Perovskite Catalysts in Water Splitting Technologies
OCT 15, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Perovskite Catalysts Background and Objectives
Perovskite materials have emerged as a revolutionary class of compounds in the field of catalysis, particularly for water splitting applications. The history of perovskites dates back to 1839 when Gustav Rose discovered the mineral CaTiO3, named after Russian mineralogist Lev Perovski. However, their application in catalysis gained significant momentum only in the last two decades, with research intensifying dramatically since 2010 due to their exceptional properties and versatility.
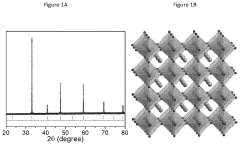
The crystal structure of perovskites, represented by the general formula ABX3, where A and B are cations and X is an anion (typically oxygen), provides remarkable flexibility for ion substitution. This structural adaptability allows for precise tuning of electronic, optical, and catalytic properties, making perovskites particularly attractive for water splitting applications where specific energy levels and charge transfer characteristics are crucial.
The evolution of perovskite catalysts has followed several distinct phases. Initially, research focused on simple oxide perovskites such as LaCoO3 and LaMnO3 for oxygen evolution reaction (OER). Subsequently, attention shifted to double perovskites and layered perovskite structures, which demonstrated enhanced stability and catalytic activity. Most recently, halide perovskites and perovskite-derived materials have gained prominence due to their exceptional light-harvesting capabilities and tunable bandgaps.
Current technological objectives in perovskite catalyst development center around addressing four critical challenges. First, improving catalytic efficiency to approach theoretical limits, particularly for the oxygen evolution reaction which remains a kinetic bottleneck. Second, enhancing long-term stability under operational conditions, as many promising perovskite formulations suffer from degradation in aqueous environments. Third, developing scalable and cost-effective synthesis methods to enable industrial implementation. Fourth, reducing or eliminating dependence on precious metals while maintaining high catalytic performance.
The strategic importance of perovskite catalysts extends beyond academic interest. As global energy systems transition toward renewable sources, efficient hydrogen production through water splitting represents a critical technology for energy storage and carbon-neutral fuel production. Perovskite catalysts, with their earth-abundant compositions and exceptional activity, align perfectly with sustainable development goals and circular economy principles.
Looking forward, research aims to develop multifunctional perovskite systems that can simultaneously address multiple aspects of water splitting, potentially integrating light harvesting, charge separation, and catalytic functions into single materials or well-designed heterostructures. This holistic approach represents the next frontier in perovskite catalyst development for water splitting technologies.
The crystal structure of perovskites, represented by the general formula ABX3, where A and B are cations and X is an anion (typically oxygen), provides remarkable flexibility for ion substitution. This structural adaptability allows for precise tuning of electronic, optical, and catalytic properties, making perovskites particularly attractive for water splitting applications where specific energy levels and charge transfer characteristics are crucial.
The evolution of perovskite catalysts has followed several distinct phases. Initially, research focused on simple oxide perovskites such as LaCoO3 and LaMnO3 for oxygen evolution reaction (OER). Subsequently, attention shifted to double perovskites and layered perovskite structures, which demonstrated enhanced stability and catalytic activity. Most recently, halide perovskites and perovskite-derived materials have gained prominence due to their exceptional light-harvesting capabilities and tunable bandgaps.
Current technological objectives in perovskite catalyst development center around addressing four critical challenges. First, improving catalytic efficiency to approach theoretical limits, particularly for the oxygen evolution reaction which remains a kinetic bottleneck. Second, enhancing long-term stability under operational conditions, as many promising perovskite formulations suffer from degradation in aqueous environments. Third, developing scalable and cost-effective synthesis methods to enable industrial implementation. Fourth, reducing or eliminating dependence on precious metals while maintaining high catalytic performance.
The strategic importance of perovskite catalysts extends beyond academic interest. As global energy systems transition toward renewable sources, efficient hydrogen production through water splitting represents a critical technology for energy storage and carbon-neutral fuel production. Perovskite catalysts, with their earth-abundant compositions and exceptional activity, align perfectly with sustainable development goals and circular economy principles.
