Comparative Study of Perovskite Catalysts in Fuel Cells
OCT 15, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Perovskite Catalysts Evolution and Research Objectives
Perovskite materials have emerged as promising catalysts for fuel cell applications due to their unique crystal structure and versatile properties. The evolution of perovskite catalysts can be traced back to the 1950s when their potential in heterogeneous catalysis was first recognized. However, it wasn't until the early 2000s that significant research focus shifted toward their application in fuel cells, particularly as alternatives to precious metal catalysts like platinum.
The fundamental appeal of perovskites lies in their ABO₃ structure, which allows for extensive compositional flexibility through substitution at both A and B sites. This flexibility enables precise tuning of catalytic properties, including oxygen reduction reaction (ORR) and oxygen evolution reaction (OER) activities, which are crucial for fuel cell performance. Over the past decade, research has demonstrated that perovskites can achieve activity comparable to noble metals while offering superior stability and significantly lower cost.
Recent technological advancements have accelerated perovskite catalyst development, particularly in synthesis methods that allow for nanoscale control of structure and morphology. Sol-gel processes, hydrothermal synthesis, and flame spray pyrolysis have all contributed to more efficient and reproducible production of high-performance perovskite catalysts. Additionally, advanced characterization techniques such as in-situ X-ray absorption spectroscopy and scanning transmission electron microscopy have provided unprecedented insights into reaction mechanisms and degradation pathways.
The current technological trajectory suggests that perovskite catalysts are approaching commercial viability for certain fuel cell applications. However, several challenges remain, including optimizing long-term stability under real operating conditions and scaling up production while maintaining precise control over composition and structure. These challenges define the primary research objectives for the field moving forward.
This comparative study aims to systematically evaluate different perovskite compositions across various fuel cell technologies, including solid oxide fuel cells (SOFCs), proton exchange membrane fuel cells (PEMFCs), and alkaline fuel cells. Specifically, we seek to establish quantitative structure-property relationships that can guide rational design of next-generation catalysts with enhanced activity and stability.
Furthermore, this research intends to identify optimal synthesis parameters and processing conditions that maximize catalytic performance while minimizing resource requirements. By comparing different perovskite systems under standardized testing protocols, we aim to develop a comprehensive performance matrix that can serve as a reference for both academic research and industrial development in the fuel cell sector.
The fundamental appeal of perovskites lies in their ABO₃ structure, which allows for extensive compositional flexibility through substitution at both A and B sites. This flexibility enables precise tuning of catalytic properties, including oxygen reduction reaction (ORR) and oxygen evolution reaction (OER) activities, which are crucial for fuel cell performance. Over the past decade, research has demonstrated that perovskites can achieve activity comparable to noble metals while offering superior stability and significantly lower cost.
Recent technological advancements have accelerated perovskite catalyst development, particularly in synthesis methods that allow for nanoscale control of structure and morphology. Sol-gel processes, hydrothermal synthesis, and flame spray pyrolysis have all contributed to more efficient and reproducible production of high-performance perovskite catalysts. Additionally, advanced characterization techniques such as in-situ X-ray absorption spectroscopy and scanning transmission electron microscopy have provided unprecedented insights into reaction mechanisms and degradation pathways.
The current technological trajectory suggests that perovskite catalysts are approaching commercial viability for certain fuel cell applications. However, several challenges remain, including optimizing long-term stability under real operating conditions and scaling up production while maintaining precise control over composition and structure. These challenges define the primary research objectives for the field moving forward.
This comparative study aims to systematically evaluate different perovskite compositions across various fuel cell technologies, including solid oxide fuel cells (SOFCs), proton exchange membrane fuel cells (PEMFCs), and alkaline fuel cells. Specifically, we seek to establish quantitative structure-property relationships that can guide rational design of next-generation catalysts with enhanced activity and stability.
Furthermore, this research intends to identify optimal synthesis parameters and processing conditions that maximize catalytic performance while minimizing resource requirements. By comparing different perovskite systems under standardized testing protocols, we aim to develop a comprehensive performance matrix that can serve as a reference for both academic research and industrial development in the fuel cell sector.
