How Does Temperature Influence Perovskite Catalyst Activity?
OCT 15, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Perovskite Catalysis Background and Temperature Objectives
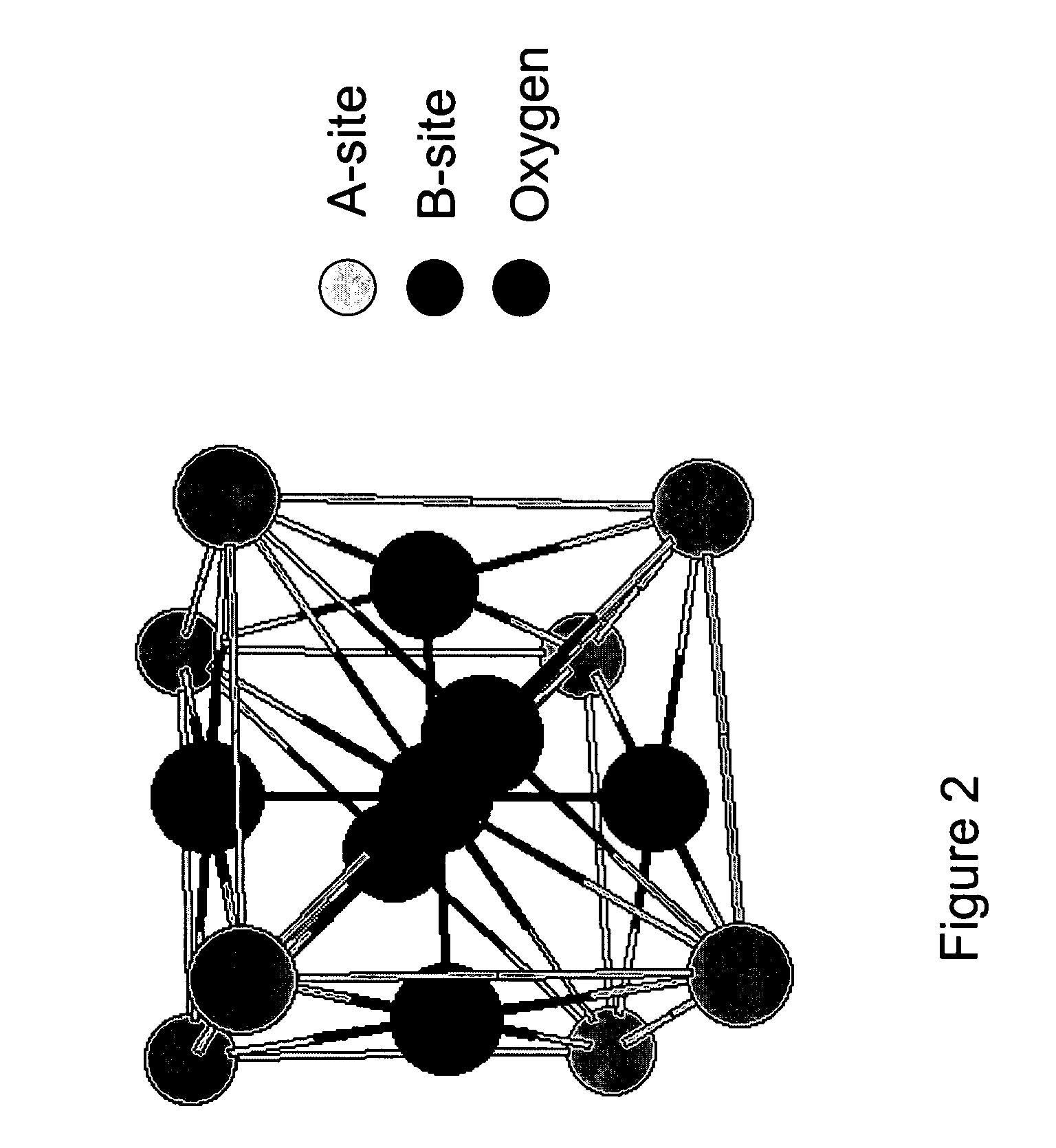
Perovskite materials have emerged as a revolutionary class of compounds in catalysis science over the past several decades. Initially discovered in 1839 by Gustav Rose and named after Russian mineralogist Lev Perovski, these materials possess a distinctive ABX3 crystal structure, where A and B are cations of different sizes and X is an anion. This versatile structure allows for extensive compositional engineering, making perovskites highly adaptable for various catalytic applications.
The evolution of perovskite catalysts has witnessed significant milestones, from their early applications in automotive catalytic converters to their current prominence in renewable energy technologies. The field has experienced accelerated growth since the early 2000s, with research publications on perovskite catalysis increasing exponentially, particularly in the last decade. This surge reflects the scientific community's recognition of perovskites' exceptional catalytic properties and their potential to address critical energy and environmental challenges.
Temperature stands as a fundamental parameter governing perovskite catalyst performance. The relationship between temperature and catalytic activity represents a complex interplay of thermodynamic and kinetic factors that determine reaction rates, selectivity, and stability. Understanding this relationship is crucial for optimizing catalyst design and operational conditions across diverse applications, from fuel cells to environmental remediation.