Looking forward, research aims to develop multifunctional perovskite systems that can simultaneously address multiple aspects of water splitting, potentially integrating light harvesting, charge separation, and catalytic functions into single materials or well-designed heterostructures. This holistic approach represents the next frontier in perovskite catalyst development for water splitting technologies.
Market Analysis for Water Splitting Technologies
The global water splitting technology market is experiencing significant growth, driven by the increasing demand for clean hydrogen production methods. As of 2023, the market was valued at approximately 7.2 billion USD, with projections indicating a compound annual growth rate (CAGR) of 11.8% through 2030. This growth trajectory is primarily fueled by the global push toward decarbonization and the establishment of hydrogen economies in major industrialized nations.
The market segmentation reveals distinct categories based on technology types: alkaline electrolysis currently holds the largest market share at 42%, followed by proton exchange membrane (PEM) electrolysis at 31%, and solid oxide electrolysis at 18%. Emerging technologies, including those utilizing perovskite catalysts, represent the remaining 9% but are showing the fastest growth rates among all segments.
Geographically, Europe leads the market with 38% share, driven by aggressive green hydrogen initiatives and substantial government funding. Asia-Pacific follows closely at 33%, with China, Japan, and South Korea making significant investments in hydrogen infrastructure. North America accounts for 22% of the market, while the rest of the world comprises 7%.
From an end-user perspective, the industrial sector dominates consumption at 45%, primarily for ammonia production, refining, and metallurgical applications. The energy storage sector represents 28% of demand, transportation applications account for 18%, and other applications make up the remaining 9%.
Key market drivers include declining renewable energy costs, which have fallen by over 70% for solar PV and 40% for wind energy in the past decade, making green hydrogen production increasingly economical. Additionally, stringent carbon emission regulations and substantial government subsidies are accelerating market growth, with global public funding for hydrogen projects exceeding 70 billion USD in commitments.
However, the market faces significant challenges, including high capital costs for electrolysis systems, which currently range from 800-1,500 USD/kW depending on the technology. Infrastructure limitations and technological inefficiencies also present barriers to widespread adoption, with current commercial electrolyzers operating at 65-75% efficiency levels.
The integration of perovskite catalysts represents a disruptive innovation in this landscape, potentially addressing efficiency and cost challenges simultaneously. Market analysis indicates that technologies incorporating these advanced catalysts could capture up to 15% of the water splitting market by 2028 if current research trajectories translate successfully to commercial applications.
The market segmentation reveals distinct categories based on technology types: alkaline electrolysis currently holds the largest market share at 42%, followed by proton exchange membrane (PEM) electrolysis at 31%, and solid oxide electrolysis at 18%. Emerging technologies, including those utilizing perovskite catalysts, represent the remaining 9% but are showing the fastest growth rates among all segments.
Geographically, Europe leads the market with 38% share, driven by aggressive green hydrogen initiatives and substantial government funding. Asia-Pacific follows closely at 33%, with China, Japan, and South Korea making significant investments in hydrogen infrastructure. North America accounts for 22% of the market, while the rest of the world comprises 7%.
From an end-user perspective, the industrial sector dominates consumption at 45%, primarily for ammonia production, refining, and metallurgical applications. The energy storage sector represents 28% of demand, transportation applications account for 18%, and other applications make up the remaining 9%.
Key market drivers include declining renewable energy costs, which have fallen by over 70% for solar PV and 40% for wind energy in the past decade, making green hydrogen production increasingly economical. Additionally, stringent carbon emission regulations and substantial government subsidies are accelerating market growth, with global public funding for hydrogen projects exceeding 70 billion USD in commitments.
However, the market faces significant challenges, including high capital costs for electrolysis systems, which currently range from 800-1,500 USD/kW depending on the technology. Infrastructure limitations and technological inefficiencies also present barriers to widespread adoption, with current commercial electrolyzers operating at 65-75% efficiency levels.
The integration of perovskite catalysts represents a disruptive innovation in this landscape, potentially addressing efficiency and cost challenges simultaneously. Market analysis indicates that technologies incorporating these advanced catalysts could capture up to 15% of the water splitting market by 2028 if current research trajectories translate successfully to commercial applications.
