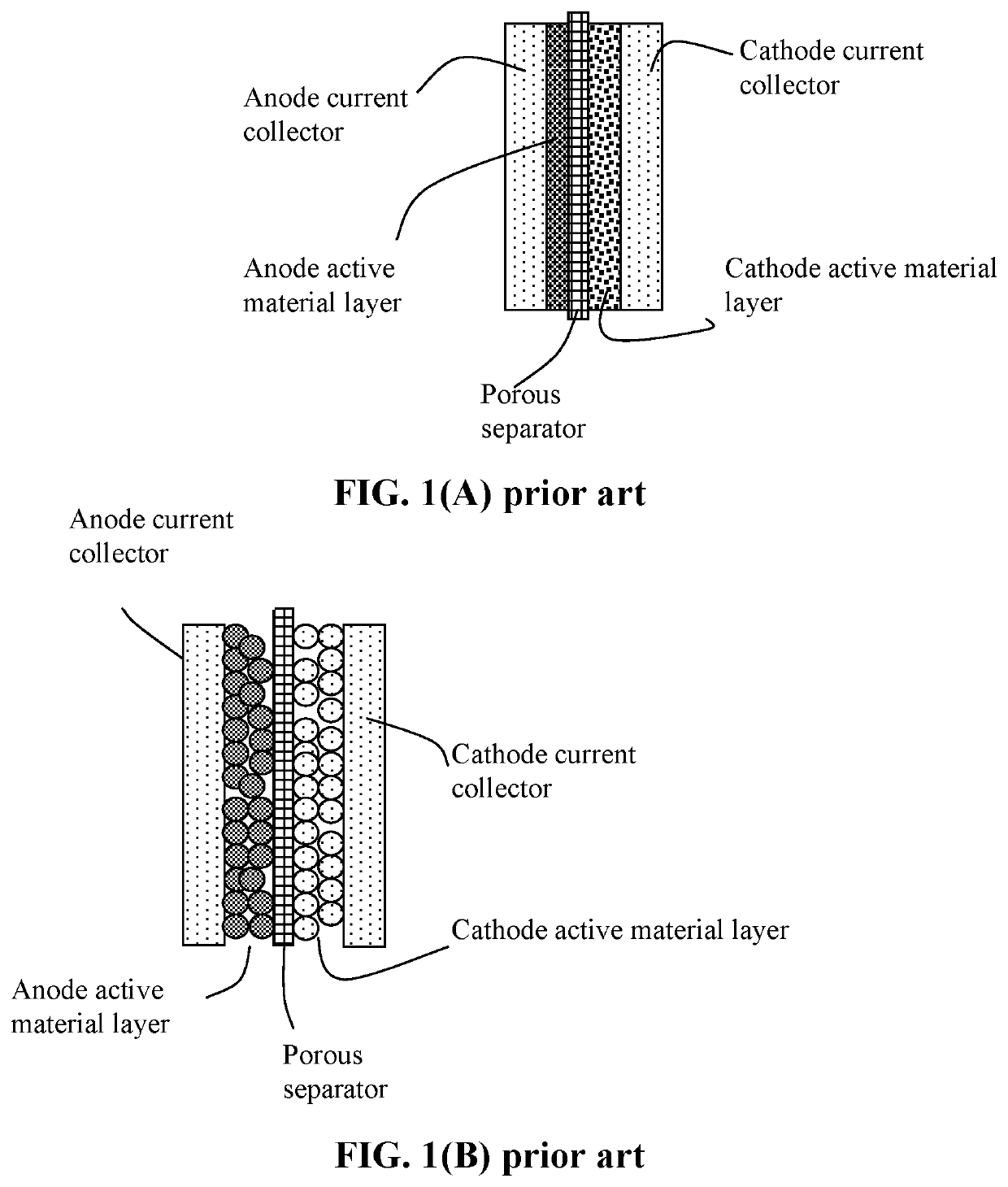
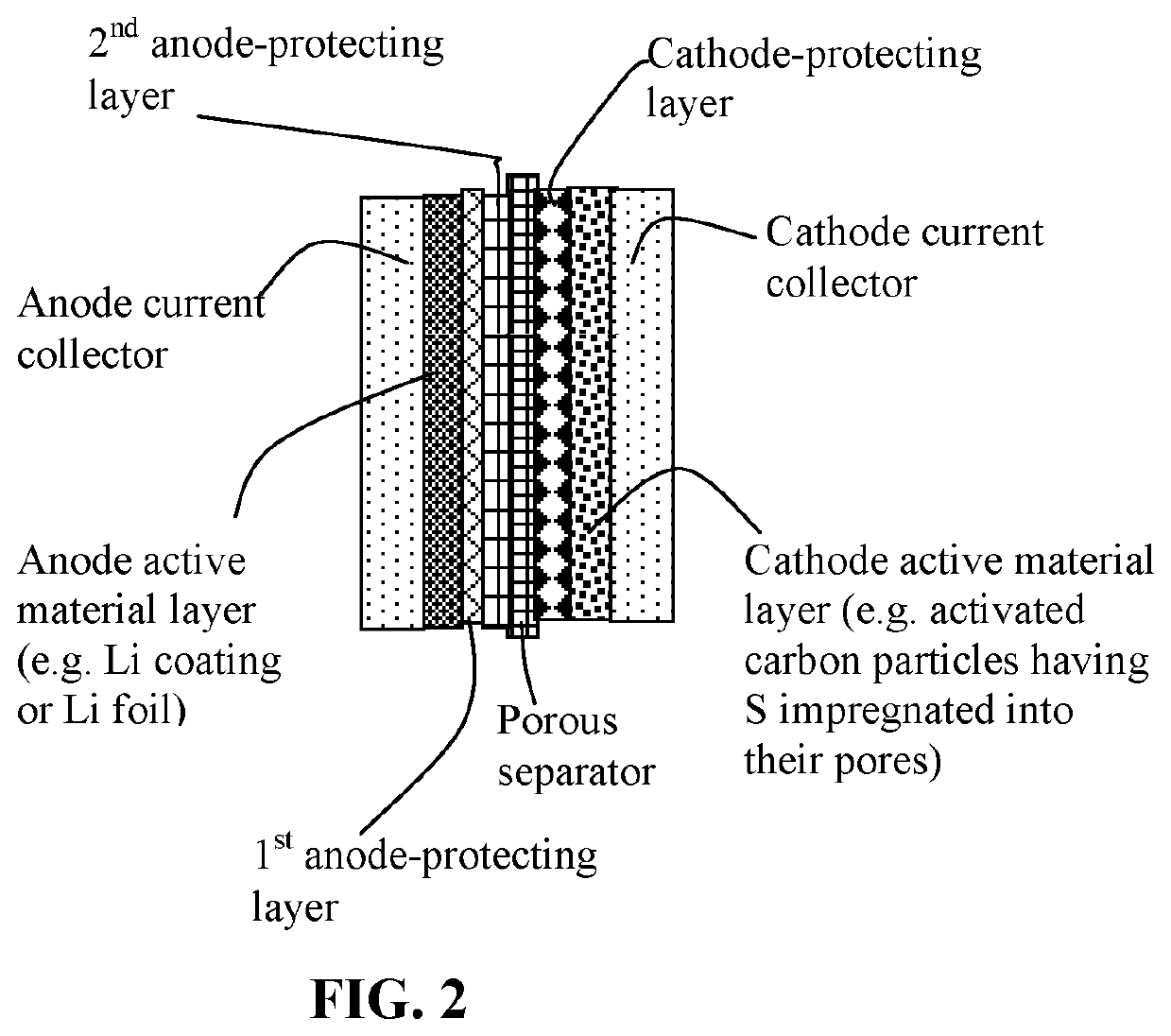
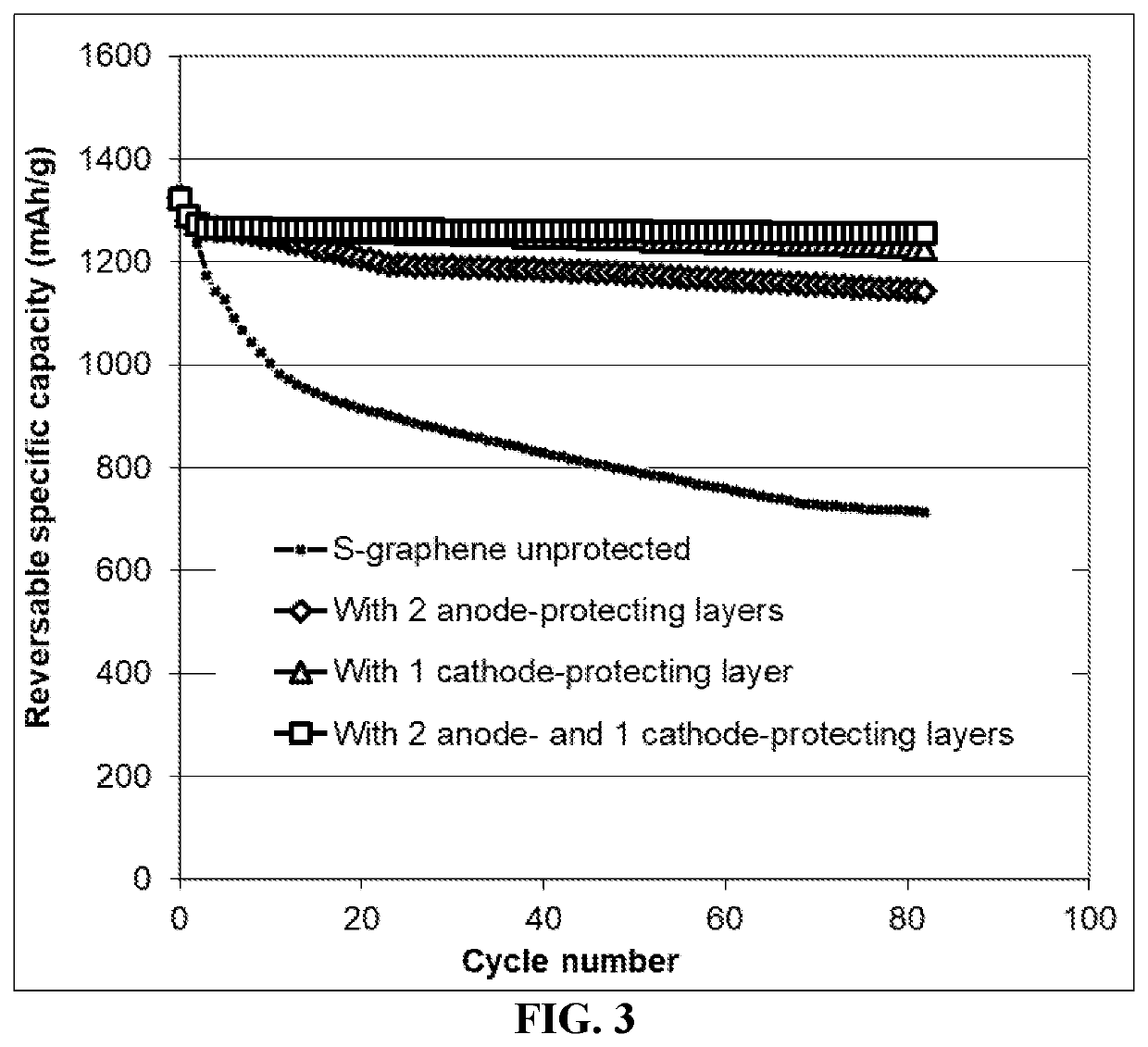
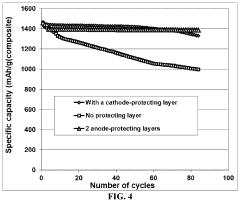
Anode Protection Techniques For Prolonged Li-S Cycling
AUG 22, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Li-S Battery Technology Background and Objectives
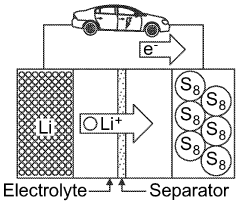
Lithium-sulfur (Li-S) batteries have emerged as a promising next-generation energy storage technology due to their theoretical energy density of 2600 Wh/kg, which significantly surpasses the capabilities of conventional lithium-ion batteries. The development of Li-S battery technology can be traced back to the 1960s, but significant research momentum has only been gained in the past two decades as the limitations of traditional lithium-ion batteries became increasingly apparent for advanced applications.
The evolution of Li-S technology has been characterized by several key breakthroughs, particularly in cathode design and electrolyte formulations. However, the protection of the lithium metal anode has consistently remained one of the most challenging aspects of Li-S battery development. The highly reactive nature of lithium metal with polysulfide species creates a complex electrochemical environment that leads to rapid capacity fading and shortened cycle life.
Current technological objectives in Li-S battery development focus primarily on addressing the "shuttle effect" - the migration of polysulfide intermediates between electrodes - and protecting the lithium metal anode from degradation. Specifically for anode protection, research aims to develop techniques that can effectively prevent dendrite formation, minimize electrolyte decomposition, and create stable solid-electrolyte interphases (SEI) that can withstand the mechanical stresses of repeated cycling.
