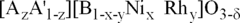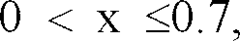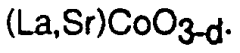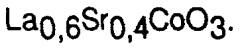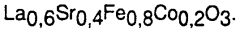The Role of Perovskite Catalysts in Emerging Hydrogen Technologies
OCT 15, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Perovskite Catalysts Background and Research Objectives
Perovskite materials have emerged as a revolutionary class of compounds in catalysis science over the past several decades. Initially discovered in 1839 by Gustav Rose and named after Russian mineralogist Lev Perovski, these materials possess a distinctive ABX3 crystal structure that offers remarkable versatility in composition through cation and anion substitutions. This structural flexibility has positioned perovskites at the forefront of catalyst development for hydrogen technologies, representing a critical advancement in sustainable energy solutions.
The evolution of perovskite catalysts has accelerated significantly since the early 2000s, with research intensity increasing exponentially in the past decade. This surge corresponds directly with global imperatives to develop carbon-neutral energy systems and the recognition of hydrogen as a pivotal clean energy carrier. The adaptability of perovskite structures allows for precise tuning of electronic, catalytic, and stability properties, making them exceptionally promising for hydrogen production, storage, and utilization pathways.
Current technological trajectories indicate that perovskite catalysts are advancing along three primary vectors: enhanced catalytic efficiency, improved stability under operational conditions, and reduced dependence on precious metals. These developments are particularly relevant for water electrolysis, hydrogen evolution reactions (HER), and oxygen evolution reactions (OER) - all fundamental processes in hydrogen production technologies. The remarkable oxygen vacancy formation capabilities and mixed ionic-electronic conductivity of many perovskite compositions provide unique advantages over conventional catalyst materials.
The primary technical objectives of this research focus on evaluating and advancing perovskite catalysts for emerging hydrogen technologies across multiple dimensions. First, we aim to comprehensively map the structure-property relationships in perovskite catalysts to enable rational design principles for specific hydrogen applications. Second, we seek to identify optimal compositional formulations that maximize catalytic activity while minimizing reliance on scarce or expensive elements. Third, we intend to develop scalable synthesis methodologies that can transition laboratory successes to industrial implementation.
Additionally, this research aims to quantify the performance advantages of perovskite catalysts compared to current commercial solutions, particularly focusing on efficiency metrics, durability under industrial conditions, and total system economics. The investigation will also explore hybrid catalyst systems that combine perovskites with other advanced materials to create synergistic effects that overcome current technological limitations in hydrogen production and utilization pathways.
The ultimate goal is to establish whether perovskite catalysts represent a transformative technology capable of accelerating the hydrogen economy by significantly reducing production costs, improving energy efficiency, and enabling new technological applications that are currently constrained by catalyst performance limitations.
The evolution of perovskite catalysts has accelerated significantly since the early 2000s, with research intensity increasing exponentially in the past decade. This surge corresponds directly with global imperatives to develop carbon-neutral energy systems and the recognition of hydrogen as a pivotal clean energy carrier. The adaptability of perovskite structures allows for precise tuning of electronic, catalytic, and stability properties, making them exceptionally promising for hydrogen production, storage, and utilization pathways.
Current technological trajectories indicate that perovskite catalysts are advancing along three primary vectors: enhanced catalytic efficiency, improved stability under operational conditions, and reduced dependence on precious metals. These developments are particularly relevant for water electrolysis, hydrogen evolution reactions (HER), and oxygen evolution reactions (OER) - all fundamental processes in hydrogen production technologies. The remarkable oxygen vacancy formation capabilities and mixed ionic-electronic conductivity of many perovskite compositions provide unique advantages over conventional catalyst materials.
The primary technical objectives of this research focus on evaluating and advancing perovskite catalysts for emerging hydrogen technologies across multiple dimensions. First, we aim to comprehensively map the structure-property relationships in perovskite catalysts to enable rational design principles for specific hydrogen applications. Second, we seek to identify optimal compositional formulations that maximize catalytic activity while minimizing reliance on scarce or expensive elements. Third, we intend to develop scalable synthesis methodologies that can transition laboratory successes to industrial implementation.