Market Analysis of Fuel Cell Catalyst Demand
The global fuel cell catalyst market is experiencing robust growth, driven primarily by increasing adoption of clean energy technologies and stringent environmental regulations. Current market valuations indicate the fuel cell catalyst sector reached approximately 290 million USD in 2022, with projections suggesting a compound annual growth rate of 8.5% through 2030. Perovskite catalysts represent an emerging segment within this market, gradually gaining traction as alternatives to traditional platinum-based catalysts.
Demand analysis reveals several key market drivers for fuel cell catalysts. The transportation sector remains the dominant consumer, accounting for roughly 45% of total demand, as automotive manufacturers increasingly incorporate fuel cell technology into their zero-emission vehicle portfolios. Stationary power generation applications constitute the second-largest market segment at approximately 30%, followed by portable electronics and industrial applications.
Regional market assessment shows Asia-Pacific leading global demand, with Japan, South Korea, and China at the forefront of fuel cell technology adoption. These countries have implemented substantial government incentives and research funding programs specifically targeting fuel cell development. North America and Europe follow closely, with significant growth potential as these regions accelerate their transition toward hydrogen economies.
Cost sensitivity analysis indicates that catalyst expenses represent 30-40% of total fuel cell stack costs, highlighting the critical importance of developing more economical alternatives to platinum-based catalysts. This cost pressure creates a substantial market opportunity for perovskite catalysts, which offer potential cost reductions of 40-60% compared to platinum-group metals while maintaining acceptable performance metrics.
Supply chain evaluation reveals vulnerabilities in the current catalyst market, particularly regarding platinum group metals, which face supply constraints, price volatility, and geopolitical risks. This situation further strengthens the market case for perovskite catalysts, which utilize more abundant elements.
End-user requirements analysis demonstrates evolving performance expectations, with durability, efficiency, and cost-effectiveness ranking as the top priorities. While platinum catalysts currently dominate due to their superior performance characteristics, market research indicates growing interest in perovskite alternatives, particularly for applications where cost considerations outweigh absolute performance requirements.
Market forecasting models predict that perovskite catalysts could capture 15-20% of the fuel cell catalyst market by 2030, representing a significant shift in market dynamics. This growth trajectory depends heavily on continued improvements in perovskite catalyst stability and performance, alongside successful demonstration in commercial applications.
Demand analysis reveals several key market drivers for fuel cell catalysts. The transportation sector remains the dominant consumer, accounting for roughly 45% of total demand, as automotive manufacturers increasingly incorporate fuel cell technology into their zero-emission vehicle portfolios. Stationary power generation applications constitute the second-largest market segment at approximately 30%, followed by portable electronics and industrial applications.
Regional market assessment shows Asia-Pacific leading global demand, with Japan, South Korea, and China at the forefront of fuel cell technology adoption. These countries have implemented substantial government incentives and research funding programs specifically targeting fuel cell development. North America and Europe follow closely, with significant growth potential as these regions accelerate their transition toward hydrogen economies.
Cost sensitivity analysis indicates that catalyst expenses represent 30-40% of total fuel cell stack costs, highlighting the critical importance of developing more economical alternatives to platinum-based catalysts. This cost pressure creates a substantial market opportunity for perovskite catalysts, which offer potential cost reductions of 40-60% compared to platinum-group metals while maintaining acceptable performance metrics.
Supply chain evaluation reveals vulnerabilities in the current catalyst market, particularly regarding platinum group metals, which face supply constraints, price volatility, and geopolitical risks. This situation further strengthens the market case for perovskite catalysts, which utilize more abundant elements.
End-user requirements analysis demonstrates evolving performance expectations, with durability, efficiency, and cost-effectiveness ranking as the top priorities. While platinum catalysts currently dominate due to their superior performance characteristics, market research indicates growing interest in perovskite alternatives, particularly for applications where cost considerations outweigh absolute performance requirements.