The strategic importance of advancing Li-S battery technology lies in its potential applications across multiple high-value sectors. Electric vehicles represent the most significant market opportunity, where the theoretical energy density of Li-S could enable driving ranges exceeding 500 miles on a single charge. Additionally, aerospace applications, grid-scale energy storage, and portable electronics could all benefit substantially from the weight reduction and energy density improvements offered by Li-S technology.
Recent technological trends indicate a convergence of multiple protection strategies, including artificial SEI layers, electrolyte additives, and physical barriers such as solid-state electrolytes. The integration of nanotechnology has also opened new avenues for anode protection, with nanostructured materials showing promise in controlling lithium deposition morphology and preventing dendrite formation.
The ultimate goal of current Li-S research is to develop commercially viable batteries with energy densities above 500 Wh/kg, cycle lives exceeding 1000 cycles, and sufficient safety characteristics for mainstream adoption. Achieving these targets requires fundamental breakthroughs in anode protection techniques that can effectively address the multifaceted degradation mechanisms at the lithium-electrolyte interface.
The evolution of Li-S technology has been characterized by several key breakthroughs, particularly in cathode design and electrolyte formulations. However, the protection of the lithium metal anode has consistently remained one of the most challenging aspects of Li-S battery development. The highly reactive nature of lithium metal with polysulfide species creates a complex electrochemical environment that leads to rapid capacity fading and shortened cycle life.
Current technological objectives in Li-S battery development focus primarily on addressing the "shuttle effect" - the migration of polysulfide intermediates between electrodes - and protecting the lithium metal anode from degradation. Specifically for anode protection, research aims to develop techniques that can effectively prevent dendrite formation, minimize electrolyte decomposition, and create stable solid-electrolyte interphases (SEI) that can withstand the mechanical stresses of repeated cycling.
The strategic importance of advancing Li-S battery technology lies in its potential applications across multiple high-value sectors. Electric vehicles represent the most significant market opportunity, where the theoretical energy density of Li-S could enable driving ranges exceeding 500 miles on a single charge. Additionally, aerospace applications, grid-scale energy storage, and portable electronics could all benefit substantially from the weight reduction and energy density improvements offered by Li-S technology.
Recent technological trends indicate a convergence of multiple protection strategies, including artificial SEI layers, electrolyte additives, and physical barriers such as solid-state electrolytes. The integration of nanotechnology has also opened new avenues for anode protection, with nanostructured materials showing promise in controlling lithium deposition morphology and preventing dendrite formation.
The ultimate goal of current Li-S research is to develop commercially viable batteries with energy densities above 500 Wh/kg, cycle lives exceeding 1000 cycles, and sufficient safety characteristics for mainstream adoption. Achieving these targets requires fundamental breakthroughs in anode protection techniques that can effectively address the multifaceted degradation mechanisms at the lithium-electrolyte interface.
Market Analysis for Li-S Battery Applications
The lithium-sulfur (Li-S) battery market is experiencing significant growth potential due to the technology's theoretical energy density of 2600 Wh/kg, which far exceeds that of conventional lithium-ion batteries (typically 250-300 Wh/kg). This substantial energy density advantage positions Li-S batteries as a promising solution for applications requiring high energy storage capacity with minimal weight constraints.
The electric vehicle (EV) sector represents the largest potential market for Li-S batteries, with projections indicating that the global EV battery market will reach $95 billion by 2028. Within this market, Li-S technology could capture a growing share as manufacturers seek solutions to extend vehicle range while reducing battery weight. The aviation industry also presents substantial opportunities, particularly for electric aircraft and drones where the weight-to-energy ratio is critical for performance.
Consumer electronics constitutes another significant market segment, with demand for longer-lasting portable devices driving interest in advanced battery technologies. The global consumer electronics battery market is expected to grow at a CAGR of 7.3% through 2027, creating opportunities for Li-S battery integration in premium devices where performance outweighs cost considerations.
Grid storage applications represent an emerging market for Li-S technology, particularly in regions investing heavily in renewable energy infrastructure. The stationary energy storage market is projected to reach $19.7 billion by 2027, with Li-S batteries potentially capturing specialized niches within this sector.
Market adoption faces several challenges, including the current high production costs compared to established lithium-ion technologies. Manufacturing costs for Li-S batteries remain 30-40% higher than conventional alternatives, limiting immediate commercial viability to premium applications. Additionally, concerns regarding cycle life stability—directly related to anode protection techniques—have slowed widespread adoption.