Additionally, this research aims to quantify the performance advantages of perovskite catalysts compared to current commercial solutions, particularly focusing on efficiency metrics, durability under industrial conditions, and total system economics. The investigation will also explore hybrid catalyst systems that combine perovskites with other advanced materials to create synergistic effects that overcome current technological limitations in hydrogen production and utilization pathways.
The ultimate goal is to establish whether perovskite catalysts represent a transformative technology capable of accelerating the hydrogen economy by significantly reducing production costs, improving energy efficiency, and enabling new technological applications that are currently constrained by catalyst performance limitations.
Hydrogen Economy Market Analysis and Growth Projections
The global hydrogen economy is experiencing unprecedented growth, driven by the urgent need for clean energy alternatives and decarbonization efforts across industries. Current market valuations place the hydrogen market at approximately $130 billion in 2023, with projections indicating potential expansion to $500-700 billion by 2050 according to the Hydrogen Council. This represents a compound annual growth rate (CAGR) of 6-8% over the next three decades, significantly outpacing traditional energy sector growth.
The transportation sector presents the most immediate growth opportunity, with hydrogen fuel cell vehicles gaining traction particularly in commercial fleets, heavy-duty trucks, and public transportation. The market for hydrogen mobility solutions is expected to reach $140 billion by 2035, with Asia-Pacific leading adoption rates followed by Europe and North America.
Industrial applications constitute the largest current hydrogen consumption segment, accounting for over 70% of produced hydrogen. Traditional uses in petroleum refining and ammonia production are being supplemented by emerging applications in steel manufacturing, where hydrogen-based direct reduction processes could capture 10-15% of global steel production by 2040.
Energy storage represents another high-growth segment, with hydrogen increasingly viewed as a solution for long-duration energy storage to complement intermittent renewable sources. The power generation sector is projected to consume 400-500 TWh of hydrogen annually by 2050, representing approximately 15% of global electricity production.
Regional analysis reveals distinct market development patterns. Europe leads in policy support through initiatives like the European Clean Hydrogen Alliance and €470 billion investment commitments by 2050. Asia-Pacific, particularly Japan, South Korea, and China, demonstrates aggressive hydrogen adoption strategies with combined investments exceeding $60 billion announced through 2030.
The integration of perovskite catalysts into hydrogen technologies is expected to accelerate market growth by addressing key economic barriers. By potentially reducing electrolysis costs by 30-40% through improved efficiency and reduced precious metal requirements, perovskite catalysts could accelerate hydrogen price competitiveness with conventional fuels by 5-7 years compared to current projections.
Investment trends show rapidly increasing capital flows, with venture capital and private equity investments in hydrogen technologies reaching $11 billion in 2022, a threefold increase from 2019. Public funding commitments from governments worldwide now exceed $70 billion through 2030, creating a robust foundation for continued market expansion and technological advancement in hydrogen solutions.
The transportation sector presents the most immediate growth opportunity, with hydrogen fuel cell vehicles gaining traction particularly in commercial fleets, heavy-duty trucks, and public transportation. The market for hydrogen mobility solutions is expected to reach $140 billion by 2035, with Asia-Pacific leading adoption rates followed by Europe and North America.
Industrial applications constitute the largest current hydrogen consumption segment, accounting for over 70% of produced hydrogen. Traditional uses in petroleum refining and ammonia production are being supplemented by emerging applications in steel manufacturing, where hydrogen-based direct reduction processes could capture 10-15% of global steel production by 2040.
Energy storage represents another high-growth segment, with hydrogen increasingly viewed as a solution for long-duration energy storage to complement intermittent renewable sources. The power generation sector is projected to consume 400-500 TWh of hydrogen annually by 2050, representing approximately 15% of global electricity production.
Regional analysis reveals distinct market development patterns. Europe leads in policy support through initiatives like the European Clean Hydrogen Alliance and €470 billion investment commitments by 2050. Asia-Pacific, particularly Japan, South Korea, and China, demonstrates aggressive hydrogen adoption strategies with combined investments exceeding $60 billion announced through 2030.
The integration of perovskite catalysts into hydrogen technologies is expected to accelerate market growth by addressing key economic barriers. By potentially reducing electrolysis costs by 30-40% through improved efficiency and reduced precious metal requirements, perovskite catalysts could accelerate hydrogen price competitiveness with conventional fuels by 5-7 years compared to current projections.
