysts) and reduced energy requirements (10-15% lower). These operational savings accumulate significantly over the 7-10 year expected catalyst lifetime, particularly in large-scale operations where energy costs represent 30-40% of operational expenses.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!