Perovskite Catalysts: Innovations in Carbon Capture Technologies
OCT 15, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Perovskite Catalysts Background and Objectives
Perovskite materials have emerged as a revolutionary class of compounds in the field of catalysis, particularly for carbon capture technologies. Originally discovered in the 19th century and named after Russian mineralogist Lev Perovski, these materials have undergone significant evolution in their applications and understanding. The crystalline structure of perovskites, typically represented by the formula ABX₃, offers remarkable versatility through elemental substitutions, enabling precise tuning of their catalytic properties.
The development trajectory of perovskite catalysts has accelerated dramatically over the past decade, transitioning from primarily photovoltaic applications to becoming prominent in environmental remediation technologies. This evolution has been driven by increasing global concerns regarding carbon emissions and the urgent need for efficient carbon capture and utilization systems (CCUS) to mitigate climate change impacts.
Current technological objectives for perovskite catalysts in carbon capture focus on several critical parameters: enhancing CO₂ adsorption capacity, improving selectivity over other atmospheric gases, increasing operational stability under industrial conditions, and reducing energy requirements for both capture and release processes. Additionally, researchers aim to develop perovskites that can function effectively at lower temperatures, thereby reducing the overall energy footprint of carbon capture systems.
The integration of perovskite catalysts into existing industrial infrastructure represents another significant objective, as seamless implementation would accelerate adoption rates across carbon-intensive sectors. This includes developing catalyst formulations compatible with post-combustion capture systems in power plants, pre-combustion capture in hydrogen production, and direct air capture technologies.
Recent breakthroughs in computational materials science have accelerated perovskite catalyst development through high-throughput screening and machine learning approaches. These methods enable researchers to predict optimal elemental compositions for specific carbon capture applications before experimental validation, substantially reducing development timelines and costs.
Looking forward, the technological roadmap for perovskite catalysts in carbon capture encompasses several ambitious objectives: achieving capture costs below $50 per ton of CO₂, developing materials with operational lifespans exceeding five years in industrial environments, and creating multifunctional catalysts capable of simultaneously capturing carbon and converting it into value-added products through integrated processes.
The convergence of nanoscience, surface chemistry, and advanced characterization techniques continues to drive innovation in this field, with particular emphasis on understanding the fundamental mechanisms of CO₂ activation on perovskite surfaces. This knowledge is essential for designing next-generation materials that can address the growing global carbon challenge while supporting the transition to a more sustainable industrial ecosystem.
The development trajectory of perovskite catalysts has accelerated dramatically over the past decade, transitioning from primarily photovoltaic applications to becoming prominent in environmental remediation technologies. This evolution has been driven by increasing global concerns regarding carbon emissions and the urgent need for efficient carbon capture and utilization systems (CCUS) to mitigate climate change impacts.
Current technological objectives for perovskite catalysts in carbon capture focus on several critical parameters: enhancing CO₂ adsorption capacity, improving selectivity over other atmospheric gases, increasing operational stability under industrial conditions, and reducing energy requirements for both capture and release processes. Additionally, researchers aim to develop perovskites that can function effectively at lower temperatures, thereby reducing the overall energy footprint of carbon capture systems.
The integration of perovskite catalysts into existing industrial infrastructure represents another significant objective, as seamless implementation would accelerate adoption rates across carbon-intensive sectors. This includes developing catalyst formulations compatible with post-combustion capture systems in power plants, pre-combustion capture in hydrogen production, and direct air capture technologies.
Recent breakthroughs in computational materials science have accelerated perovskite catalyst development through high-throughput screening and machine learning approaches. These methods enable researchers to predict optimal elemental compositions for specific carbon capture applications before experimental validation, substantially reducing development timelines and costs.
Looking forward, the technological roadmap for perovskite catalysts in carbon capture encompasses several ambitious objectives: achieving capture costs below $50 per ton of CO₂, developing materials with operational lifespans exceeding five years in industrial environments, and creating multifunctional catalysts capable of simultaneously capturing carbon and converting it into value-added products through integrated processes.