Investment trends show rapidly increasing capital flows, with venture capital and private equity investments in hydrogen technologies reaching $11 billion in 2022, a threefold increase from 2019. Public funding commitments from governments worldwide now exceed $70 billion through 2030, creating a robust foundation for continued market expansion and technological advancement in hydrogen solutions.
Current Challenges in Perovskite Catalyst Development
Despite significant advancements in perovskite catalyst research for hydrogen technologies, several critical challenges continue to impede widespread commercial implementation. The inherent stability issues of perovskite materials represent perhaps the most significant barrier, particularly under the harsh operating conditions required for hydrogen production and utilization. When exposed to high temperatures, humidity, or acidic/alkaline environments typical in electrolyzers and fuel cells, many promising perovskite formulations undergo structural degradation, phase separation, or surface poisoning, resulting in diminished catalytic performance over time.
Compositional optimization remains another substantial challenge. While the ABO₃ perovskite structure offers remarkable flexibility for elemental substitution, identifying the optimal combination of A-site and B-site cations to maximize catalytic activity while maintaining stability requires extensive experimental iterations. The vast compositional space makes systematic exploration time-consuming and resource-intensive, with current high-throughput screening methods still insufficient to fully map structure-property relationships.
Scalable and cost-effective synthesis methods present additional hurdles. Laboratory-scale techniques that produce high-performance perovskite catalysts often involve complex procedures, expensive precursors, or energy-intensive processing steps that become economically prohibitive at industrial scales. The transition from milligram-scale research samples to kilogram-scale production batches frequently results in compromised material properties and inconsistent performance.
Surface engineering and interface control represent emerging challenges as researchers seek to enhance catalytic activity. The precise control of surface defects, oxygen vacancies, and electronic states at the atomic level remains difficult to achieve reproducibly. Furthermore, integrating perovskite catalysts with support materials or electrode structures while maintaining optimal interfacial contact and electron transfer pathways requires sophisticated fabrication techniques not yet fully developed.
Mechanistic understanding gaps further complicate development efforts. Despite extensive research, the fundamental reaction mechanisms and rate-determining steps for many hydrogen-related processes on perovskite surfaces remain incompletely understood. This knowledge deficit hampers rational catalyst design and optimization, forcing researchers to rely heavily on empirical approaches rather than theory-guided development.
Finally, standardization and benchmarking issues create difficulties in comparing results across different research groups. Variations in testing protocols, electrode preparation methods, and performance metrics make it challenging to objectively evaluate the true potential of novel perovskite catalysts against existing alternatives or established benchmarks in the hydrogen technology landscape.
Compositional optimization remains another substantial challenge. While the ABO₃ perovskite structure offers remarkable flexibility for elemental substitution, identifying the optimal combination of A-site and B-site cations to maximize catalytic activity while maintaining stability requires extensive experimental iterations. The vast compositional space makes systematic exploration time-consuming and resource-intensive, with current high-throughput screening methods still insufficient to fully map structure-property relationships.
Scalable and cost-effective synthesis methods present additional hurdles. Laboratory-scale techniques that produce high-performance perovskite catalysts often involve complex procedures, expensive precursors, or energy-intensive processing steps that become economically prohibitive at industrial scales. The transition from milligram-scale research samples to kilogram-scale production batches frequently results in compromised material properties and inconsistent performance.
Surface engineering and interface control represent emerging challenges as researchers seek to enhance catalytic activity. The precise control of surface defects, oxygen vacancies, and electronic states at the atomic level remains difficult to achieve reproducibly. Furthermore, integrating perovskite catalysts with support materials or electrode structures while maintaining optimal interfacial contact and electron transfer pathways requires sophisticated fabrication techniques not yet fully developed.
Mechanistic understanding gaps further complicate development efforts. Despite extensive research, the fundamental reaction mechanisms and rate-determining steps for many hydrogen-related processes on perovskite surfaces remain incompletely understood. This knowledge deficit hampers rational catalyst design and optimization, forcing researchers to rely heavily on empirical approaches rather than theory-guided development.
Finally, standardization and benchmarking issues create difficulties in comparing results across different research groups. Variations in testing protocols, electrode preparation methods, and performance metrics make it challenging to objectively evaluate the true potential of novel perovskite catalysts against existing alternatives or established benchmarks in the hydrogen technology landscape.