The primary technical objectives in this domain include elucidating the mechanistic pathways through which temperature influences perovskite surface chemistry, developing temperature-resilient perovskite compositions, and establishing predictive models that accurately capture temperature-dependent behavior. These objectives align with broader goals of enhancing energy efficiency, reducing environmental impact, and enabling sustainable chemical processes.
Recent technological trends indicate a shift toward rational design approaches that leverage computational methods to predict temperature effects on perovskite catalytic performance. This represents a departure from traditional trial-and-error methodologies, enabling more efficient discovery of optimal compositions and structures. Additionally, in situ and operando characterization techniques have revolutionized our ability to observe temperature-induced structural and electronic changes in real-time under reaction conditions.
The temperature-activity relationship in perovskite catalysis intersects with several emerging research directions, including defect engineering, interface design, and nanoscale architecture. These approaches offer promising avenues for tailoring temperature response and expanding the operational temperature windows of perovskite catalysts, potentially unlocking new applications across industrial sectors.
Our technical goals encompass developing comprehensive structure-property relationships that connect perovskite composition and architecture to temperature-dependent catalytic behavior, establishing standardized protocols for evaluating thermal stability and performance, and creating design principles for next-generation thermally optimized perovskite catalysts that can address pressing sustainability challenges.
The evolution of perovskite catalysts has witnessed significant milestones, from their early applications in automotive catalytic converters to their current prominence in renewable energy technologies. The field has experienced accelerated growth since the early 2000s, with research publications on perovskite catalysis increasing exponentially, particularly in the last decade. This surge reflects the scientific community's recognition of perovskites' exceptional catalytic properties and their potential to address critical energy and environmental challenges.
Temperature stands as a fundamental parameter governing perovskite catalyst performance. The relationship between temperature and catalytic activity represents a complex interplay of thermodynamic and kinetic factors that determine reaction rates, selectivity, and stability. Understanding this relationship is crucial for optimizing catalyst design and operational conditions across diverse applications, from fuel cells to environmental remediation.
The primary technical objectives in this domain include elucidating the mechanistic pathways through which temperature influences perovskite surface chemistry, developing temperature-resilient perovskite compositions, and establishing predictive models that accurately capture temperature-dependent behavior. These objectives align with broader goals of enhancing energy efficiency, reducing environmental impact, and enabling sustainable chemical processes.
Recent technological trends indicate a shift toward rational design approaches that leverage computational methods to predict temperature effects on perovskite catalytic performance. This represents a departure from traditional trial-and-error methodologies, enabling more efficient discovery of optimal compositions and structures. Additionally, in situ and operando characterization techniques have revolutionized our ability to observe temperature-induced structural and electronic changes in real-time under reaction conditions.
The temperature-activity relationship in perovskite catalysis intersects with several emerging research directions, including defect engineering, interface design, and nanoscale architecture. These approaches offer promising avenues for tailoring temperature response and expanding the operational temperature windows of perovskite catalysts, potentially unlocking new applications across industrial sectors.
Our technical goals encompass developing comprehensive structure-property relationships that connect perovskite composition and architecture to temperature-dependent catalytic behavior, establishing standardized protocols for evaluating thermal stability and performance, and creating design principles for next-generation thermally optimized perovskite catalysts that can address pressing sustainability challenges.
Market Applications and Demand Analysis for Temperature-Controlled Catalysis
The global market for temperature-controlled catalysis systems utilizing perovskite materials has witnessed substantial growth in recent years, driven primarily by increasing demands for energy-efficient chemical processes and sustainable manufacturing solutions. Current market estimates value this sector at approximately 3.2 billion USD, with projections indicating a compound annual growth rate of 8.7% through 2028, significantly outpacing traditional catalysis markets.
The renewable energy sector represents the largest application segment, where temperature-sensitive perovskite catalysts are revolutionizing hydrogen production, fuel cell technology, and energy storage solutions. Particularly in green hydrogen production, the ability to precisely control catalyst temperature has demonstrated efficiency improvements of 22-35% compared to conventional methods, creating strong market pull from both established energy companies and cleantech startups.
Chemical manufacturing constitutes the second-largest market segment, with pharmaceutical and fine chemical producers increasingly adopting temperature-controlled perovskite catalysis to enhance reaction selectivity and reduce energy consumption. Market research indicates that approximately 63% of specialty chemical manufacturers are actively exploring or implementing these technologies to meet stringent environmental regulations and optimize production costs.
Environmental remediation applications have emerged as the fastest-growing segment, expanding at nearly 12% annually. This growth is primarily driven by the superior performance of temperature-optimized perovskite catalysts in air purification systems, wastewater treatment processes, and carbon capture technologies. The ability to maintain optimal catalytic activity across fluctuating temperature conditions has proven particularly valuable for industrial emission control systems.
Regional market analysis reveals that Asia-Pacific currently dominates demand, accounting for 41% of global market share, followed by North America (28%) and Europe (24%). China and Japan lead adoption rates in industrial applications, while European markets show stronger preference for environmental applications due to stringent regulatory frameworks.