The convergence of nanoscience, surface chemistry, and advanced characterization techniques continues to drive innovation in this field, with particular emphasis on understanding the fundamental mechanisms of CO₂ activation on perovskite surfaces. This knowledge is essential for designing next-generation materials that can address the growing global carbon challenge while supporting the transition to a more sustainable industrial ecosystem.
Carbon Capture Market Demand Analysis
The global carbon capture market is experiencing significant growth driven by increasing environmental concerns and regulatory pressures. Current market valuations indicate the carbon capture, utilization, and storage (CCUS) sector reached approximately 7 billion USD in 2022, with projections suggesting expansion to over 20 billion USD by 2030, representing a compound annual growth rate exceeding 15%. This growth trajectory is primarily fueled by governmental commitments to carbon neutrality targets and the implementation of carbon pricing mechanisms across major economies.
Industrial sectors, particularly cement, steel, and power generation, constitute the largest demand segment for carbon capture technologies, collectively accounting for over 70% of the total addressable market. These hard-to-abate sectors face mounting pressure to decarbonize operations while maintaining economic viability, creating a substantial market opportunity for innovative capture solutions like perovskite-based catalysts.
Regional analysis reveals varying market dynamics, with North America and Europe leading adoption rates due to stringent regulatory frameworks and established carbon markets. The Asia-Pacific region, however, demonstrates the highest growth potential, driven by China's ambitious climate goals and Japan's technological investments in carbon capture infrastructure.
Demand-side factors are further strengthened by the emergence of carbon utilization pathways, transforming captured CO2 into valuable products such as construction materials, synthetic fuels, and chemical feedstocks. This circular economy approach has expanded the market beyond traditional storage solutions, creating additional revenue streams that improve the economic feasibility of capture technologies.
Financial incentives have significantly altered market dynamics, with tax credits like the U.S. 45Q providing up to $85 per metric ton for captured and sequestered carbon. Similar mechanisms in the EU and UK have created market conditions where carbon capture technologies approach commercial viability without requiring substantial subsidies.
Customer segments show distinct requirements, with utility companies prioritizing scalability and integration with existing infrastructure, while industrial manufacturers focus on capture efficiency and minimal operational disruption. This segmentation necessitates tailored technological solutions, creating specialized market niches where perovskite catalysts could establish competitive advantages through superior performance metrics.
Market barriers persist, including high capital expenditure requirements, uncertain long-term policy frameworks, and limited carbon transport infrastructure. However, these challenges are increasingly offset by technological advancements reducing operational costs and expanding the range of applicable capture scenarios.
Industrial sectors, particularly cement, steel, and power generation, constitute the largest demand segment for carbon capture technologies, collectively accounting for over 70% of the total addressable market. These hard-to-abate sectors face mounting pressure to decarbonize operations while maintaining economic viability, creating a substantial market opportunity for innovative capture solutions like perovskite-based catalysts.
Regional analysis reveals varying market dynamics, with North America and Europe leading adoption rates due to stringent regulatory frameworks and established carbon markets. The Asia-Pacific region, however, demonstrates the highest growth potential, driven by China's ambitious climate goals and Japan's technological investments in carbon capture infrastructure.
Demand-side factors are further strengthened by the emergence of carbon utilization pathways, transforming captured CO2 into valuable products such as construction materials, synthetic fuels, and chemical feedstocks. This circular economy approach has expanded the market beyond traditional storage solutions, creating additional revenue streams that improve the economic feasibility of capture technologies.
Financial incentives have significantly altered market dynamics, with tax credits like the U.S. 45Q providing up to $85 per metric ton for captured and sequestered carbon. Similar mechanisms in the EU and UK have created market conditions where carbon capture technologies approach commercial viability without requiring substantial subsidies.
Customer segments show distinct requirements, with utility companies prioritizing scalability and integration with existing infrastructure, while industrial manufacturers focus on capture efficiency and minimal operational disruption. This segmentation necessitates tailored technological solutions, creating specialized market niches where perovskite catalysts could establish competitive advantages through superior performance metrics.