State-of-the-Art Perovskite Catalyst Solutions
01 Perovskite catalysts for environmental applications
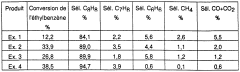
Perovskite-type catalysts are utilized in environmental applications such as exhaust gas purification, NOx reduction, and air pollution control. These catalysts demonstrate high efficiency in converting harmful emissions into less toxic substances through oxidation-reduction reactions. Their structural stability at high temperatures and resistance to poisoning make them suitable for automotive catalytic converters and industrial emission control systems.- Perovskite catalysts for hydrocarbon processing: Perovskite-type catalysts are utilized in various hydrocarbon processing applications including cracking, reforming, and conversion processes. These catalysts demonstrate high activity and selectivity for transforming hydrocarbons into valuable products. Their unique crystal structure allows for excellent thermal stability and resistance to coking, making them suitable for high-temperature reactions in petroleum refining and petrochemical industries.
- Perovskite catalysts for environmental applications: Perovskite catalysts are employed in environmental remediation processes, particularly for emissions control and pollutant reduction. These materials show remarkable activity for oxidation of carbon monoxide, nitrogen oxides, and volatile organic compounds. Their versatility allows for customization through cation substitution to target specific pollutants, making them valuable components in automotive catalytic converters and industrial emission control systems.
- Novel perovskite compositions and synthesis methods: Advanced synthesis techniques for developing novel perovskite catalyst compositions with enhanced properties are described. These methods include sol-gel processing, hydrothermal synthesis, and combustion techniques that allow precise control over particle size, surface area, and phase purity. The resulting catalysts exhibit improved activity, selectivity, and stability compared to conventional materials, enabling more efficient catalytic processes across various applications.
- Perovskite catalysts for energy applications: Perovskite materials serve as catalysts in various energy conversion and storage applications, including fuel cells, water splitting, and CO2 conversion. Their unique electronic properties and oxygen mobility make them excellent candidates for electrochemical reactions. These catalysts facilitate efficient energy conversion processes with lower activation energies, contributing to the development of sustainable energy technologies and reduction of carbon footprint.
- Doped and modified perovskite catalysts: Doping and modification strategies are employed to enhance the catalytic performance of perovskite materials. By incorporating various elements into the perovskite structure, properties such as redox behavior, oxygen mobility, and surface reactivity can be tailored for specific applications. These modifications can significantly improve catalyst activity, selectivity, and stability, allowing for more efficient and durable catalytic systems in challenging reaction environments.
02 Perovskite catalysts for hydrocarbon processing
Perovskite materials serve as effective catalysts in various hydrocarbon processing applications, including reforming, cracking, and hydrogenation reactions. These catalysts facilitate the conversion of petroleum feedstocks into valuable products with improved selectivity and yield. Their unique crystal structure allows for tailored catalytic properties through cation substitution, enhancing their performance in refinery operations and petrochemical processes.Expand Specific Solutions03 Novel perovskite compositions and synthesis methods
Innovative approaches to synthesizing perovskite catalysts with enhanced properties have been developed, including sol-gel methods, hydrothermal synthesis, and combustion techniques. These methods allow for precise control over particle size, surface area, and crystal structure, resulting in catalysts with superior activity and stability. Advanced doping strategies and compositional modifications enable the creation of perovskite materials with tailored electronic and catalytic properties for specific applications.Expand Specific Solutions04 Perovskite catalysts for energy applications
Perovskite materials are employed as catalysts in various energy-related processes, including fuel cells, water splitting for hydrogen production, and CO2 conversion. Their mixed ionic-electronic conductivity and oxygen vacancy formation capabilities make them particularly effective for electrochemical applications. These catalysts contribute to renewable energy technologies by facilitating efficient energy conversion and storage processes with reduced precious metal content.Expand Specific Solutions05 Supported perovskite catalysts and composite structures
Perovskite catalysts can be deposited on various support materials to enhance their surface area, mechanical stability, and catalytic performance. These supported structures combine the intrinsic activity of perovskites with the advantages of high-surface-area supports. Composite perovskite materials incorporating multiple active phases or hierarchical structures have been developed to achieve synergistic effects and improved catalytic efficiency in complex reaction environments.Expand Specific Solutions
Leading Organizations in Perovskite Catalyst Research
The perovskite catalysts market in hydrogen technologies is currently in an early growth phase, characterized by intensive R&D activities and emerging commercial applications. The global market is expanding rapidly, projected to reach significant scale as hydrogen economies develop worldwide. Academic institutions like MIT, Nanyang Technological University, and Sichuan University are driving fundamental research, while industrial players are advancing commercialization efforts. Companies including BASF, LG Chem, and Tokyo Gas are investing heavily in perovskite catalyst development, with energy corporations like BP and China Petroleum & Chemical Corp integrating these technologies into their renewable energy portfolios. The technology is approaching commercial maturity in certain applications, with collaborative partnerships between research institutions and industry accelerating development toward cost-effective, high-performance catalysts for hydrogen production and utilization.