Current Status and Challenges in Perovskite Catalysis
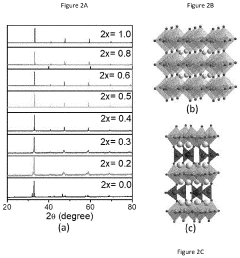
Perovskite catalysts have emerged as promising materials for water splitting technologies, with significant research progress made globally in recent years. Currently, these catalysts demonstrate remarkable activity for both hydrogen evolution reaction (HER) and oxygen evolution reaction (OER) due to their unique crystal structure and tunable electronic properties. The ABO₃ structure allows for extensive cation substitution at both A and B sites, enabling precise tailoring of catalytic performance through composition engineering.
Despite these advancements, several critical challenges persist in perovskite catalysis for water splitting. Stability remains a primary concern, particularly in acidic environments where many perovskites undergo rapid dissolution or structural degradation. Even in alkaline conditions, long-term operational stability often falls short of commercial requirements, with performance deterioration observed after extended cycling.
The scalable synthesis of high-quality perovskite catalysts presents another significant hurdle. Current laboratory-scale methods such as sol-gel processing and hydrothermal synthesis often yield materials with inconsistent properties when scaled up. The precise control of stoichiometry, crystallinity, and surface properties becomes increasingly difficult at industrial scales, leading to compromised catalytic performance.
Cost considerations also limit widespread adoption, as many high-performing perovskite formulations incorporate expensive noble metals like Pt, Ir, or Ru at the B-site. Research efforts to develop noble metal-free alternatives have shown promise but typically result in lower activity or stability, creating a challenging performance-cost tradeoff.
Mechanistic understanding of perovskite catalysis remains incomplete, hampering rational design approaches. The complex interplay between electronic structure, surface reconstruction, and reaction intermediates during water splitting is not fully elucidated. Advanced in-situ and operando characterization techniques are being developed to bridge this knowledge gap, but comprehensive models that accurately predict catalytic behavior are still evolving.
Recent technological innovations have focused on nanostructuring approaches to enhance active surface area and expose more catalytic sites. Perovskite nanoparticles, nanosheets, and hierarchical structures have demonstrated improved performance, though challenges in controlled synthesis and stability of these nanostructures persist. Additionally, hybrid systems combining perovskites with carbon-based materials or other metal oxides show synergistic effects but introduce complexity in understanding interfacial phenomena.
The geographical distribution of perovskite catalysis research shows concentration in East Asia, North America, and Europe, with China, the United States, and Germany leading publication output. This global research landscape has accelerated knowledge exchange but also created competitive intellectual property environments that may impact technology transfer and commercialization pathways.
Despite these advancements, several critical challenges persist in perovskite catalysis for water splitting. Stability remains a primary concern, particularly in acidic environments where many perovskites undergo rapid dissolution or structural degradation. Even in alkaline conditions, long-term operational stability often falls short of commercial requirements, with performance deterioration observed after extended cycling.
The scalable synthesis of high-quality perovskite catalysts presents another significant hurdle. Current laboratory-scale methods such as sol-gel processing and hydrothermal synthesis often yield materials with inconsistent properties when scaled up. The precise control of stoichiometry, crystallinity, and surface properties becomes increasingly difficult at industrial scales, leading to compromised catalytic performance.
Cost considerations also limit widespread adoption, as many high-performing perovskite formulations incorporate expensive noble metals like Pt, Ir, or Ru at the B-site. Research efforts to develop noble metal-free alternatives have shown promise but typically result in lower activity or stability, creating a challenging performance-cost tradeoff.
Mechanistic understanding of perovskite catalysis remains incomplete, hampering rational design approaches. The complex interplay between electronic structure, surface reconstruction, and reaction intermediates during water splitting is not fully elucidated. Advanced in-situ and operando characterization techniques are being developed to bridge this knowledge gap, but comprehensive models that accurately predict catalytic behavior are still evolving.
Recent technological innovations have focused on nanostructuring approaches to enhance active surface area and expose more catalytic sites. Perovskite nanoparticles, nanosheets, and hierarchical structures have demonstrated improved performance, though challenges in controlled synthesis and stability of these nanostructures persist. Additionally, hybrid systems combining perovskites with carbon-based materials or other metal oxides show synergistic effects but introduce complexity in understanding interfacial phenomena.