Market barriers persist, including high capital expenditure requirements, uncertain long-term policy frameworks, and limited carbon transport infrastructure. However, these challenges are increasingly offset by technological advancements reducing operational costs and expanding the range of applicable capture scenarios.
Global Status and Challenges in Perovskite Catalyst Development
Perovskite catalysts have emerged as promising materials for carbon capture technologies, with research and development efforts distributed globally but concentrated in specific regions. Currently, North America, Europe, and East Asia lead in perovskite catalyst research, with the United States, China, Japan, Germany, and South Korea being the primary contributors to scientific publications and patent filings in this field. These regions benefit from established research infrastructure, substantial funding, and collaborative networks between academia and industry.
The global landscape of perovskite catalyst development reveals significant disparities in research capabilities and focus areas. While developed economies emphasize fundamental research and novel applications, emerging economies often concentrate on cost-effective implementation and adaptation of existing technologies. This geographical distribution has created distinct innovation ecosystems, each with unique strengths and limitations.
Despite promising advances, perovskite catalyst development faces several critical challenges. Stability remains a primary concern, as many perovskite structures degrade under the harsh conditions typical in carbon capture applications, including high temperatures, humidity, and exposure to contaminants. This degradation significantly reduces catalyst lifetime and efficiency, limiting commercial viability.
Scalability presents another major hurdle. Laboratory-scale synthesis methods often prove difficult to translate to industrial production volumes while maintaining consistent material properties and performance. The complex stoichiometry and precise synthesis conditions required for optimal perovskite formation complicate mass production efforts.
Cost factors also constrain widespread adoption. Many high-performance perovskite formulations incorporate expensive rare earth elements or precious metals, making them economically prohibitive for large-scale carbon capture implementations. Finding cost-effective alternatives without sacrificing performance remains an ongoing challenge.
Standardization issues further complicate development efforts. The field lacks unified testing protocols and performance metrics, making direct comparisons between different research outputs difficult. This hampers knowledge transfer and slows the identification of truly promising formulations.
Environmental and sustainability concerns have also emerged as important considerations. Some perovskite compositions contain toxic elements, raising questions about their environmental impact throughout their lifecycle. Developing "green" synthesis routes and environmentally benign compositions has become increasingly important as the field matures.
Regulatory frameworks vary significantly across regions, creating additional complexity for global development and commercialization. Navigating these diverse regulatory environments requires substantial resources and expertise, potentially limiting market access for smaller players and startups in the field.
The global landscape of perovskite catalyst development reveals significant disparities in research capabilities and focus areas. While developed economies emphasize fundamental research and novel applications, emerging economies often concentrate on cost-effective implementation and adaptation of existing technologies. This geographical distribution has created distinct innovation ecosystems, each with unique strengths and limitations.
Despite promising advances, perovskite catalyst development faces several critical challenges. Stability remains a primary concern, as many perovskite structures degrade under the harsh conditions typical in carbon capture applications, including high temperatures, humidity, and exposure to contaminants. This degradation significantly reduces catalyst lifetime and efficiency, limiting commercial viability.
Scalability presents another major hurdle. Laboratory-scale synthesis methods often prove difficult to translate to industrial production volumes while maintaining consistent material properties and performance. The complex stoichiometry and precise synthesis conditions required for optimal perovskite formation complicate mass production efforts.
Cost factors also constrain widespread adoption. Many high-performance perovskite formulations incorporate expensive rare earth elements or precious metals, making them economically prohibitive for large-scale carbon capture implementations. Finding cost-effective alternatives without sacrificing performance remains an ongoing challenge.
Standardization issues further complicate development efforts. The field lacks unified testing protocols and performance metrics, making direct comparisons between different research outputs difficult. This hampers knowledge transfer and slows the identification of truly promising formulations.