Market forecasting models predict that perovskite catalysts could capture 15-20% of the fuel cell catalyst market by 2030, representing a significant shift in market dynamics. This growth trajectory depends heavily on continued improvements in perovskite catalyst stability and performance, alongside successful demonstration in commercial applications.
Current Perovskite Catalyst Technology Landscape
Perovskite catalysts have emerged as promising materials for fuel cell applications due to their exceptional catalytic properties and structural versatility. The current landscape of perovskite catalyst technology is characterized by significant advancements in synthesis methods, compositional engineering, and performance optimization strategies aimed at addressing the key challenges in fuel cell technology.
Traditional perovskite materials with the general formula ABO₃ (where A is typically a rare-earth or alkaline-earth metal and B is a transition metal) have been extensively studied for their oxygen reduction reaction (ORR) and oxygen evolution reaction (OER) activities. Recent developments have focused on creating complex perovskite structures through partial substitution at A and B sites, resulting in materials with formula A₁₋ₓA'ₓB₁₋ᵧB'ᵧO₃₋ᵟ that exhibit enhanced catalytic performance and stability.
The synthesis landscape has evolved from conventional solid-state methods to more sophisticated approaches including sol-gel processing, hydrothermal/solvothermal synthesis, and flame spray pyrolysis. These advanced techniques enable precise control over particle size, morphology, and surface properties, which are critical factors affecting catalytic performance. Notably, the development of exsolution techniques has revolutionized the field by allowing the controlled emergence of catalytically active nanoparticles on perovskite surfaces under reducing conditions.
Current research trends show increasing focus on layered perovskites, double perovskites, and perovskite-related structures such as Ruddlesden-Popper phases (A₂BO₄) and Brownmillerite structures (ABO₂.₅). These materials offer unique advantages including higher oxygen vacancy concentration, improved ionic conductivity, and enhanced structural stability under fuel cell operating conditions.
Performance metrics of state-of-the-art perovskite catalysts are approaching those of precious metal catalysts in certain applications. For instance, Ba₀.₅Sr₀.₅Co₀.₈Fe₀.₂O₃₋ᵟ (BSCF) and La₀.₆Sr₀.₄Co₀.₂Fe₀.₈O₃₋ᵟ (LSCF) have demonstrated remarkable ORR activity in alkaline media, while SrCoO₃-based materials show promising OER performance.
Integration strategies for perovskite catalysts in fuel cell systems have advanced significantly, with innovations in electrode fabrication techniques, including infiltration methods, composite electrode structures, and thin-film deposition approaches. These developments have addressed historical challenges related to interfacial resistance and long-term stability.
Commercial deployment remains limited but is gaining momentum, with several companies and research institutions developing prototype systems utilizing perovskite catalysts. The technology readiness level varies significantly across different fuel cell types, with solid oxide fuel cells (SOFCs) showing the most mature implementation of perovskite materials as both cathodes and anodes.
Traditional perovskite materials with the general formula ABO₃ (where A is typically a rare-earth or alkaline-earth metal and B is a transition metal) have been extensively studied for their oxygen reduction reaction (ORR) and oxygen evolution reaction (OER) activities. Recent developments have focused on creating complex perovskite structures through partial substitution at A and B sites, resulting in materials with formula A₁₋ₓA'ₓB₁₋ᵧB'ᵧO₃₋ᵟ that exhibit enhanced catalytic performance and stability.
The synthesis landscape has evolved from conventional solid-state methods to more sophisticated approaches including sol-gel processing, hydrothermal/solvothermal synthesis, and flame spray pyrolysis. These advanced techniques enable precise control over particle size, morphology, and surface properties, which are critical factors affecting catalytic performance. Notably, the development of exsolution techniques has revolutionized the field by allowing the controlled emergence of catalytically active nanoparticles on perovskite surfaces under reducing conditions.