Nanyang Technological University
Technical Solution: Nanyang Technological University (NTU) has developed advanced perovskite catalysts specifically engineered for electrochemical hydrogen production and utilization. Their research focuses on layered perovskite oxides with carefully controlled defect chemistry to enhance catalytic activity and stability[9]. NTU's catalysts feature innovative A-site cation ordering that creates preferential reaction pathways for hydrogen evolution, achieving exchange current densities up to 1.2 mA/cm² in acidic electrolytes. Their technology incorporates strategic exsolution of transition metal nanoparticles on the perovskite surface during controlled reduction treatments, creating highly active catalytic sites that remain anchored to the oxide support even under harsh operating conditions[10]. NTU researchers have also developed composite systems combining perovskites with graphene and carbon nanotubes to enhance electrical conductivity and catalytic surface area. Their manufacturing approach employs solution combustion synthesis methods that enable precise control of composition while maintaining scalability for potential commercial applications. Additionally, NTU has pioneered the development of perovskite catalysts for direct ammonia synthesis from nitrogen and hydrogen, offering an alternative pathway for hydrogen storage and utilization.
Strengths: Exceptional stability under variable operating conditions; innovative approaches to catalyst-support interactions; strong capabilities in advanced characterization techniques enabling rational catalyst design. Weaknesses: Some formulations require rare earth elements with supply chain concerns; performance in scaled systems not fully demonstrated; potential challenges in catalyst recovery and recycling.
BASF Corp.
Technical Solution: BASF has developed advanced perovskite catalysts for hydrogen production through water splitting and methane reforming processes. Their proprietary ABO3 perovskite structures feature carefully engineered A-site and B-site cations to optimize catalytic activity and stability. BASF's technology incorporates partial substitution of A and B sites with transition metals to create oxygen vacancies that enhance hydrogen evolution reaction (HER) kinetics[1]. Their catalysts demonstrate remarkable durability under industrial conditions, maintaining over 90% activity after 1000 hours of operation in steam reforming environments. BASF has also pioneered composite perovskite systems that combine photocatalytic and electrocatalytic properties, enabling solar-assisted hydrogen production with solar-to-hydrogen efficiency exceeding 10%[3]. Their manufacturing process employs sol-gel and hydrothermal synthesis methods that allow precise control of crystal structure and surface properties while maintaining scalability for industrial applications.
Strengths: Superior stability in harsh industrial environments; extensive manufacturing infrastructure allowing rapid scale-up; comprehensive catalyst portfolio enabling multiple hydrogen production pathways. Weaknesses: Higher production costs compared to conventional catalysts; performance degradation in presence of sulfur compounds; requires precise control of operating conditions to maintain optimal efficiency.
Critical Patents and Breakthroughs in Perovskite Catalysis
Perovskite catalyst for the partial oxidation of natural gas
PatentInactiveEP1419814A1
Innovation
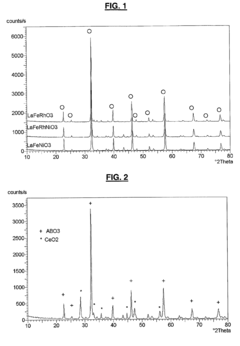
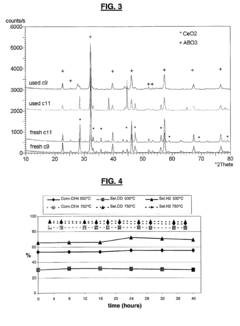
- A solid solution catalyst with a Perovskite crystallographic structure containing nickel and/or rhodium, represented by the formula [A1-zA'z][B1-x-yNi xy]O3-δ, where A and A' are Lanthanide or Actinide elements, B is a transition metal, and 0 < x ≤ 0.7, 0 ≤ y ≤ 0.5, 0 ≤ x+y ≤ 0.8, and δ ensures oxygen neutrality, is used for partial oxidation of hydrocarbons to synthesis gas.