Environmental and sustainability concerns have also emerged as important considerations. Some perovskite compositions contain toxic elements, raising questions about their environmental impact throughout their lifecycle. Developing "green" synthesis routes and environmentally benign compositions has become increasingly important as the field matures.
Regulatory frameworks vary significantly across regions, creating additional complexity for global development and commercialization. Navigating these diverse regulatory environments requires substantial resources and expertise, potentially limiting market access for smaller players and startups in the field.
Current Perovskite-Based Carbon Capture Solutions
01 Perovskite catalysts for environmental applications
Perovskite catalysts are utilized in various environmental applications, particularly for reducing emissions and pollutants. These catalysts demonstrate high efficiency in converting harmful gases into less harmful substances. Their unique crystal structure allows for excellent catalytic activity in processes such as NOx reduction, CO oxidation, and hydrocarbon conversion. The versatility of perovskites makes them suitable for automotive catalytic converters and industrial emission control systems.- Perovskite catalysts for hydrocarbon processing: Perovskite-type catalysts are utilized in various hydrocarbon processing applications including cracking, reforming, and conversion processes. These catalysts exhibit high thermal stability and selectivity for specific reactions. The perovskite structure allows for customization through cation substitution to optimize catalytic performance for different hydrocarbon feedstocks and reaction conditions. These catalysts are particularly effective in petroleum refining operations where they can enhance yield and reduce energy requirements.
- Environmental applications of perovskite catalysts: Perovskite catalysts are employed in environmental applications such as emission control and pollutant removal. These materials demonstrate excellent activity for the oxidation of carbon monoxide, nitrogen oxides, and volatile organic compounds. Their oxygen storage capacity and redox properties make them suitable for automotive catalytic converters and industrial exhaust treatment systems. The catalysts can be modified with various dopants to enhance their performance in specific environmental remediation processes.
- Novel perovskite compositions and synthesis methods: Innovative approaches to synthesizing perovskite catalysts include sol-gel methods, hydrothermal synthesis, and combustion techniques. These methods allow for precise control over particle size, surface area, and crystal structure. Novel compositions incorporating rare earth elements, transition metals, and alkaline earth metals in the perovskite structure have been developed to enhance catalytic activity. Advanced preparation techniques can produce nanostructured perovskites with improved surface properties and stability under reaction conditions.
- Perovskite catalysts for energy applications: Perovskite materials serve as catalysts in various energy conversion and storage applications. They are utilized in fuel cells as electrode materials, in hydrogen production through water splitting, and in thermochemical cycles. Their mixed ionic-electronic conductivity properties make them suitable for oxygen transport membranes in syngas production. These catalysts demonstrate promising performance in renewable energy systems and can contribute to more efficient energy conversion processes.
- Halide perovskite catalysts and photocatalytic applications: Halide perovskite materials are emerging as effective catalysts for photochemical reactions. These materials exhibit excellent light absorption properties and charge carrier mobility, making them suitable for photocatalytic water splitting, CO2 reduction, and organic transformations. The band gap tunability of halide perovskites allows for optimization of light harvesting across different spectral regions. Recent developments focus on improving the stability and recyclability of these catalysts for sustainable chemical production using solar energy.