Current research trends show increasing focus on layered perovskites, double perovskites, and perovskite-related structures such as Ruddlesden-Popper phases (A₂BO₄) and Brownmillerite structures (ABO₂.₅). These materials offer unique advantages including higher oxygen vacancy concentration, improved ionic conductivity, and enhanced structural stability under fuel cell operating conditions.
Performance metrics of state-of-the-art perovskite catalysts are approaching those of precious metal catalysts in certain applications. For instance, Ba₀.₅Sr₀.₅Co₀.₈Fe₀.₂O₃₋ᵟ (BSCF) and La₀.₆Sr₀.₄Co₀.₂Fe₀.₈O₃₋ᵟ (LSCF) have demonstrated remarkable ORR activity in alkaline media, while SrCoO₃-based materials show promising OER performance.
Integration strategies for perovskite catalysts in fuel cell systems have advanced significantly, with innovations in electrode fabrication techniques, including infiltration methods, composite electrode structures, and thin-film deposition approaches. These developments have addressed historical challenges related to interfacial resistance and long-term stability.
Commercial deployment remains limited but is gaining momentum, with several companies and research institutions developing prototype systems utilizing perovskite catalysts. The technology readiness level varies significantly across different fuel cell types, with solid oxide fuel cells (SOFCs) showing the most mature implementation of perovskite materials as both cathodes and anodes.
Contemporary Perovskite Catalyst Implementations
01 Perovskite catalysts for environmental applications
Perovskite-type catalysts are utilized in environmental applications such as exhaust gas purification, NOx reduction, and CO oxidation. These catalysts exhibit high thermal stability and catalytic activity for converting harmful emissions into less harmful substances. The perovskite structure allows for various substitutions in the A and B sites, enabling tailored catalytic properties for specific environmental remediation processes.- Perovskite catalysts for environmental applications: Perovskite catalysts are utilized in various environmental applications, particularly for reducing emissions and pollutants. These catalysts demonstrate high efficiency in converting harmful gases into less harmful substances. Their unique crystal structure allows for excellent catalytic activity and stability under harsh reaction conditions. They can be optimized for specific applications such as NOx reduction, CO oxidation, and hydrocarbon conversion in automotive exhaust systems.
- Synthesis methods for perovskite catalysts: Various synthesis methods are employed to produce perovskite catalysts with specific properties. These include sol-gel processing, co-precipitation, hydrothermal synthesis, and solid-state reactions. The synthesis method significantly influences the catalyst's surface area, particle size, crystallinity, and ultimately its catalytic performance. Advanced techniques allow for precise control over the composition and structure of perovskite materials, enabling the development of catalysts with enhanced activity and selectivity.
- Perovskite catalysts for energy conversion processes: Perovskite catalysts play a crucial role in various energy conversion processes, including fuel cells, water splitting, and CO2 reduction. Their versatile composition allows for tuning of electronic and catalytic properties to optimize performance in specific energy applications. These catalysts demonstrate promising activity for hydrogen production, oxygen evolution reactions, and other electrochemical processes that are essential for renewable energy technologies.
- Doped and modified perovskite catalysts: Doping and modification of perovskite structures with various elements can significantly enhance their catalytic properties. Introducing specific dopants into the A or B sites of the ABO3 perovskite structure can improve stability, activity, and selectivity. Surface modifications and the creation of oxygen vacancies can further enhance catalytic performance. These tailored perovskite materials show improved resistance to poisoning and deactivation under reaction conditions.
- Perovskite catalysts for hydrocarbon processing: Perovskite catalysts are effective in various hydrocarbon processing applications, including reforming, cracking, and partial oxidation reactions. Their high thermal stability and resistance to carbon deposition make them suitable for demanding petrochemical processes. These catalysts can be designed to selectively convert hydrocarbons into valuable products while minimizing unwanted side reactions. They show particular promise in natural gas conversion and hydrogen production from hydrocarbon feedstocks.