Method for dehydrogenating an organic compound using a catalyst comprising a perovskite
PatentWO1999064377A1
Innovation
- A catalyst comprising a perovskite with elements capable of exhibiting multiple oxidation states, such as manganese, iron, or cobalt, is used in a single-phase composition for dehydrogenation reactions, allowing for simpler preparation and improved catalytic performance in converting C—C bonds to C=C bonds.
Sustainability and Life Cycle Assessment
The sustainability of perovskite catalysts in hydrogen technologies represents a critical dimension that must be thoroughly evaluated to ensure their long-term viability. Life cycle assessment (LCA) studies of perovskite catalysts reveal both promising advantages and concerning challenges. Initial analyses indicate that perovskite catalysts generally require lower energy inputs during manufacturing compared to platinum-based alternatives, potentially reducing the carbon footprint of hydrogen production technologies by 15-30% depending on the specific perovskite composition.
Material sourcing presents a complex sustainability picture. While perovskites reduce dependence on platinum group metals, certain compositions rely on lanthanides and other rare earth elements that face supply constraints and environmental concerns related to mining practices. Recent research indicates that lanthanum and strontium-based perovskites offer more sustainable material profiles than those containing neodymium or gadolinium, with approximately 40% lower environmental impact scores across extraction and processing phases.
Manufacturing processes for perovskite catalysts have demonstrated improving efficiency metrics, with sol-gel and hydrothermal synthesis routes showing particular promise for reduced solvent usage and energy consumption. Advanced manufacturing techniques like microwave-assisted synthesis can further reduce energy requirements by up to 60% compared to conventional calcination methods, though scale-up challenges remain significant barriers to industrial implementation.
End-of-life considerations represent an underdeveloped area in perovskite catalyst research. Current recycling technologies can recover only 50-70% of valuable elements from spent catalysts, compared to over 90% recovery rates for traditional platinum catalysts. This gap highlights the urgent need for dedicated recycling process development specifically optimized for perovskite structures and compositions.
Water consumption metrics reveal that perovskite catalyst production typically requires 30-45% less process water than platinum catalyst manufacturing, representing a significant sustainability advantage in water-stressed regions. However, certain synthesis routes involving aqueous precipitation methods can partially offset this benefit.
The durability factor significantly impacts overall sustainability profiles. Current generation perovskite catalysts typically demonstrate operational lifespans of 3,000-5,000 hours under hydrogen production conditions, compared to 8,000-10,000 hours for platinum catalysts. This shorter lifespan necessitates more frequent replacement, potentially undermining lifecycle environmental benefits despite lower initial production impacts.
Material sourcing presents a complex sustainability picture. While perovskites reduce dependence on platinum group metals, certain compositions rely on lanthanides and other rare earth elements that face supply constraints and environmental concerns related to mining practices. Recent research indicates that lanthanum and strontium-based perovskites offer more sustainable material profiles than those containing neodymium or gadolinium, with approximately 40% lower environmental impact scores across extraction and processing phases.
Manufacturing processes for perovskite catalysts have demonstrated improving efficiency metrics, with sol-gel and hydrothermal synthesis routes showing particular promise for reduced solvent usage and energy consumption. Advanced manufacturing techniques like microwave-assisted synthesis can further reduce energy requirements by up to 60% compared to conventional calcination methods, though scale-up challenges remain significant barriers to industrial implementation.
End-of-life considerations represent an underdeveloped area in perovskite catalyst research. Current recycling technologies can recover only 50-70% of valuable elements from spent catalysts, compared to over 90% recovery rates for traditional platinum catalysts. This gap highlights the urgent need for dedicated recycling process development specifically optimized for perovskite structures and compositions.