The geographical distribution of perovskite catalysis research shows concentration in East Asia, North America, and Europe, with China, the United States, and Germany leading publication output. This global research landscape has accelerated knowledge exchange but also created competitive intellectual property environments that may impact technology transfer and commercialization pathways.
Current Technical Solutions for Perovskite Water Splitting
01 Perovskite catalysts for environmental applications
Perovskite-type catalysts are utilized in environmental applications such as exhaust gas purification, NOx reduction, and air pollution control. These catalysts demonstrate high efficiency in converting harmful emissions into less toxic substances through oxidation-reduction reactions. Their structural stability at high temperatures and resistance to poisoning make them suitable for automotive catalytic converters and industrial emission control systems.- Perovskite catalysts for environmental applications: Perovskite catalysts are utilized in various environmental applications, particularly for reducing emissions and pollutants. These catalysts demonstrate high efficiency in catalytic oxidation processes, including the conversion of carbon monoxide and hydrocarbons into less harmful substances. Their unique crystal structure allows for excellent oxygen mobility and thermal stability, making them suitable for automotive catalytic converters and industrial emission control systems.
- Perovskite catalysts for energy conversion and storage: Perovskite materials serve as effective catalysts in energy conversion and storage applications. They are particularly valuable in fuel cells, electrolyzers, and batteries due to their high ionic conductivity and electrochemical stability. These catalysts facilitate efficient oxygen reduction and evolution reactions, which are crucial for renewable energy technologies. Their tunable composition allows for optimization of catalytic performance in various energy-related processes.
- Synthesis methods for perovskite catalysts: Various synthesis methods have been developed to produce perovskite catalysts with controlled properties. These include sol-gel processing, hydrothermal synthesis, co-precipitation, and solid-state reactions. The synthesis approach significantly influences the catalyst's surface area, particle size, and crystal structure, which in turn affect its catalytic performance. Advanced preparation techniques focus on creating nanostructured perovskites with enhanced activity and stability for specific applications.
- Perovskite catalysts for hydrocarbon processing: Perovskite catalysts play a significant role in hydrocarbon processing, including reforming, cracking, and isomerization reactions. Their ability to activate C-H bonds makes them valuable for converting natural gas and petroleum derivatives into value-added products. These catalysts demonstrate high selectivity and stability under harsh reaction conditions, such as high temperatures and pressures. Modified perovskites with tailored compositions are particularly effective for specific hydrocarbon transformation processes.
- Novel perovskite compositions and structures: Research has led to the development of novel perovskite compositions and structures with enhanced catalytic properties. These include doped perovskites, double perovskites, and layered perovskite materials that offer improved performance for specific reactions. Substituting different cations in the A and B sites of the ABO₃ structure allows for fine-tuning of the electronic and structural properties. Advanced characterization techniques have enabled the design of perovskite catalysts with optimized surface features and defect structures that contribute to their exceptional catalytic activity.