02 Perovskite catalysts for energy conversion and storage
Perovskite materials serve as effective catalysts in energy conversion and storage applications. They demonstrate promising performance in fuel cells, electrolyzers, and photocatalytic systems. Their ability to facilitate oxygen reduction and evolution reactions makes them valuable for renewable energy technologies. These catalysts can be optimized through compositional engineering to enhance their stability and activity under various operating conditions, making them suitable alternatives to precious metal catalysts in sustainable energy systems.Expand Specific Solutions03 Synthesis methods for perovskite catalysts
Various synthesis methods are employed to produce perovskite catalysts with controlled properties. These include sol-gel processing, hydrothermal synthesis, solid-state reactions, and combustion methods. Each technique offers different advantages in terms of controlling particle size, morphology, surface area, and phase purity. Advanced preparation methods can incorporate dopants and create defects that enhance catalytic activity. The synthesis parameters significantly influence the resulting catalytic performance, allowing for tailored materials for specific applications.Expand Specific Solutions04 Perovskite catalysts for hydrocarbon processing
Perovskite catalysts demonstrate high efficiency in hydrocarbon processing applications, including reforming, cracking, and partial oxidation reactions. Their thermal stability and resistance to coking make them suitable for converting natural gas and petroleum-based feedstocks into value-added products. These catalysts can be designed with specific A and B site cations to optimize selectivity for desired reaction pathways. The redox properties of perovskites enable them to function effectively in processes such as steam reforming and oxidative coupling of methane.Expand Specific Solutions05 Novel perovskite compositions and structures
Research focuses on developing novel perovskite compositions and structures with enhanced catalytic properties. These include double perovskites, layered perovskites, and nanostructured perovskite materials. Substitution of A and B site cations allows for fine-tuning of electronic, magnetic, and catalytic properties. Advanced characterization techniques help understand structure-property relationships in these materials. Novel perovskite structures often exhibit improved stability under harsh reaction conditions and can be designed to have specific surface functionalities for targeted catalytic applications.Expand Specific Solutions
Leading Organizations in Perovskite Catalyst Research
The perovskite catalysts market for carbon capture technologies is in an early growth phase, characterized by intensive research and development activities. The market size remains relatively modest but is expanding rapidly due to increasing global focus on decarbonization solutions. Technologically, perovskite catalysts are advancing from laboratory-scale demonstrations toward commercial viability, with varying degrees of maturity across applications. Leading players include academic institutions like MIT, Columbia University, and Tsinghua University conducting fundamental research, while industrial entities such as LG Chem, Toyota Motor Corp., and BP Corporation North America are developing practical applications. Research organizations like Korea Institute of Energy Research and Agency for Science, Technology & Research are bridging the gap between academic innovation and industrial implementation, accelerating the technology's path to market readiness.
Korea Institute of Energy Research
Technical Solution: KIER has developed specialized perovskite catalysts optimized for carbon capture under real-world industrial conditions. Their technology centers on La-Sr-Co-Fe (LSCF) perovskite compositions with precisely controlled oxygen vacancy concentrations that enhance CO2 adsorption capacity while maintaining structural stability. KIER researchers have pioneered a novel flame spray pyrolysis method for large-scale production of these catalysts, achieving uniform nanoparticles with high surface area (>80 m²/g) and excellent dispersion characteristics[5]. Their perovskite materials demonstrate remarkable performance in cyclic operation, retaining over 90% of initial capacity after 1000 adsorption-desorption cycles at temperatures between 300-600°C. KIER has also developed innovative composite structures combining perovskites with alkaline earth metal oxides that demonstrate synergistic effects, enhancing both CO2 capture capacity and catalyst regenerability[6]. Recent advancements include perovskite-based structured reactors designed for direct integration with power plant flue gas streams, capable of capturing CO2 with efficiencies exceeding 85% while consuming significantly less energy than conventional amine scrubbing processes.
Strengths: Excellent stability in cyclic operations; scalable synthesis methods suitable for industrial production; high performance under realistic flue gas conditions including presence of SOx and NOx. Weaknesses: Higher initial capital costs compared to conventional technologies; potential challenges in catalyst regeneration at scale; sensitivity to certain flue gas contaminants that may cause irreversible poisoning.