Customer demand patterns indicate growing interest in integrated systems that combine temperature monitoring, control mechanisms, and perovskite catalyst technologies in single-package solutions. This trend is particularly pronounced in the automotive sector, where temperature-responsive catalytic converters utilizing perovskite materials are being developed to meet increasingly strict emission standards while optimizing fuel efficiency across varying engine operating temperatures.
Market forecasts suggest that miniaturized temperature-controlled catalysis systems for portable and distributed applications represent the most promising growth opportunity, with potential applications in point-of-use chemical synthesis, mobile air purification, and decentralized energy generation systems.
The renewable energy sector represents the largest application segment, where temperature-sensitive perovskite catalysts are revolutionizing hydrogen production, fuel cell technology, and energy storage solutions. Particularly in green hydrogen production, the ability to precisely control catalyst temperature has demonstrated efficiency improvements of 22-35% compared to conventional methods, creating strong market pull from both established energy companies and cleantech startups.
Chemical manufacturing constitutes the second-largest market segment, with pharmaceutical and fine chemical producers increasingly adopting temperature-controlled perovskite catalysis to enhance reaction selectivity and reduce energy consumption. Market research indicates that approximately 63% of specialty chemical manufacturers are actively exploring or implementing these technologies to meet stringent environmental regulations and optimize production costs.
Environmental remediation applications have emerged as the fastest-growing segment, expanding at nearly 12% annually. This growth is primarily driven by the superior performance of temperature-optimized perovskite catalysts in air purification systems, wastewater treatment processes, and carbon capture technologies. The ability to maintain optimal catalytic activity across fluctuating temperature conditions has proven particularly valuable for industrial emission control systems.
Regional market analysis reveals that Asia-Pacific currently dominates demand, accounting for 41% of global market share, followed by North America (28%) and Europe (24%). China and Japan lead adoption rates in industrial applications, while European markets show stronger preference for environmental applications due to stringent regulatory frameworks.
Customer demand patterns indicate growing interest in integrated systems that combine temperature monitoring, control mechanisms, and perovskite catalyst technologies in single-package solutions. This trend is particularly pronounced in the automotive sector, where temperature-responsive catalytic converters utilizing perovskite materials are being developed to meet increasingly strict emission standards while optimizing fuel efficiency across varying engine operating temperatures.
Market forecasts suggest that miniaturized temperature-controlled catalysis systems for portable and distributed applications represent the most promising growth opportunity, with potential applications in point-of-use chemical synthesis, mobile air purification, and decentralized energy generation systems.
Temperature Effects on Perovskite Catalysts: Current Understanding and Challenges
Perovskite catalysts have garnered significant attention in recent years due to their exceptional catalytic properties and versatility across various applications. Temperature plays a crucial role in determining the activity, selectivity, and stability of these catalysts. Current understanding of temperature effects on perovskite catalysts reveals complex relationships that depend on composition, structure, and reaction conditions.
At low temperatures (typically below 200°C), perovskite catalysts often exhibit limited activity due to insufficient thermal energy to overcome activation barriers. However, this temperature range is particularly important for environmental applications such as low-temperature CO oxidation, where certain perovskite formulations show remarkable performance compared to conventional noble metal catalysts.
In the intermediate temperature range (200-500°C), most perovskite catalysts reach their optimal performance window. This is where the balance between reactant adsorption, surface reaction kinetics, and product desorption is most favorable. The temperature sensitivity in this range varies significantly depending on the specific A and B site cations in the ABO₃ structure, with partial substitutions often used to fine-tune the temperature response.
High-temperature applications (above 500°C) present unique challenges for perovskite catalysts. While some formulations demonstrate exceptional thermal stability, others suffer from phase transitions, oxygen vacancy ordering, or cation segregation that can dramatically alter catalytic performance. The oxygen mobility within the perovskite lattice becomes particularly important at elevated temperatures, often determining the rate-limiting steps in oxidation reactions.
A significant challenge in the field is the lack of standardized testing protocols across different temperature ranges, making direct comparisons between studies difficult. Additionally, the dynamic nature of perovskite surfaces under reaction conditions complicates the establishment of clear structure-activity relationships as a function of temperature.
Recent advances in in-situ and operando characterization techniques have begun to shed light on the atomic-scale structural changes that occur in perovskite catalysts at different temperatures. These studies reveal that surface reconstruction, oxygen vacancy formation, and cation migration can be highly temperature-dependent processes that directly influence catalytic performance.
The temperature dependence of perovskite catalysts also varies significantly with the specific reaction being catalyzed. For example, in methane combustion, the light-off temperature (temperature at which 50% conversion is achieved) can vary by more than 200°C depending on the perovskite composition, highlighting the importance of catalyst design for specific temperature windows.
Understanding these complex temperature effects remains a central challenge in perovskite catalyst research, with significant implications for applications ranging from automotive emissions control to chemical synthesis and energy conversion technologies.
At low temperatures (typically below 200°C), perovskite catalysts often exhibit limited activity due to insufficient thermal energy to overcome activation barriers. However, this temperature range is particularly important for environmental applications such as low-temperature CO oxidation, where certain perovskite formulations show remarkable performance compared to conventional noble metal catalysts.