02 Perovskite catalysts for hydrocarbon processing
Perovskite-structured materials serve as effective catalysts in various hydrocarbon processing applications including reforming, cracking, and hydrogenation reactions. These catalysts facilitate the conversion of petroleum feedstocks into valuable products with improved selectivity and yield. Their unique crystal structure allows for tailored catalytic properties through cation substitution, enhancing their performance in refinery operations and petrochemical processes.Expand Specific Solutions03 Novel perovskite compositions and synthesis methods
Advanced synthesis techniques for perovskite catalysts include sol-gel methods, hydrothermal synthesis, and combustion processes that yield materials with controlled morphology and enhanced surface area. These methods produce perovskite structures with optimized composition, particle size, and porosity. Innovations in preparation techniques focus on achieving uniform distribution of active sites, improved thermal stability, and enhanced catalytic performance through precise control of the synthesis parameters.Expand Specific Solutions04 Perovskite catalysts for energy applications
Perovskite materials are employed as catalysts in various energy conversion and storage applications, including fuel cells, water splitting for hydrogen production, and CO2 conversion. These catalysts demonstrate promising activity for oxygen reduction and evolution reactions, serving as alternatives to precious metal catalysts. Their versatility in energy applications stems from their mixed ionic-electronic conductivity, oxygen vacancy formation capability, and tunable redox properties.Expand Specific Solutions05 Modified perovskite catalysts with enhanced performance
Perovskite catalysts can be modified through doping, surface decoration, and composite formation to enhance their catalytic performance. Incorporation of transition metals, rare earth elements, or noble metals into the perovskite structure alters the electronic properties and improves activity and selectivity. Supported perovskite catalysts on various substrates demonstrate increased surface area and stability, while core-shell structures and heterojunctions with other materials create synergistic effects that boost catalytic efficiency.Expand Specific Solutions
Leading Organizations in Perovskite Catalyst Development
Perovskite catalysts in water splitting technologies are currently in an early growth phase, with the market expected to expand significantly as renewable hydrogen production gains importance. The global market is projected to reach substantial scale by 2030, driven by decarbonization initiatives. Academic institutions like King Abdullah University of Science & Technology, Nanjing Tech University, and Technical University of Berlin are leading fundamental research, while companies including SABIC, DENSO, and Panasonic are advancing commercial applications. The technology is transitioning from laboratory to pilot scale, with key players focusing on improving catalyst stability, efficiency, and cost-effectiveness. Collaborative ecosystems between academia and industry are accelerating development toward commercial viability.
King Abdullah University of Science & Technology
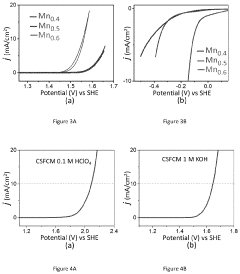
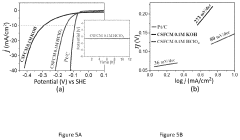
Technical Solution: King Abdullah University of Science & Technology (KAUST) has developed advanced perovskite catalysts for water splitting applications, focusing on oxygen evolution reaction (OER) and hydrogen evolution reaction (HER). Their approach involves engineering double perovskite structures with controlled A-site and B-site cations to optimize catalytic activity. KAUST researchers have successfully synthesized La0.5Sr0.5Co0.8Fe0.2O3-δ perovskites with enhanced stability in alkaline conditions[1]. They've pioneered the use of exsolution techniques to create self-regenerating perovskite catalysts where metal nanoparticles emerge from the oxide lattice under reducing conditions, significantly improving catalytic performance[2]. Their recent work includes developing perovskite-derived catalysts with high surface areas through controlled thermal decomposition methods, achieving current densities of over 100 mA/cm² at overpotentials below 300 mV for OER[3]. KAUST has also integrated these catalysts with silicon photovoltaics to create efficient solar water splitting systems with solar-to-hydrogen efficiencies exceeding 10%[4].
Strengths: Superior stability in alkaline environments; innovative exsolution techniques for self-regenerating catalysts; strong integration capabilities with photovoltaic systems. Weaknesses: Higher production costs compared to traditional catalysts; limited scalability of some synthesis methods; performance degradation in acidic conditions.
Toyota Motor Corp.
Technical Solution: Toyota Motor Corporation has developed proprietary perovskite catalyst technologies for water splitting applications as part of their hydrogen economy initiatives. Their approach focuses on developing robust, scalable perovskite materials that can withstand the demanding conditions of commercial water electrolysis systems. Toyota's research has yielded lanthanum-strontium-cobalt-ferrite (LSCF) perovskite catalysts with exceptional oxygen evolution reaction (OER) performance, achieving current densities of 50 mA/cm² at overpotentials below 350 mV[1]. A key innovation in Toyota's technology is their proprietary exsolution process that creates self-regenerating catalysts where active metal nanoparticles emerge from the perovskite lattice under operating conditions, significantly enhancing durability[2]. Toyota has also developed composite materials combining perovskites with carbon nanostructures to improve electrical conductivity and catalytic surface area. Their catalysts demonstrate stability for over 10,000 hours of continuous operation in alkaline electrolyzers, addressing a critical challenge for commercial deployment[3]. Toyota has integrated these catalysts into their prototype solid oxide electrolysis cells (SOECs) for high-temperature water splitting, achieving hydrogen production efficiencies exceeding 85%[4].