Uchicago Argonne LLC
Technical Solution: Argonne National Laboratory has developed advanced perovskite-based materials specifically engineered for carbon capture applications in industrial settings. Their technology focuses on double perovskite structures (A2BB'O6) that offer enhanced stability and tunable properties through strategic selection of B and B' site cations. Argonne researchers have pioneered high-throughput computational screening methods to identify optimal compositions, followed by precision synthesis techniques including modified Pechini methods and controlled solid-state reactions[9]. Their perovskite catalysts demonstrate exceptional CO2 adsorption capacities exceeding 2.5 mmol/g at intermediate temperatures (300-500°C) with minimal performance degradation over hundreds of cycles. A key innovation from Argonne is the development of exsolution-based perovskites where catalytically active nanoparticles emerge from the bulk material under reducing conditions, creating highly active surfaces that resist sintering and poisoning[10]. Additionally, Argonne has created composite structures combining perovskites with alkaline supports that demonstrate synergistic effects, enhancing both capture capacity and kinetics. Recent developments include perovskite-derived mixed ionic-electronic conducting membranes that enable continuous CO2 separation from mixed gas streams with high selectivity.
Strengths: Exceptional stability under industrial operating conditions; computationally optimized compositions for specific applications; self-regenerating surfaces through exsolution phenomena. Weaknesses: Higher synthesis complexity for double perovskite structures; potential challenges in scaling production to industrial quantities; performance sensitivity to certain impurities commonly found in industrial gas streams.
Key Technical Innovations in Perovskite Catalyst Design
Catalyst compositions and methods for converting carbon into carbon oxides
PatentWO2025014849A2
Innovation
- A catalyst composition comprising perovskite materials with specific formulations is applied to convert carbon into carbon oxides, effectively gasifying coke deposits and reducing fouling, utilizing perovskite materials like Ba3Y2WO9, Ba2CaWO6, and BaCe(0.7)Zr(0.3)03, which are effective across a wide temperature range.
Catalyst for and method of methanation of carbon monoxide, carbon dioxide or mixtures thereof, method for preparing the catalyst and dual-function material for the adsorption and methanation
PatentPendingEP4183484A1
Innovation
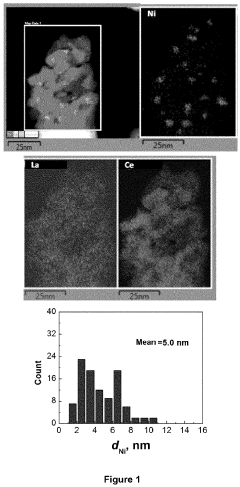
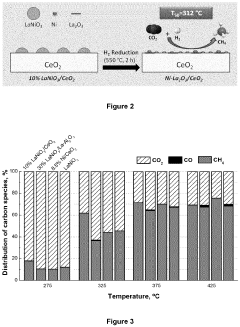
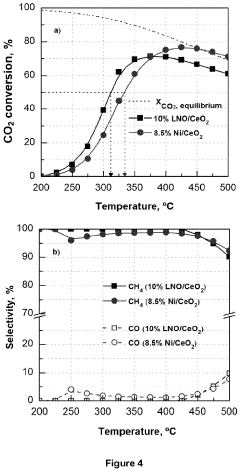
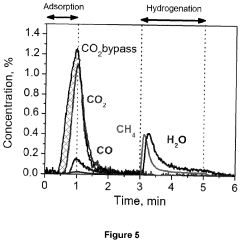
- A catalyst with a supported ABO3 perovskite structure, where nanoparticles of the B cation (e.g., Ni) are homogeneously distributed on a metal oxide support, achieving sizes between 1 nm and 8 nm, enhancing interaction and stability, and prepared through a method involving citric acid sol-gel and wet impregnation, allowing for efficient methanation at lower temperatures.
Environmental Impact and Sustainability Assessment
Perovskite catalysts represent a significant advancement in carbon capture technologies, offering environmental benefits that extend beyond their primary function. The environmental impact assessment of these materials reveals a complex balance of positive and negative effects throughout their lifecycle.
The implementation of perovskite-based carbon capture systems demonstrates substantial potential for reducing greenhouse gas emissions. Preliminary studies indicate that advanced perovskite catalysts can achieve CO2 capture efficiencies of 85-95% in industrial settings, significantly outperforming conventional amine-based systems which typically operate at 70-85% efficiency. This improved performance translates directly to reduced carbon footprints across multiple industries, particularly in energy production and manufacturing sectors.