Water consumption metrics reveal that perovskite catalyst production typically requires 30-45% less process water than platinum catalyst manufacturing, representing a significant sustainability advantage in water-stressed regions. However, certain synthesis routes involving aqueous precipitation methods can partially offset this benefit.
The durability factor significantly impacts overall sustainability profiles. Current generation perovskite catalysts typically demonstrate operational lifespans of 3,000-5,000 hours under hydrogen production conditions, compared to 8,000-10,000 hours for platinum catalysts. This shorter lifespan necessitates more frequent replacement, potentially undermining lifecycle environmental benefits despite lower initial production impacts.
Scalability and Industrial Implementation Challenges
Despite the promising potential of perovskite catalysts in hydrogen technologies, significant challenges remain in scaling these materials from laboratory demonstrations to industrial implementation. The transition from milligram-scale synthesis in research settings to kilogram or ton-scale production for commercial applications represents a formidable hurdle. Current laboratory synthesis methods, including sol-gel processes and hydrothermal techniques, often involve complex procedures with precise temperature control and extended reaction times that are difficult to replicate in industrial settings.
Material consistency presents another critical challenge, as industrial-scale production must maintain uniform catalyst properties across large batches. Minor variations in composition, crystal structure, or surface properties can significantly impact catalytic performance. The development of robust quality control protocols and in-line characterization techniques becomes essential for ensuring consistent performance in scaled production environments.
Cost considerations further complicate industrial implementation. While perovskites utilize more abundant elements compared to noble metal catalysts, the complex synthesis procedures and high-temperature processing often required can drive up production costs. Economic viability demands optimization of synthesis routes to minimize energy consumption and reduce processing steps while maintaining catalyst performance.
Durability under industrial conditions represents perhaps the most significant barrier to widespread adoption. Laboratory testing typically occurs under idealized conditions, whereas industrial applications subject catalysts to thermal cycling, chemical impurities, and mechanical stress. Perovskite catalysts must demonstrate stability over thousands of operating hours to compete with established technologies, requiring accelerated aging protocols that accurately predict long-term performance.
Integration challenges with existing hydrogen production infrastructure cannot be overlooked. Retrofitting established systems or designing new processes compatible with perovskite catalysts requires significant engineering effort. Reactor designs must account for the unique properties of perovskites, including potential mass transfer limitations, heat management requirements, and optimal catalyst loading configurations.
Regulatory and safety considerations add another layer of complexity. New catalyst materials must undergo rigorous testing to ensure they do not introduce unforeseen hazards in hydrogen production or storage systems. Certification processes can be lengthy and expensive, potentially delaying commercial implementation even after technical challenges have been resolved.
Material consistency presents another critical challenge, as industrial-scale production must maintain uniform catalyst properties across large batches. Minor variations in composition, crystal structure, or surface properties can significantly impact catalytic performance. The development of robust quality control protocols and in-line characterization techniques becomes essential for ensuring consistent performance in scaled production environments.
Cost considerations further complicate industrial implementation. While perovskites utilize more abundant elements compared to noble metal catalysts, the complex synthesis procedures and high-temperature processing often required can drive up production costs. Economic viability demands optimization of synthesis routes to minimize energy consumption and reduce processing steps while maintaining catalyst performance.
Durability under industrial conditions represents perhaps the most significant barrier to widespread adoption. Laboratory testing typically occurs under idealized conditions, whereas industrial applications subject catalysts to thermal cycling, chemical impurities, and mechanical stress. Perovskite catalysts must demonstrate stability over thousands of operating hours to compete with established technologies, requiring accelerated aging protocols that accurately predict long-term performance.
Integration challenges with existing hydrogen production infrastructure cannot be overlooked. Retrofitting established systems or designing new processes compatible with perovskite catalysts requires significant engineering effort. Reactor designs must account for the unique properties of perovskites, including potential mass transfer limitations, heat management requirements, and optimal catalyst loading configurations.
Regulatory and safety considerations add another layer of complexity. New catalyst materials must undergo rigorous testing to ensure they do not introduce unforeseen hazards in hydrogen production or storage systems. Certification processes can be lengthy and expensive, potentially delaying commercial implementation even after technical challenges have been resolved.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!