Regional market analysis indicates that Asia-Pacific, particularly China, South Korea, and Japan, leads in Li-S battery research and development investments. North America follows with significant research initiatives at both academic and commercial levels, while Europe has established strategic funding programs specifically targeting sulfur-based battery technologies.
Market forecasts suggest that with continued improvements in anode protection techniques extending cycle life beyond 1000 cycles, Li-S batteries could achieve a compound annual growth rate of 35% between 2025-2030, potentially reaching commercial viability for mainstream applications by mid-decade. This growth trajectory depends heavily on successful resolution of the lithium metal anode degradation issues that currently limit long-term cycling performance.
The electric vehicle (EV) sector represents the largest potential market for Li-S batteries, with projections indicating that the global EV battery market will reach $95 billion by 2028. Within this market, Li-S technology could capture a growing share as manufacturers seek solutions to extend vehicle range while reducing battery weight. The aviation industry also presents substantial opportunities, particularly for electric aircraft and drones where the weight-to-energy ratio is critical for performance.
Consumer electronics constitutes another significant market segment, with demand for longer-lasting portable devices driving interest in advanced battery technologies. The global consumer electronics battery market is expected to grow at a CAGR of 7.3% through 2027, creating opportunities for Li-S battery integration in premium devices where performance outweighs cost considerations.
Grid storage applications represent an emerging market for Li-S technology, particularly in regions investing heavily in renewable energy infrastructure. The stationary energy storage market is projected to reach $19.7 billion by 2027, with Li-S batteries potentially capturing specialized niches within this sector.
Market adoption faces several challenges, including the current high production costs compared to established lithium-ion technologies. Manufacturing costs for Li-S batteries remain 30-40% higher than conventional alternatives, limiting immediate commercial viability to premium applications. Additionally, concerns regarding cycle life stability—directly related to anode protection techniques—have slowed widespread adoption.
Regional market analysis indicates that Asia-Pacific, particularly China, South Korea, and Japan, leads in Li-S battery research and development investments. North America follows with significant research initiatives at both academic and commercial levels, while Europe has established strategic funding programs specifically targeting sulfur-based battery technologies.
Market forecasts suggest that with continued improvements in anode protection techniques extending cycle life beyond 1000 cycles, Li-S batteries could achieve a compound annual growth rate of 35% between 2025-2030, potentially reaching commercial viability for mainstream applications by mid-decade. This growth trajectory depends heavily on successful resolution of the lithium metal anode degradation issues that currently limit long-term cycling performance.
Anode Protection Challenges in Li-S Batteries
Lithium-sulfur (Li-S) batteries face significant challenges related to anode protection that severely limit their commercial viability despite their promising theoretical energy density of 2600 Wh/kg. The lithium metal anode, while offering high specific capacity (3860 mAh/g), suffers from several critical degradation mechanisms during cycling that compromise battery performance and longevity.
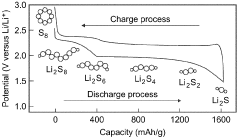
The primary challenge stems from the polysulfide shuttle effect, where dissolved lithium polysulfides migrate from the cathode to the anode during cycling. These species react with the lithium metal anode, forming insoluble Li2S and Li2S2 precipitates on the anode surface. This parasitic reaction not only consumes active lithium but also creates an unstable solid electrolyte interphase (SEI), leading to continuous electrolyte decomposition and capacity fade.
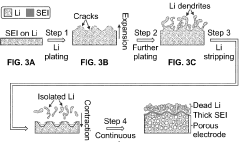
Dendrite formation represents another major obstacle for lithium metal anodes in Li-S systems. During repeated charging cycles, lithium tends to deposit unevenly on the anode surface, forming needle-like structures that can penetrate the separator and cause internal short circuits. This safety hazard is exacerbated in Li-S batteries due to the complex electrolyte environment containing various sulfur species that interfere with uniform lithium plating.
The volume expansion of lithium metal during cycling (approximately 80%) creates mechanical stress that fractures the SEI layer. This continuous breaking and reforming of the SEI consumes both lithium and electrolyte, accelerating capacity fade. The problem is particularly severe in Li-S batteries where the electrolyte must simultaneously accommodate sulfur chemistry at the cathode and lithium metal protection at the anode.