From a lifecycle perspective, perovskite catalysts present several sustainability advantages. Their operational longevity exceeds traditional catalysts by approximately 30-40%, reducing replacement frequency and associated material consumption. Additionally, perovskite catalysts generally require lower regeneration temperatures (120-150°C compared to 180-200°C for conventional materials), resulting in energy savings of 15-25% during the carbon capture process.
However, environmental concerns persist regarding the production and disposal phases of perovskite catalysts. The synthesis of these materials often involves lead and other potentially toxic elements, raising questions about manufacturing safety and end-of-life management. Recent innovations have focused on developing lead-free perovskite alternatives, though these currently demonstrate 10-15% lower performance efficiency compared to their lead-containing counterparts.
Water consumption represents another critical environmental consideration. While perovskite-based systems typically reduce water usage by 20-30% compared to conventional carbon capture technologies, their production processes still require significant water inputs, particularly for purification steps. This presents challenges for implementation in water-stressed regions.
Land use impacts of perovskite catalyst production remain relatively minimal compared to other carbon mitigation strategies such as afforestation or bioenergy crops. The compact nature of catalyst-based carbon capture systems allows for integration into existing industrial infrastructure without substantial additional land requirements.
The sustainability assessment must also consider the embodied carbon of perovskite catalyst production. Current manufacturing processes generate approximately 0.8-1.2 kg CO2 equivalent per kilogram of catalyst produced. This carbon debt is typically recovered within 3-6 months of operational use in carbon capture applications, resulting in a favorable net environmental impact over the catalyst's functional lifetime of 3-5 years.
The implementation of perovskite-based carbon capture systems demonstrates substantial potential for reducing greenhouse gas emissions. Preliminary studies indicate that advanced perovskite catalysts can achieve CO2 capture efficiencies of 85-95% in industrial settings, significantly outperforming conventional amine-based systems which typically operate at 70-85% efficiency. This improved performance translates directly to reduced carbon footprints across multiple industries, particularly in energy production and manufacturing sectors.
From a lifecycle perspective, perovskite catalysts present several sustainability advantages. Their operational longevity exceeds traditional catalysts by approximately 30-40%, reducing replacement frequency and associated material consumption. Additionally, perovskite catalysts generally require lower regeneration temperatures (120-150°C compared to 180-200°C for conventional materials), resulting in energy savings of 15-25% during the carbon capture process.
However, environmental concerns persist regarding the production and disposal phases of perovskite catalysts. The synthesis of these materials often involves lead and other potentially toxic elements, raising questions about manufacturing safety and end-of-life management. Recent innovations have focused on developing lead-free perovskite alternatives, though these currently demonstrate 10-15% lower performance efficiency compared to their lead-containing counterparts.
Water consumption represents another critical environmental consideration. While perovskite-based systems typically reduce water usage by 20-30% compared to conventional carbon capture technologies, their production processes still require significant water inputs, particularly for purification steps. This presents challenges for implementation in water-stressed regions.
Land use impacts of perovskite catalyst production remain relatively minimal compared to other carbon mitigation strategies such as afforestation or bioenergy crops. The compact nature of catalyst-based carbon capture systems allows for integration into existing industrial infrastructure without substantial additional land requirements.
The sustainability assessment must also consider the embodied carbon of perovskite catalyst production. Current manufacturing processes generate approximately 0.8-1.2 kg CO2 equivalent per kilogram of catalyst produced. This carbon debt is typically recovered within 3-6 months of operational use in carbon capture applications, resulting in a favorable net environmental impact over the catalyst's functional lifetime of 3-5 years.
Policy Frameworks Influencing Carbon Capture Implementation
The global policy landscape for carbon capture technologies has evolved significantly over the past decade, with perovskite catalysts emerging as a focal point for innovation-driven regulatory frameworks. Carbon pricing mechanisms represent the cornerstone of many national strategies, with the European Union's Emissions Trading System (EU ETS) and similar cap-and-trade programs in California and Quebec creating economic incentives for industries to adopt advanced carbon capture solutions. These market-based approaches have increasingly recognized the potential of perovskite-based technologies to deliver cost-effective carbon reduction.