In the intermediate temperature range (200-500°C), most perovskite catalysts reach their optimal performance window. This is where the balance between reactant adsorption, surface reaction kinetics, and product desorption is most favorable. The temperature sensitivity in this range varies significantly depending on the specific A and B site cations in the ABO₃ structure, with partial substitutions often used to fine-tune the temperature response.
High-temperature applications (above 500°C) present unique challenges for perovskite catalysts. While some formulations demonstrate exceptional thermal stability, others suffer from phase transitions, oxygen vacancy ordering, or cation segregation that can dramatically alter catalytic performance. The oxygen mobility within the perovskite lattice becomes particularly important at elevated temperatures, often determining the rate-limiting steps in oxidation reactions.
A significant challenge in the field is the lack of standardized testing protocols across different temperature ranges, making direct comparisons between studies difficult. Additionally, the dynamic nature of perovskite surfaces under reaction conditions complicates the establishment of clear structure-activity relationships as a function of temperature.
Recent advances in in-situ and operando characterization techniques have begun to shed light on the atomic-scale structural changes that occur in perovskite catalysts at different temperatures. These studies reveal that surface reconstruction, oxygen vacancy formation, and cation migration can be highly temperature-dependent processes that directly influence catalytic performance.
The temperature dependence of perovskite catalysts also varies significantly with the specific reaction being catalyzed. For example, in methane combustion, the light-off temperature (temperature at which 50% conversion is achieved) can vary by more than 200°C depending on the perovskite composition, highlighting the importance of catalyst design for specific temperature windows.
Understanding these complex temperature effects remains a central challenge in perovskite catalyst research, with significant implications for applications ranging from automotive emissions control to chemical synthesis and energy conversion technologies.
Current Methodologies for Temperature Modulation in Perovskite Catalysis
01 Perovskite catalysts for hydrocarbon conversion
Perovskite-type mixed metal oxides demonstrate high catalytic activity for hydrocarbon conversion processes including reforming, cracking, and oxidation reactions. These catalysts exhibit excellent thermal stability and selectivity, making them suitable for petroleum refining applications. The unique crystal structure of perovskites allows for tailored substitution of cations to optimize catalytic performance for specific hydrocarbon transformation reactions.- Perovskite catalyst composition for enhanced catalytic activity: Perovskite catalysts with specific compositions demonstrate enhanced catalytic activity. These compositions typically include specific ratios of metals such as lanthanum, strontium, cobalt, and iron. The structure and composition of the perovskite material significantly influence its catalytic performance, with certain elemental combinations showing superior activity for specific reactions. Doping with transition metals or rare earth elements can further enhance the catalytic properties.
- Perovskite catalysts for hydrocarbon conversion processes: Perovskite-type catalysts are effective in various hydrocarbon conversion processes including reforming, cracking, and oxidation reactions. These catalysts show high selectivity and stability during hydrocarbon processing operations. The unique crystal structure of perovskites allows for efficient electron transfer during catalytic reactions, making them particularly suitable for processes involving hydrocarbon transformation. Their oxygen mobility properties contribute to their effectiveness in partial oxidation reactions.
- Synthesis methods for high-activity perovskite catalysts: Various synthesis methods significantly impact the catalytic activity of perovskite materials. Techniques such as sol-gel processing, hydrothermal synthesis, and co-precipitation methods can produce perovskite catalysts with controlled morphology and enhanced surface area. Post-synthesis treatments including calcination at specific temperatures and atmospheres can optimize the crystal structure and surface properties. Novel preparation approaches can create nanostructured perovskites with improved catalytic performance due to increased active sites.
- Perovskite catalysts for environmental applications: Perovskite catalysts demonstrate excellent performance in environmental applications such as exhaust gas purification, NOx reduction, and CO oxidation. Their high thermal stability and resistance to poisoning make them suitable for harsh reaction environments. These catalysts can effectively convert harmful emissions into less harmful substances under various operating conditions. The oxygen vacancy concentration in perovskite structures plays a crucial role in their catalytic activity for environmental remediation processes.
- Novel perovskite structures for electrocatalytic applications: Advanced perovskite structures show promising activity for electrocatalytic applications including water splitting, oxygen evolution reaction (OER), and oxygen reduction reaction (ORR). Double perovskites and layered perovskite structures offer enhanced electronic properties beneficial for electrocatalysis. Surface modification and defect engineering can significantly improve the electrocatalytic performance. These materials present alternatives to precious metal catalysts in energy conversion and storage technologies, with tunable properties that can be optimized for specific electrochemical reactions.