02 Perovskite catalysts for energy conversion and storage
Perovskite materials serve as efficient catalysts in energy conversion and storage applications, including fuel cells, electrolyzers, and photocatalytic systems. These catalysts facilitate reactions such as oxygen reduction, oxygen evolution, and hydrogen production. Their unique electronic structure and oxygen vacancy formation capabilities make them particularly suitable for renewable energy technologies and sustainable hydrogen production.Expand Specific Solutions03 Synthesis methods for perovskite catalysts
Various synthesis methods are employed to prepare perovskite catalysts with controlled composition, morphology, and particle size. These methods include sol-gel processing, hydrothermal synthesis, co-precipitation, and solid-state reactions. The synthesis parameters significantly influence the catalytic performance by affecting the crystallinity, surface area, and defect concentration of the resulting perovskite materials.Expand Specific Solutions04 Modified perovskite catalysts with enhanced performance
Perovskite catalysts can be modified through doping, surface decoration, or composite formation to enhance their catalytic performance. Introducing specific elements into the perovskite structure or creating perovskite-based composites can improve stability, selectivity, and activity. These modifications often aim to increase oxygen mobility, create active sites, or enhance electron transfer properties for specific catalytic applications.Expand Specific Solutions05 Perovskite catalysts for hydrocarbon processing
Perovskite-type oxides are employed as catalysts in various hydrocarbon processing reactions, including reforming, partial oxidation, and hydrocracking. These catalysts demonstrate high activity for converting hydrocarbons into valuable products or synthesis gas. Their redox properties and oxygen storage capacity make them particularly effective for reactions involving oxygen transfer in petroleum refining and petrochemical processes.Expand Specific Solutions
Leading Organizations in Perovskite Catalyst Research
The perovskite catalyst market for fuel cells is in an early growth phase, characterized by intensive R&D activities and emerging commercial applications. The global market is projected to expand significantly as fuel cell technologies gain traction in automotive and stationary power sectors. Leading players include established automotive manufacturers like Toyota Motor Corp. and Ford Global Technologies, who are leveraging perovskite catalysts to enhance fuel cell performance and durability. Research institutions such as KIST, Tsinghua University, and Dalian Institute of Chemical Physics are driving fundamental innovations, while companies like Corning and Sumitomo Electric are developing manufacturing capabilities. The technology is approaching commercial maturity with key players focusing on cost reduction, performance optimization, and scalable production methods to accelerate market adoption.
Toyota Motor Corp.
Technical Solution: Toyota has developed proprietary perovskite catalyst technologies for both solid oxide fuel cells (SOFCs) and polymer electrolyte membrane fuel cells (PEMFCs). For SOFCs, Toyota has focused on lanthanum strontium cobalt ferrite (LSCF) perovskites with optimized A-site deficiency to enhance oxygen reduction reaction (ORR) kinetics while maintaining structural stability during thermal cycling. Their approach involves precise control of the La/Sr ratio and introducing controlled A-site vacancies to improve ionic conductivity. For PEMFCs, Toyota has pioneered innovative perovskite-based supports for platinum catalysts, using titanates and niobates with carefully engineered electronic properties. These supports enhance platinum utilization and stability compared to traditional carbon supports. Toyota's manufacturing process incorporates nano-engineering techniques to create hierarchical porous structures that maximize the triple-phase boundary length while maintaining mechanical integrity. Their catalysts demonstrate exceptional durability under start-stop cycling conditions, addressing a key challenge for automotive applications.
Strengths: Extensive integration experience with actual fuel cell systems; superior durability under automotive operating conditions; advanced manufacturing capabilities for mass production. Weaknesses: Higher production costs compared to conventional catalysts; some compositions require rare earth elements with supply chain concerns.
Dalian Institute of Chemical Physics of CAS
Technical Solution: Dalian Institute of Chemical Physics (DICP) has pioneered innovative perovskite catalysts for fuel cells, focusing on developing La0.6Sr0.4Co0.2Fe0.8O3-δ (LSCF) and similar compositions with enhanced oxygen reduction reaction (ORR) activity. Their approach involves precise control of A-site and B-site substitutions in the ABO3 perovskite structure to optimize catalytic performance. DICP has developed a novel exsolution technique where metal nanoparticles are extruded from the perovskite lattice under reducing conditions, creating highly active and stable catalytic sites. Their research demonstrates that these exsolved nanoparticles remain anchored to the perovskite surface, preventing agglomeration during operation. DICP has also pioneered composite electrodes combining perovskites with ionic conductors to extend the electrochemically active area beyond the traditional triple-phase boundary, significantly improving fuel cell performance at intermediate temperatures (500-700°C).