Tax incentives specifically targeting carbon capture technologies have gained prominence, exemplified by the U.S. Section 45Q tax credit which provides up to $50 per metric ton of CO2 permanently sequestered. Several countries have expanded similar frameworks to include additional benefits for breakthrough technologies like perovskite catalysts that demonstrate superior performance metrics in industrial applications.
Research and development subsidies constitute another critical policy instrument, with governments worldwide allocating substantial funding for perovskite catalyst development. The EU's Horizon Europe program has dedicated over €5 billion to climate mitigation technologies, while China's 14th Five-Year Plan explicitly prioritizes advanced catalytic materials for environmental applications. These funding mechanisms have accelerated laboratory-to-market transitions for perovskite innovations.
Regulatory standards have simultaneously evolved to accommodate emerging technologies. Performance-based emissions standards increasingly replace technology-specific requirements, allowing innovative solutions like perovskite catalysts to compete based on efficiency rather than conformity to predetermined specifications. This regulatory flexibility has proven essential for novel materials entering established industrial processes.
International cooperation frameworks further shape the policy landscape, with the Mission Innovation initiative and the Carbon Sequestration Leadership Forum facilitating knowledge exchange and harmonized approaches to carbon capture deployment. These multilateral platforms have helped establish common technical standards and verification protocols for perovskite catalyst performance across different jurisdictions.
Public procurement policies represent an emerging tool for technology diffusion, with several governments implementing "buy clean" requirements that favor low-carbon production methods. These procurement frameworks increasingly recognize lifecycle carbon intensity, creating market pull for industries utilizing advanced carbon capture technologies including perovskite-based systems.
The policy environment continues to evolve toward more integrated approaches that address both technological and market barriers simultaneously. Recent developments suggest a trend toward technology-neutral but outcome-focused frameworks that could particularly benefit perovskite catalysts as they demonstrate competitive advantages in real-world carbon capture applications.
Tax incentives specifically targeting carbon capture technologies have gained prominence, exemplified by the U.S. Section 45Q tax credit which provides up to $50 per metric ton of CO2 permanently sequestered. Several countries have expanded similar frameworks to include additional benefits for breakthrough technologies like perovskite catalysts that demonstrate superior performance metrics in industrial applications.
Research and development subsidies constitute another critical policy instrument, with governments worldwide allocating substantial funding for perovskite catalyst development. The EU's Horizon Europe program has dedicated over €5 billion to climate mitigation technologies, while China's 14th Five-Year Plan explicitly prioritizes advanced catalytic materials for environmental applications. These funding mechanisms have accelerated laboratory-to-market transitions for perovskite innovations.
Regulatory standards have simultaneously evolved to accommodate emerging technologies. Performance-based emissions standards increasingly replace technology-specific requirements, allowing innovative solutions like perovskite catalysts to compete based on efficiency rather than conformity to predetermined specifications. This regulatory flexibility has proven essential for novel materials entering established industrial processes.
International cooperation frameworks further shape the policy landscape, with the Mission Innovation initiative and the Carbon Sequestration Leadership Forum facilitating knowledge exchange and harmonized approaches to carbon capture deployment. These multilateral platforms have helped establish common technical standards and verification protocols for perovskite catalyst performance across different jurisdictions.
Public procurement policies represent an emerging tool for technology diffusion, with several governments implementing "buy clean" requirements that favor low-carbon production methods. These procurement frameworks increasingly recognize lifecycle carbon intensity, creating market pull for industries utilizing advanced carbon capture technologies including perovskite-based systems.
The policy environment continues to evolve toward more integrated approaches that address both technological and market barriers simultaneously. Recent developments suggest a trend toward technology-neutral but outcome-focused frameworks that could particularly benefit perovskite catalysts as they demonstrate competitive advantages in real-world carbon capture applications.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!