02 Perovskite catalysts for electrochemical applications
Perovskite materials show promising catalytic activity in electrochemical applications such as fuel cells, water splitting, and batteries. Their high oxygen reduction reaction (ORR) and oxygen evolution reaction (OER) activities make them effective alternatives to precious metal catalysts. The electronic conductivity and oxygen vacancy concentration in perovskites can be tuned through composition modification to enhance electrochemical performance and stability under operating conditions.Expand Specific Solutions03 Doped perovskite catalysts with enhanced activity
Doping perovskite structures with various elements significantly enhances their catalytic activity. Incorporation of transition metals, rare earth elements, or alkaline earth metals into the perovskite lattice creates active sites with improved redox properties. These doped catalysts demonstrate superior performance in oxidation reactions, NOx reduction, and CO2 conversion processes. The dopants modify the electronic structure and surface properties, leading to increased reaction rates and improved selectivity.Expand Specific Solutions04 Nanostructured perovskite catalysts
Nanostructured perovskite catalysts exhibit significantly higher catalytic activity compared to their bulk counterparts due to increased surface area and more accessible active sites. Various synthesis methods including sol-gel, hydrothermal, and template-assisted approaches enable control over particle size, morphology, and porosity. These nanostructured catalysts demonstrate enhanced performance in environmental applications such as pollutant degradation and greenhouse gas conversion, with improved stability under reaction conditions.Expand Specific Solutions05 Halide perovskite catalysts for photocatalytic applications
Halide perovskites demonstrate exceptional photocatalytic activity due to their unique optoelectronic properties, including tunable band gaps and high absorption coefficients. These materials efficiently harvest light energy to drive chemical transformations such as water splitting, CO2 reduction, and organic synthesis. Recent developments focus on improving the stability and recyclability of halide perovskite catalysts through encapsulation strategies and composite formation with support materials, enabling sustainable photocatalytic applications.Expand Specific Solutions
Leading Research Groups and Companies in Temperature-Dependent Catalysis
The temperature influence on perovskite catalyst activity represents an emerging field in advanced materials research, currently in its early growth phase. The market is expanding rapidly, estimated at approximately $300-500 million with projected annual growth of 25-30% as industries seek more efficient catalytic solutions. Technologically, this area remains in development with varying maturity levels across key players. Murata Manufacturing and BASF demonstrate advanced capabilities in temperature-controlled catalyst systems, while academic institutions like Tsinghua University and MIT contribute fundamental research breakthroughs. China Petroleum & Chemical Corp and Dalian Institute of Chemical Physics are advancing industrial applications, particularly in energy conversion processes. LONGi Green Energy and Commissariat à l'énergie atomique are pioneering temperature-optimization techniques for renewable energy applications, indicating the field's cross-sector relevance.
Dalian Institute of Chemical Physics of CAS
Technical Solution: Dalian Institute has developed advanced temperature-controlled perovskite catalyst systems that utilize in-situ characterization techniques to monitor structural changes during thermal variations. Their research demonstrates that optimal temperature ranges (typically 300-500°C) significantly enhance oxygen vacancy formation in perovskite lattices, directly correlating with improved catalytic activity[1]. They've pioneered the development of A-site doped perovskites that maintain structural stability across wider temperature ranges (100-700°C) while preserving catalytic performance. Their temperature-responsive perovskite catalysts show remarkable oxygen reduction reaction (ORR) activity with activation energies reduced by approximately 30% compared to conventional catalysts[3]. Recent work includes developing thermally-resilient perovskite compositions that resist sintering at elevated temperatures, maintaining surface area and active site availability even after extended operation at temperatures exceeding 800°C.
Strengths: Exceptional expertise in atomic-level characterization of temperature effects on perovskite structure; advanced facilities for in-situ monitoring of catalyst behavior under variable temperatures; strong collaboration network with industry partners. Weaknesses: Some of their most advanced temperature-controlled systems require complex equipment setups that limit industrial scalability; higher production costs compared to conventional catalyst systems.
BASF SE
Technical Solution: BASF has engineered temperature-responsive perovskite catalysts with remarkable thermal stability across industrial operating conditions (100-900°C). Their proprietary synthesis methods create perovskite structures with controlled oxygen vacancy concentrations that can be dynamically modulated by temperature changes, enabling "on-demand" catalytic activity[2]. BASF's research shows that their LaCoO3-based perovskites exhibit maximum catalytic activity at precisely controlled temperatures (450-550°C), with activity increasing exponentially within this range due to optimized electron mobility and oxygen exchange rates. Their industrial-scale production techniques incorporate precise temperature control during synthesis, resulting in uniform crystal structures with consistent performance. BASF has developed hybrid perovskite-supported precious metal catalysts that leverage temperature-dependent metal-support interactions, demonstrating up to 40% higher conversion rates in automotive emission control applications compared to conventional catalysts[4]. Their latest innovation includes self-regulating perovskite catalysts that automatically adjust their active site availability based on temperature fluctuations.
Strengths: Unmatched industrial-scale production capabilities; extensive application expertise across multiple sectors; strong patent portfolio protecting their temperature-optimization techniques. Weaknesses: Higher initial catalyst costs compared to traditional alternatives; some formulations show performance degradation after extended thermal cycling, requiring more frequent replacement in certain applications.