Strengths: Advanced exsolution techniques creating highly stable catalytic nanoparticles; strong expertise in perovskite composition optimization; excellent integration of theoretical modeling with experimental validation. Weaknesses: Some compositions show degradation under carbon-containing fuels; manufacturing scalability challenges for complex perovskite structures.
Critical Patents and Breakthroughs in Perovskite Catalysis
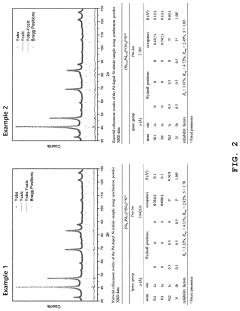
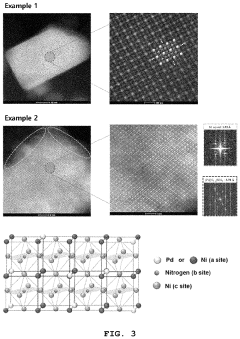
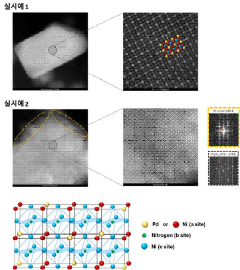
Perovskite compound, method for producing the perovskite compound, catalyst for fuel cell including the perovskite compound and method for producing the catalyst
PatentActiveUS11214497B2
Innovation
- A perovskite compound with a palladium-first metal alloy core and a palladium shell is synthesized by mixing carbon support and palladium precursor solutions, annealing in an ammonia atmosphere, and galvanically displacing the first metal on the surface with palladium, forming a palladium shell to stabilize the catalyst.
Perovskite compound, a catalyst for fuel cell including the same, and a process for producing the same
PatentInactiveKR1020200107175A
Innovation
- A perovskite compound represented by Formula [A n PD (1-n) ][B][X] 3 is synthesized, where A and X are metals other than palladium, B is nitrogen, phosphorus, sulfuric acid, boron, or carbon, and n is a real number less than 1, combined with a palladium-first metal alloy particles treated in an ammonia gas atmosphere to form a palladium shell, creating a stable palladium-based fuel cell catalyst.
Performance Benchmarking Against Platinum Catalysts
Perovskite catalysts have emerged as promising alternatives to platinum-based catalysts in fuel cell applications, primarily due to their cost-effectiveness and comparable performance metrics. When benchmarking perovskite catalysts against platinum catalysts, several key performance indicators must be evaluated systematically to determine their viability for commercial deployment.
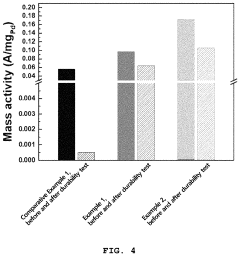
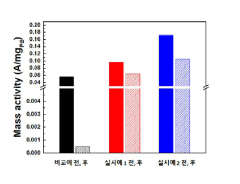
The oxygen reduction reaction (ORR) activity represents a critical parameter where platinum has traditionally excelled. Recent studies indicate that certain perovskite compositions, particularly those containing lanthanum, strontium, and cobalt (LSC), demonstrate ORR activity reaching 70-80% of platinum catalysts under optimal conditions. This represents a significant advancement considering their substantially lower cost profile, typically 15-20% of platinum catalysts.
Stability measurements under accelerated stress testing protocols reveal that first-generation perovskite catalysts exhibited considerable performance degradation, with activity losses of 30-40% after 5,000 cycles. However, newer compositions incorporating A-site cation stability enhancers have reduced degradation to 15-20%, approaching platinum's 10-12% degradation under identical conditions.