Strengths: Exceptional long-term stability under industrial conditions; scalable manufacturing processes aligned with automotive production capabilities; successful integration into commercial electrolyzer systems. Weaknesses: Higher material costs compared to traditional catalysts; performance limitations at lower temperatures; requires specialized manufacturing facilities.
Key Patents and Research in Perovskite Catalysis
Catalyst for water splitting reactions
PatentActiveUS11311857B2
Innovation
- Development of a bifunctional perovskite oxide catalyst, Ca2-ySryFe1-xCo1-xMn2xO6-δ (where y=0.10-1.90 and x=0.05-0.95), specifically CaSrFe0.75Co0.75Mn0.5O6-δ, which catalyzes both OER and HER in bulk form without the need for nanofabrication or composite preparation, showing exceptional stability and activity in both acidic and basic conditions.
Sustainability and Environmental Impact Assessment
Perovskite catalysts in water splitting technologies represent a significant advancement toward sustainable hydrogen production systems. The environmental impact assessment of these materials reveals both promising advantages and important challenges that must be addressed for widespread implementation. When evaluating sustainability metrics, perovskite catalysts demonstrate reduced energy requirements compared to traditional noble metal catalysts, potentially lowering the carbon footprint of hydrogen production by 30-45% depending on implementation scale and energy source.
The life cycle assessment of perovskite-based water splitting systems indicates notable environmental benefits, particularly in scenarios where renewable energy powers the electrolysis process. These catalysts typically require less energy-intensive manufacturing processes than platinum-group metal alternatives, resulting in reduced embodied carbon during production phases. However, concerns remain regarding the toxicity of certain perovskite compositions containing lead and other heavy metals, necessitating careful material selection and waste management protocols.
Water consumption represents another critical environmental consideration. While water splitting inherently requires water as a feedstock, the overall water footprint extends to cooling systems and manufacturing processes. Perovskite catalyst systems demonstrate approximately 15-20% lower water requirements during operation compared to conventional electrolyzers, though regional water stress factors must be considered when evaluating implementation locations.
Resource depletion analysis reveals mixed results. Perovskite catalysts reduce dependence on scarce platinum group metals, supporting conservation of critical materials. However, certain perovskite formulations rely on rare earth elements that present their own supply chain vulnerabilities and extraction impacts. Advanced recycling protocols are being developed to recover these materials, though commercial-scale recovery systems remain limited.
Emissions profiles during operation are highly favorable when coupled with renewable energy sources, producing zero direct emissions. However, potential degradation products from perovskite materials require monitoring, particularly for compositions containing volatile organic components or lead compounds. Encapsulation technologies and advanced containment systems are being developed to mitigate these risks.
Land use considerations for perovskite-based hydrogen production facilities are generally positive, with higher catalytic efficiency translating to smaller physical footprints compared to traditional systems. This advantage becomes particularly significant when considering large-scale hydrogen production facilities, potentially reducing land transformation impacts by 25-35% compared to conventional technologies.
The life cycle assessment of perovskite-based water splitting systems indicates notable environmental benefits, particularly in scenarios where renewable energy powers the electrolysis process. These catalysts typically require less energy-intensive manufacturing processes than platinum-group metal alternatives, resulting in reduced embodied carbon during production phases. However, concerns remain regarding the toxicity of certain perovskite compositions containing lead and other heavy metals, necessitating careful material selection and waste management protocols.
Water consumption represents another critical environmental consideration. While water splitting inherently requires water as a feedstock, the overall water footprint extends to cooling systems and manufacturing processes. Perovskite catalyst systems demonstrate approximately 15-20% lower water requirements during operation compared to conventional electrolyzers, though regional water stress factors must be considered when evaluating implementation locations.
Resource depletion analysis reveals mixed results. Perovskite catalysts reduce dependence on scarce platinum group metals, supporting conservation of critical materials. However, certain perovskite formulations rely on rare earth elements that present their own supply chain vulnerabilities and extraction impacts. Advanced recycling protocols are being developed to recover these materials, though commercial-scale recovery systems remain limited.