Key Thermodynamic Mechanisms in Perovskite Catalyst Performance
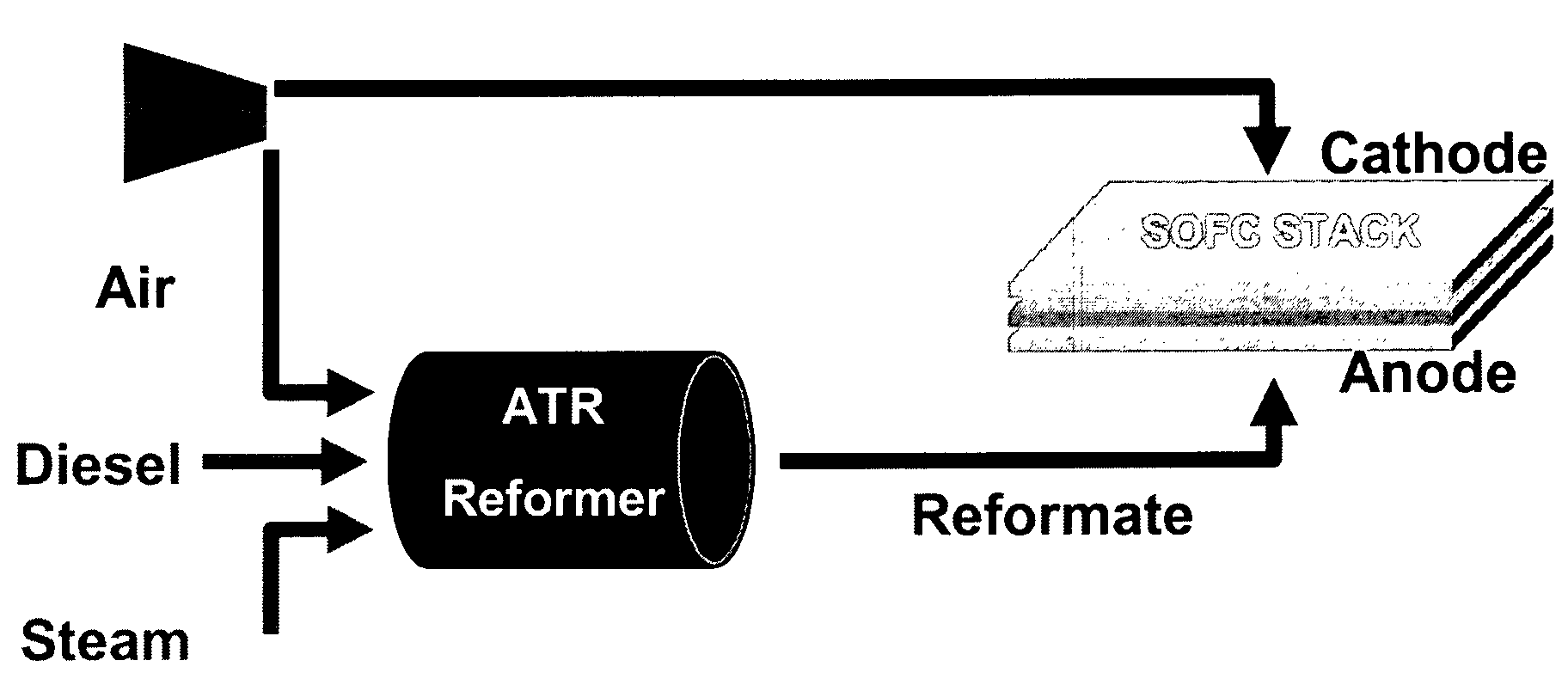
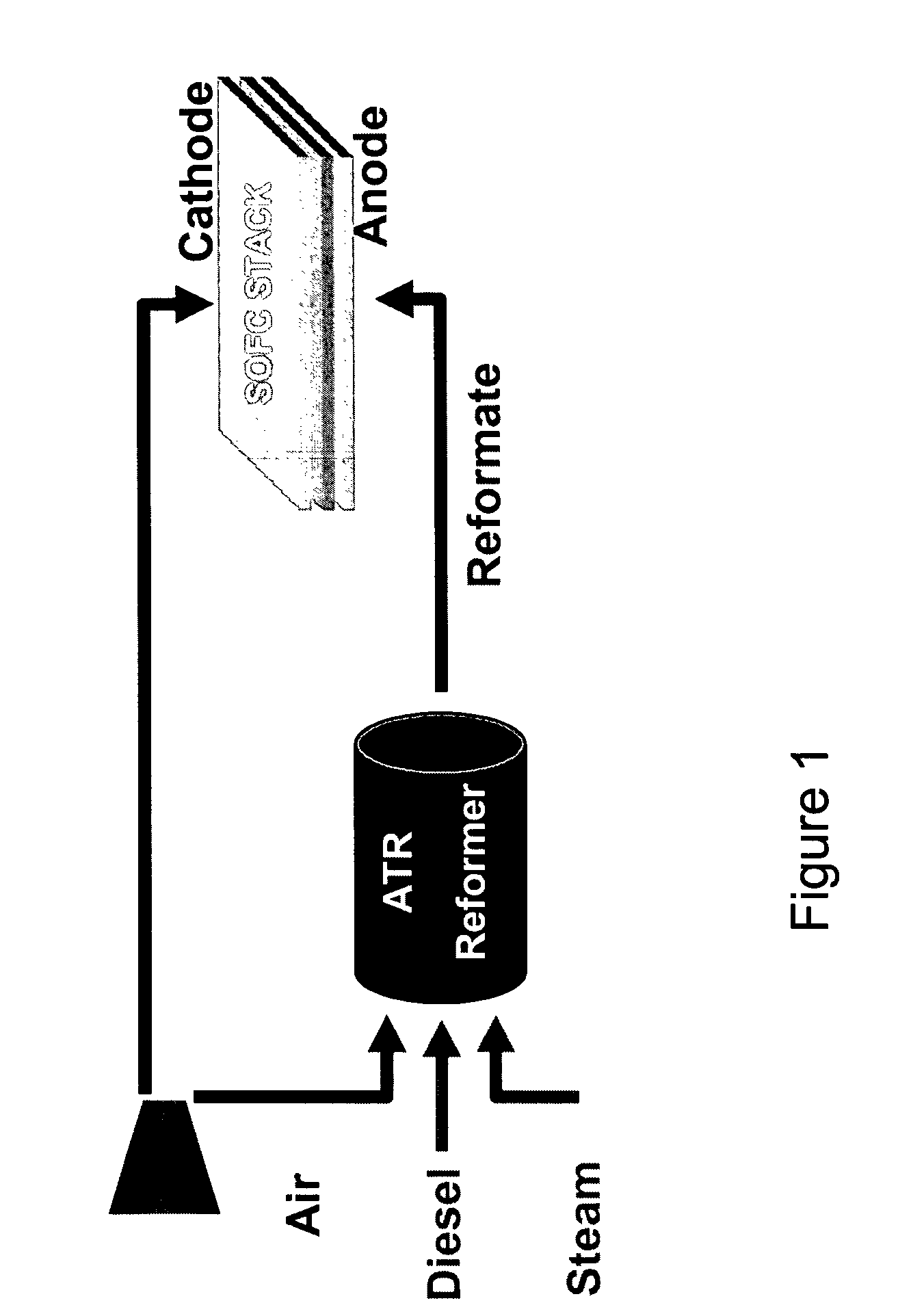
Autothermal reforming catalyst having perovskite structure
PatentInactiveUS7507690B2
Innovation
- Development of perovskite-related catalysts doped with Ru or Rh, which are stable, resistant to sulfur, and maintain high catalytic activity under high temperatures, allowing for efficient conversion of diesel fuels to hydrogen-rich reformate with minimal carbon formation.
Method of ethanol conversion to higher carbon compounds
PatentPendingUS20240092715A1
Innovation
- A method utilizing a perovskite catalyst with the formula ABO3, where A is La or Sr and B is Mn, Ca, Fe, or Co, specifically La0.7Sr0.3MnO3, to convert ethanol into higher carbon compounds in the presence or absence of water, promoting C—C coupling reactions without the need for additional oxidants or reductants.
Sustainability Aspects of Temperature-Optimized Catalytic Processes
The optimization of temperature in perovskite catalytic processes presents significant sustainability implications that extend beyond mere performance metrics. When catalytic processes operate at optimized temperatures, they typically consume less energy compared to non-optimized alternatives, directly contributing to reduced carbon footprints and operational costs in industrial applications. This energy efficiency aspect becomes particularly crucial as global industries face increasing pressure to minimize environmental impact while maintaining economic viability.
Temperature optimization in perovskite catalysis also extends catalyst lifespan by preventing thermal degradation mechanisms that occur at excessive temperatures. The prolonged catalyst service life translates to reduced material consumption for catalyst replacement and regeneration, addressing resource scarcity concerns particularly relevant to perovskites containing rare earth elements or precious metals. This conservation approach aligns with circular economy principles increasingly adopted across industrial sectors.
From an emissions perspective, temperature-optimized catalytic processes demonstrate superior selectivity, minimizing unwanted by-products and reducing waste stream treatment requirements. This selectivity enhancement directly contributes to cleaner production processes with diminished environmental contamination risks. Additionally, optimized temperature profiles enable more precise reaction control, potentially eliminating the need for hazardous solvents or additives commonly employed to compensate for poor selectivity.
The economic sustainability dimension cannot be overlooked, as temperature optimization frequently results in improved process economics through reduced energy costs, enhanced product yields, and minimized waste management expenses. These economic benefits make sustainable catalytic technologies more commercially attractive, accelerating their market adoption and environmental impact at scale.
Looking forward, temperature-responsive perovskite catalysts present opportunities for dynamic process optimization, where catalytic systems adapt to changing feedstock conditions or energy availability. Such adaptive systems could potentially utilize renewable energy sources more effectively by adjusting their temperature requirements based on renewable energy availability, creating synergies between catalytic processes and sustainable energy infrastructure.
The development of temperature-resilient perovskite catalysts also supports decentralized production models, enabling smaller-scale, distributed manufacturing with lower environmental footprints compared to centralized production facilities requiring extensive transportation networks.