Power density comparisons in single-cell testing environments demonstrate that state-of-the-art perovskite catalysts achieve 0.7-0.8 W/cm² at 0.6V, whereas platinum catalysts typically deliver 0.9-1.0 W/cm². This performance gap narrows at higher operating temperatures (>80°C), where perovskites exhibit enhanced kinetics and improved tolerance to carbon monoxide poisoning.
Durability testing over extended operational periods (>5,000 hours) indicates that perovskite catalysts maintain 85-90% of initial performance, compared to platinum's 92-95% retention. The performance gap is more pronounced during start-stop cycles, where perovskites currently demonstrate inferior resistance to voltage fluctuations.
Cost-performance analysis reveals that despite lower absolute performance, perovskite catalysts deliver superior performance-per-cost metrics. A detailed economic assessment indicates that fuel cells utilizing perovskite catalysts achieve approximately 3-4 times better performance-per-dollar ratios compared to platinum-based systems, particularly when considering total system costs over operational lifetimes.
Manufacturing scalability assessments demonstrate that perovskite catalysts can be produced using established industrial processes with fewer supply chain constraints than platinum, which faces geopolitical supply limitations and price volatility. This advantage translates to more predictable production costs and potentially more stable pricing for end-users.
Environmental impact comparisons indicate that perovskite catalyst production generates approximately 40% lower carbon emissions compared to platinum mining and refining processes, representing a significant sustainability advantage that aligns with broader clean energy objectives.
The oxygen reduction reaction (ORR) activity represents a critical parameter where platinum has traditionally excelled. Recent studies indicate that certain perovskite compositions, particularly those containing lanthanum, strontium, and cobalt (LSC), demonstrate ORR activity reaching 70-80% of platinum catalysts under optimal conditions. This represents a significant advancement considering their substantially lower cost profile, typically 15-20% of platinum catalysts.
Stability measurements under accelerated stress testing protocols reveal that first-generation perovskite catalysts exhibited considerable performance degradation, with activity losses of 30-40% after 5,000 cycles. However, newer compositions incorporating A-site cation stability enhancers have reduced degradation to 15-20%, approaching platinum's 10-12% degradation under identical conditions.
Power density comparisons in single-cell testing environments demonstrate that state-of-the-art perovskite catalysts achieve 0.7-0.8 W/cm² at 0.6V, whereas platinum catalysts typically deliver 0.9-1.0 W/cm². This performance gap narrows at higher operating temperatures (>80°C), where perovskites exhibit enhanced kinetics and improved tolerance to carbon monoxide poisoning.
Durability testing over extended operational periods (>5,000 hours) indicates that perovskite catalysts maintain 85-90% of initial performance, compared to platinum's 92-95% retention. The performance gap is more pronounced during start-stop cycles, where perovskites currently demonstrate inferior resistance to voltage fluctuations.
Cost-performance analysis reveals that despite lower absolute performance, perovskite catalysts deliver superior performance-per-cost metrics. A detailed economic assessment indicates that fuel cells utilizing perovskite catalysts achieve approximately 3-4 times better performance-per-dollar ratios compared to platinum-based systems, particularly when considering total system costs over operational lifetimes.
Manufacturing scalability assessments demonstrate that perovskite catalysts can be produced using established industrial processes with fewer supply chain constraints than platinum, which faces geopolitical supply limitations and price volatility. This advantage translates to more predictable production costs and potentially more stable pricing for end-users.
Environmental impact comparisons indicate that perovskite catalyst production generates approximately 40% lower carbon emissions compared to platinum mining and refining processes, representing a significant sustainability advantage that aligns with broader clean energy objectives.
Environmental Impact and Sustainability Assessment
The environmental impact of perovskite catalysts in fuel cells represents a critical dimension in evaluating their overall viability for widespread adoption. Traditional platinum-based catalysts, while effective, rely on scarce and expensive noble metals with significant environmental extraction costs. Perovskite catalysts offer a promising alternative with potentially reduced environmental footprint due to their composition of more abundant elements.