Electrolyte compatibility presents additional challenges, as conventional electrolyte formulations that work well for lithium-ion batteries often fail to form stable protective layers on lithium in the presence of polysulfides. The highly reactive nature of lithium metal, combined with the corrosive nature of polysulfides, creates a particularly harsh environment for the anode.
The cumulative effect of these challenges manifests as poor coulombic efficiency, typically below 98% for unprotected lithium anodes in Li-S batteries, compared to >99.9% needed for practical applications. This efficiency gap translates to rapid capacity decay, with many Li-S cells losing significant capacity within just 100-200 cycles, far below the 1000+ cycles required for commercial viability.
These anode protection challenges represent a critical bottleneck in Li-S battery development, with recent research increasingly focused on innovative protection strategies to address these fundamental limitations and unlock the full potential of this promising battery chemistry.
The primary challenge stems from the polysulfide shuttle effect, where dissolved lithium polysulfides migrate from the cathode to the anode during cycling. These species react with the lithium metal anode, forming insoluble Li2S and Li2S2 precipitates on the anode surface. This parasitic reaction not only consumes active lithium but also creates an unstable solid electrolyte interphase (SEI), leading to continuous electrolyte decomposition and capacity fade.
Dendrite formation represents another major obstacle for lithium metal anodes in Li-S systems. During repeated charging cycles, lithium tends to deposit unevenly on the anode surface, forming needle-like structures that can penetrate the separator and cause internal short circuits. This safety hazard is exacerbated in Li-S batteries due to the complex electrolyte environment containing various sulfur species that interfere with uniform lithium plating.
The volume expansion of lithium metal during cycling (approximately 80%) creates mechanical stress that fractures the SEI layer. This continuous breaking and reforming of the SEI consumes both lithium and electrolyte, accelerating capacity fade. The problem is particularly severe in Li-S batteries where the electrolyte must simultaneously accommodate sulfur chemistry at the cathode and lithium metal protection at the anode.
Electrolyte compatibility presents additional challenges, as conventional electrolyte formulations that work well for lithium-ion batteries often fail to form stable protective layers on lithium in the presence of polysulfides. The highly reactive nature of lithium metal, combined with the corrosive nature of polysulfides, creates a particularly harsh environment for the anode.
The cumulative effect of these challenges manifests as poor coulombic efficiency, typically below 98% for unprotected lithium anodes in Li-S batteries, compared to >99.9% needed for practical applications. This efficiency gap translates to rapid capacity decay, with many Li-S cells losing significant capacity within just 100-200 cycles, far below the 1000+ cycles required for commercial viability.
These anode protection challenges represent a critical bottleneck in Li-S battery development, with recent research increasingly focused on innovative protection strategies to address these fundamental limitations and unlock the full potential of this promising battery chemistry.
Current Anode Protection Solutions and Implementations
01 Protective coatings for lithium metal anodes
Protective coatings can be applied to lithium metal anodes to prevent direct contact with the electrolyte and mitigate the shuttle effect of polysulfides. These coatings create a physical barrier that inhibits the formation of lithium dendrites and reduces side reactions with polysulfides, thereby enhancing cycling stability. Various materials such as polymers, ceramics, and composite layers can be used as protective coatings to improve the electrochemical performance of lithium-sulfur batteries.- Protective coatings for lithium anodes: Protective coatings can be applied to lithium metal anodes to prevent direct contact with polysulfides, which cause capacity fading. These coatings create a physical barrier that inhibits the shuttle effect while maintaining ion conductivity. Materials such as polymers, ceramics, and carbon-based films can be used to form these protective layers, significantly improving cycling stability and extending battery life by preventing lithium dendrite formation and polysulfide dissolution.
- Structured carbon hosts for sulfur cathodes: Specially designed carbon structures can be used as hosts for sulfur in the cathode to improve cycling stability. These carbon frameworks, including mesoporous carbon, carbon nanotubes, and graphene, physically confine sulfur and polysulfides, preventing their dissolution into the electrolyte. The high surface area and conductivity of these carbon hosts also enhance electron transfer and accommodate volume changes during cycling, leading to improved capacity retention and longer battery life.
- Electrolyte additives for anode protection: Specific additives in the electrolyte can form a stable solid electrolyte interphase (SEI) on the lithium anode surface, protecting it from continuous reactions with the electrolyte and polysulfides. These additives, including lithium nitrate, fluorinated compounds, and certain salts, help suppress the shuttle effect and prevent lithium dendrite growth. The resulting SEI layer improves the interfacial stability between the anode and electrolyte, enhancing cycling performance and coulombic efficiency of lithium-sulfur batteries.