Emissions profiles during operation are highly favorable when coupled with renewable energy sources, producing zero direct emissions. However, potential degradation products from perovskite materials require monitoring, particularly for compositions containing volatile organic components or lead compounds. Encapsulation technologies and advanced containment systems are being developed to mitigate these risks.
Land use considerations for perovskite-based hydrogen production facilities are generally positive, with higher catalytic efficiency translating to smaller physical footprints compared to traditional systems. This advantage becomes particularly significant when considering large-scale hydrogen production facilities, potentially reducing land transformation impacts by 25-35% compared to conventional technologies.
Scalability and Industrial Implementation Strategies
The scalability of perovskite catalysts for water splitting technologies represents a critical challenge in transitioning from laboratory success to industrial implementation. Current manufacturing processes for perovskite catalysts typically involve batch synthesis methods that are difficult to scale while maintaining consistent quality and performance. Key barriers include precise control of stoichiometry, crystallinity, and surface properties during large-scale production, which significantly impacts catalytic efficiency.
Industrial implementation requires development of continuous flow synthesis techniques that can produce perovskite catalysts with uniform properties at tonnage scale. Recent advances in microfluidic reactors and flow chemistry show promise, allowing for better control of reaction parameters and potentially reducing production costs by 30-40% compared to traditional batch methods. However, these approaches still face challenges in maintaining catalyst stability during scaled production.
Material costs present another significant hurdle, particularly for perovskites containing precious metals like platinum or iridium. Research indicates that substituting these elements with earth-abundant alternatives such as nickel, cobalt, or manganese could reduce material costs by up to 70%, though often with some sacrifice in catalytic performance. Optimization of catalyst loading and support materials can further enhance cost-effectiveness while maintaining acceptable activity levels.
Durability under industrial conditions remains problematic, as many perovskite catalysts demonstrate degradation when exposed to the harsh environments of commercial electrolyzers. Engineering solutions include core-shell structures and protective coatings that have shown to extend catalyst lifetime by 2-3 times in accelerated stress tests. Integration strategies with existing hydrogen production infrastructure must also be considered, requiring standardized testing protocols that accurately predict long-term performance.
Regulatory frameworks and sustainability considerations will significantly impact implementation pathways. Life cycle assessments indicate that perovskite catalysts could reduce the environmental footprint of hydrogen production by 40-60% compared to conventional technologies, provided that manufacturing processes are optimized for energy efficiency and waste reduction. Industry partnerships between catalyst developers, electrolyzer manufacturers, and end-users are emerging as crucial vehicles for accelerating commercial adoption through shared risk and collaborative scale-up efforts.
Industrial implementation requires development of continuous flow synthesis techniques that can produce perovskite catalysts with uniform properties at tonnage scale. Recent advances in microfluidic reactors and flow chemistry show promise, allowing for better control of reaction parameters and potentially reducing production costs by 30-40% compared to traditional batch methods. However, these approaches still face challenges in maintaining catalyst stability during scaled production.
Material costs present another significant hurdle, particularly for perovskites containing precious metals like platinum or iridium. Research indicates that substituting these elements with earth-abundant alternatives such as nickel, cobalt, or manganese could reduce material costs by up to 70%, though often with some sacrifice in catalytic performance. Optimization of catalyst loading and support materials can further enhance cost-effectiveness while maintaining acceptable activity levels.
Durability under industrial conditions remains problematic, as many perovskite catalysts demonstrate degradation when exposed to the harsh environments of commercial electrolyzers. Engineering solutions include core-shell structures and protective coatings that have shown to extend catalyst lifetime by 2-3 times in accelerated stress tests. Integration strategies with existing hydrogen production infrastructure must also be considered, requiring standardized testing protocols that accurately predict long-term performance.
Regulatory frameworks and sustainability considerations will significantly impact implementation pathways. Life cycle assessments indicate that perovskite catalysts could reduce the environmental footprint of hydrogen production by 40-60% compared to conventional technologies, provided that manufacturing processes are optimized for energy efficiency and waste reduction. Industry partnerships between catalyst developers, electrolyzer manufacturers, and end-users are emerging as crucial vehicles for accelerating commercial adoption through shared risk and collaborative scale-up efforts.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!