Life cycle assessment (LCA) studies indicate that perovskite catalysts can reduce the carbon footprint of fuel cell production by 30-45% compared to platinum-based alternatives. This reduction stems primarily from decreased mining intensity and lower energy requirements during catalyst synthesis. However, certain perovskite formulations containing rare earth elements like lanthanum and strontium still present sustainability challenges related to extraction practices in environmentally sensitive regions.
Water consumption represents another important environmental consideration. Manufacturing processes for perovskite catalysts typically require 40-60% less water than conventional platinum catalyst production. This advantage becomes particularly significant in water-stressed regions where industrial water usage competes with agricultural and municipal needs.
Toxicity profiles of various perovskite formulations show mixed results. While free from platinum group metals, some perovskite compositions contain elements like lead, cobalt, or manganese that present their own environmental and health concerns if improperly managed during manufacturing or end-of-life disposal. Recent innovations in lead-free perovskite formulations demonstrate promising performance while addressing these toxicity concerns.
End-of-life management and recyclability present both challenges and opportunities. Current research indicates that approximately 70-85% of the valuable components in perovskite catalysts can be recovered through appropriate recycling processes, compared to over 95% recovery rates for platinum catalysts. This gap represents an area requiring further technological development to enhance the circular economy potential of perovskite-based fuel cells.
Carbon emissions during operation represent a significant advantage for all fuel cell technologies. When powered by hydrogen from renewable sources, fuel cells with either catalyst type produce only water as a byproduct. However, the lower operating temperatures possible with certain perovskite formulations can reduce auxiliary energy requirements, potentially improving overall system efficiency and further reducing lifecycle emissions by an additional 5-10%.
Regulatory frameworks worldwide are increasingly incorporating sustainability metrics into technology evaluation. The EU's Critical Raw Materials Act and similar initiatives in North America and Asia are creating policy environments that may accelerate the transition toward more sustainable catalyst technologies like optimized perovskite formulations, particularly those avoiding critical raw materials.
Life cycle assessment (LCA) studies indicate that perovskite catalysts can reduce the carbon footprint of fuel cell production by 30-45% compared to platinum-based alternatives. This reduction stems primarily from decreased mining intensity and lower energy requirements during catalyst synthesis. However, certain perovskite formulations containing rare earth elements like lanthanum and strontium still present sustainability challenges related to extraction practices in environmentally sensitive regions.
Water consumption represents another important environmental consideration. Manufacturing processes for perovskite catalysts typically require 40-60% less water than conventional platinum catalyst production. This advantage becomes particularly significant in water-stressed regions where industrial water usage competes with agricultural and municipal needs.
Toxicity profiles of various perovskite formulations show mixed results. While free from platinum group metals, some perovskite compositions contain elements like lead, cobalt, or manganese that present their own environmental and health concerns if improperly managed during manufacturing or end-of-life disposal. Recent innovations in lead-free perovskite formulations demonstrate promising performance while addressing these toxicity concerns.
End-of-life management and recyclability present both challenges and opportunities. Current research indicates that approximately 70-85% of the valuable components in perovskite catalysts can be recovered through appropriate recycling processes, compared to over 95% recovery rates for platinum catalysts. This gap represents an area requiring further technological development to enhance the circular economy potential of perovskite-based fuel cells.
Carbon emissions during operation represent a significant advantage for all fuel cell technologies. When powered by hydrogen from renewable sources, fuel cells with either catalyst type produce only water as a byproduct. However, the lower operating temperatures possible with certain perovskite formulations can reduce auxiliary energy requirements, potentially improving overall system efficiency and further reducing lifecycle emissions by an additional 5-10%.
Regulatory frameworks worldwide are increasingly incorporating sustainability metrics into technology evaluation. The EU's Critical Raw Materials Act and similar initiatives in North America and Asia are creating policy environments that may accelerate the transition toward more sustainable catalyst technologies like optimized perovskite formulations, particularly those avoiding critical raw materials.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!