- Interlayers and separators with functional coatings: Specialized interlayers or modified separators can be placed between the anode and cathode to block polysulfide migration while allowing lithium ion transport. These interlayers can be coated with materials such as metal oxides, conductive polymers, or carbon materials that have strong chemical interactions with polysulfides. By preventing polysulfides from reaching the lithium anode, these functional layers significantly reduce capacity fading and improve the cycling stability of lithium-sulfur batteries.
- Anode structure engineering: The physical structure of the lithium anode can be engineered to improve cycling stability. Approaches include creating 3D structured anodes, lithium alloys, or composite anodes with reinforcing materials. These structured anodes provide more uniform lithium deposition/dissolution, reduce local current density, and accommodate volume changes during cycling. By controlling the lithium deposition behavior and providing mechanical stability, these engineered anodes significantly improve cycling performance and prevent premature battery failure.
02 Structured carbon hosts for sulfur cathodes
Specially designed carbon structures can be used as hosts for sulfur in the cathode to indirectly protect the anode. These carbon materials, including porous carbon, carbon nanotubes, and graphene, can physically trap polysulfides and prevent their migration to the anode. By confining polysulfides within the cathode structure, these carbon hosts reduce the shuttle effect and subsequent anode degradation, leading to improved cycling stability of lithium-sulfur batteries.Expand Specific Solutions03 Electrolyte additives for anode protection
Specific additives can be incorporated into the electrolyte to form a stable solid electrolyte interphase (SEI) on the lithium anode surface. These additives react preferentially with the lithium surface to create a protective layer that prevents continuous electrolyte decomposition and polysulfide attack. Common additives include lithium nitrate, fluorinated compounds, and various salts that contribute to the formation of a robust SEI layer, enhancing the cycling stability of lithium-sulfur batteries.Expand Specific Solutions04 Interlayers and separators with functional coatings
Specialized interlayers or modified separators can be placed between the anode and cathode to block polysulfide migration. These components can be functionalized with materials that have strong chemical affinity for polysulfides, such as metal oxides, polymers with polar groups, or carbon materials with specific surface treatments. By intercepting polysulfides before they reach the anode, these interlayers significantly improve the cycling stability and coulombic efficiency of lithium-sulfur batteries.Expand Specific Solutions05 Anode-free and lithium alloy approaches
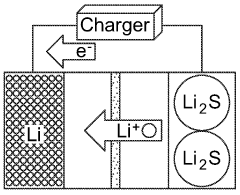
Alternative anode designs, such as anode-free configurations or lithium alloys, can be employed to address the cycling stability issues. In anode-free designs, lithium is plated onto a current collector during the first charge, eliminating initial anode degradation. Lithium alloys with metals like silicon, tin, or aluminum can reduce volume changes during cycling and suppress dendrite formation. These approaches modify the fundamental behavior of the anode material, resulting in improved cycling performance of lithium-sulfur batteries.Expand Specific Solutions
Key Industry Players in Li-S Battery Development
The lithium-sulfur (Li-S) battery market is currently in its early growth phase, characterized by significant R&D investment but limited commercial deployment. The global market size is projected to reach approximately $1 billion by 2025, with a CAGR exceeding 30%. Technologically, anode protection techniques for Li-S batteries are advancing rapidly, with key players demonstrating varying levels of maturity. Companies like Sion Power and PolyPlus Battery have achieved notable breakthroughs in lithium metal anode protection, while established corporations such as Samsung SDI and Johnson Matthey are leveraging their manufacturing expertise to scale these technologies. Academic institutions including Northwestern University and Tsinghua University are contributing fundamental research, while Global Graphene Group and Fraunhofer-Gesellschaft are developing innovative materials solutions to address the lithium dendrite formation challenge that currently limits Li-S cycling performance.
Samsung SDI Co., Ltd.
Technical Solution: Samsung SDI has developed a multi-layered protection strategy for lithium-sulfur battery anodes. Their approach combines a lithium metal anode with an artificial solid electrolyte interphase (SEI) layer composed of LiF-rich compounds that provides superior protection against polysulfide shuttling. This engineered SEI layer is created through pre-treatment of lithium metal with fluorinated electrolyte additives that form a stable, ion-conductive but electronically insulating barrier. Additionally, Samsung has implemented a functional interlayer between the anode and separator, consisting of carbon materials doped with nitrogen and sulfur that can trap polysulfides before they reach the lithium metal surface. Their comprehensive protection system also incorporates electrolyte engineering with high salt concentration and solvation structure optimization to minimize direct contact between solvent molecules and lithium metal, effectively suppressing dendrite formation and parasitic reactions[1][3].