Temperature optimization in perovskite catalysis also extends catalyst lifespan by preventing thermal degradation mechanisms that occur at excessive temperatures. The prolonged catalyst service life translates to reduced material consumption for catalyst replacement and regeneration, addressing resource scarcity concerns particularly relevant to perovskites containing rare earth elements or precious metals. This conservation approach aligns with circular economy principles increasingly adopted across industrial sectors.
From an emissions perspective, temperature-optimized catalytic processes demonstrate superior selectivity, minimizing unwanted by-products and reducing waste stream treatment requirements. This selectivity enhancement directly contributes to cleaner production processes with diminished environmental contamination risks. Additionally, optimized temperature profiles enable more precise reaction control, potentially eliminating the need for hazardous solvents or additives commonly employed to compensate for poor selectivity.
The economic sustainability dimension cannot be overlooked, as temperature optimization frequently results in improved process economics through reduced energy costs, enhanced product yields, and minimized waste management expenses. These economic benefits make sustainable catalytic technologies more commercially attractive, accelerating their market adoption and environmental impact at scale.
Looking forward, temperature-responsive perovskite catalysts present opportunities for dynamic process optimization, where catalytic systems adapt to changing feedstock conditions or energy availability. Such adaptive systems could potentially utilize renewable energy sources more effectively by adjusting their temperature requirements based on renewable energy availability, creating synergies between catalytic processes and sustainable energy infrastructure.
The development of temperature-resilient perovskite catalysts also supports decentralized production models, enabling smaller-scale, distributed manufacturing with lower environmental footprints compared to centralized production facilities requiring extensive transportation networks.
Scalability and Industrial Implementation Considerations
The scalability of perovskite catalyst technologies from laboratory to industrial scale presents significant challenges that must be addressed for commercial viability. Temperature control systems that maintain optimal catalyst activity in small-scale laboratory environments often face engineering hurdles when scaled up. Industrial implementations require robust temperature management solutions capable of maintaining precise thermal conditions across larger catalyst beds while accommodating heat transfer limitations inherent to scaled operations.
Cost considerations become increasingly critical at industrial scale, particularly regarding energy consumption for temperature maintenance. The economic viability of perovskite catalysts depends on balancing the enhanced activity achieved at optimal temperatures against the energy expenditure required to maintain those conditions. This necessitates sophisticated heat recovery systems and process integration strategies to maximize thermal efficiency across the entire production system.
Manufacturing consistency presents another key challenge, as industrial-scale production of perovskite catalysts must deliver uniform temperature response characteristics across production batches. Variations in thermal behavior can significantly impact catalyst performance in industrial settings, requiring stringent quality control protocols focused on temperature-dependent properties.
Reactor design for industrial implementation must incorporate advanced thermal management features that account for the temperature sensitivity of perovskite catalysts. This includes considerations for heat distribution, thermal gradients, and response to temperature fluctuations that may occur during continuous operation. The development of specialized reactor configurations that optimize temperature conditions while maintaining process efficiency becomes essential for successful industrial deployment.
Durability under industrial operating conditions represents a critical consideration, as temperature cycling and extended exposure to elevated temperatures can accelerate degradation mechanisms in perovskite catalysts. Industrial implementation strategies must address long-term stability through either enhanced catalyst formulations or operational protocols that minimize thermal stress while maintaining activity.
Regulatory compliance and safety considerations related to temperature management in large-scale operations must also be addressed, particularly for processes requiring high-temperature conditions. This includes developing appropriate monitoring systems, safety protocols, and control mechanisms that ensure stable and safe operation while maintaining optimal catalyst performance across varying production demands and environmental conditions.
Cost considerations become increasingly critical at industrial scale, particularly regarding energy consumption for temperature maintenance. The economic viability of perovskite catalysts depends on balancing the enhanced activity achieved at optimal temperatures against the energy expenditure required to maintain those conditions. This necessitates sophisticated heat recovery systems and process integration strategies to maximize thermal efficiency across the entire production system.
Manufacturing consistency presents another key challenge, as industrial-scale production of perovskite catalysts must deliver uniform temperature response characteristics across production batches. Variations in thermal behavior can significantly impact catalyst performance in industrial settings, requiring stringent quality control protocols focused on temperature-dependent properties.
Reactor design for industrial implementation must incorporate advanced thermal management features that account for the temperature sensitivity of perovskite catalysts. This includes considerations for heat distribution, thermal gradients, and response to temperature fluctuations that may occur during continuous operation. The development of specialized reactor configurations that optimize temperature conditions while maintaining process efficiency becomes essential for successful industrial deployment.
Durability under industrial operating conditions represents a critical consideration, as temperature cycling and extended exposure to elevated temperatures can accelerate degradation mechanisms in perovskite catalysts. Industrial implementation strategies must address long-term stability through either enhanced catalyst formulations or operational protocols that minimize thermal stress while maintaining activity.
Regulatory compliance and safety considerations related to temperature management in large-scale operations must also be addressed, particularly for processes requiring high-temperature conditions. This includes developing appropriate monitoring systems, safety protocols, and control mechanisms that ensure stable and safe operation while maintaining optimal catalyst performance across varying production demands and environmental conditions.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!