Strengths: Samsung's approach offers excellent protection against polysulfide shuttling while maintaining high ionic conductivity. The multi-layered strategy addresses multiple failure modes simultaneously. Their solution is compatible with existing battery manufacturing processes. Weaknesses: The complex protection system increases production costs and may reduce energy density due to additional inactive materials. Long-term stability of the artificial SEI in real-world conditions remains a challenge.
PolyPlus Battery Co., Inc.
Technical Solution: PolyPlus has pioneered a protected lithium electrode (PLE) technology specifically designed for lithium-sulfur batteries. Their proprietary approach utilizes a water-stable ceramic membrane that acts as a solid electrolyte interface between the lithium metal anode and the sulfur cathode. This ceramic membrane is composed of a lithium-ion conducting glass ceramic material that allows lithium ions to pass through while physically blocking polysulfide migration. The membrane is chemically bonded to the lithium metal surface through a proprietary process, creating a hermetic seal that prevents electrolyte decomposition at the anode surface. PolyPlus has further enhanced this technology by incorporating a gradient structure within the ceramic membrane, with varying porosity and composition to optimize both mechanical strength and ionic conductivity. Their latest iterations include a self-healing component that can repair minor cracks or defects that may form during cycling, significantly extending battery life beyond conventional protection methods[2][5].
Strengths: The ceramic membrane provides exceptional protection against polysulfide shuttling and dendrite formation. The hermetic seal significantly reduces side reactions at the anode surface. The technology enables extremely high cycle life compared to conventional approaches. Weaknesses: Manufacturing complexity and cost of the ceramic membrane may limit commercial scalability. The rigid ceramic interface may be susceptible to mechanical stress during cell assembly and operation.
Critical Patents and Research on Li-S Anode Protection
Method of protecting anode of a lithium-sulfur battery
PatentActiveUS20190386342A1
Innovation
- The implementation of an alkali metal-sulfur battery design featuring a sulfur-carbon hybrid cathode and a dual anode-protecting layer system, comprising a thin electron-conducting layer and an elastomer layer with high tensile elasticity, to prevent dendrite formation and enhance sulfur utilization.
High-performance lithium-sulfur batteries enabled by superior lithium anodes and sulfur cathodes
PatentWO2023205524A1
Innovation
- The use of molecular layer deposition (MLD) for coating lithium anodes and atomic layer deposition (ALD) for modifying sulfur cathodes, specifically employing TiO2 coatings to adsorb polysulfides and MLD-LiGL coatings to mitigate side reactions and dendritic growth, enhancing the performance of Li-S batteries.
Material Science Advancements for Li-S Battery Systems
Material science advancements have played a pivotal role in addressing the fundamental challenges of lithium-sulfur (Li-S) battery systems. Recent breakthroughs in material engineering have significantly improved the performance metrics of these energy storage devices, particularly in terms of cycle life and energy density. The development of novel nanostructured carbon materials with tailored porosity has enabled better sulfur containment and utilization, while simultaneously providing conductive pathways for electron transport.
Advanced polymer science has contributed substantially to the creation of functional binders and separators that mitigate polysulfide shuttling effects. These polymeric materials, often incorporating polar functional groups, can effectively trap dissolved polysulfides through chemical interactions, preventing their migration to the lithium anode. Composite materials combining the advantages of different components have emerged as promising candidates for cathode structures, offering enhanced mechanical stability and electrochemical performance.
Surface modification techniques have evolved to create protective interfaces on both electrodes, with particular emphasis on the lithium metal anode. These modifications typically involve the application of artificial solid electrolyte interphase (SEI) layers composed of inorganic compounds, polymers, or hybrid materials. Such protective layers serve as physical barriers against polysulfide attack while maintaining sufficient ionic conductivity for lithium transport.
Electrolyte engineering has witnessed significant progress with the development of localized high-concentration electrolytes and ionic liquids that suppress polysulfide dissolution. These advanced electrolyte formulations often incorporate additives that contribute to stable SEI formation on the lithium anode, thereby enhancing cycling stability and coulombic efficiency.
Nanoscale engineering approaches have enabled precise control over electrode architectures, resulting in optimized sulfur utilization and reduced volume expansion during cycling. Hierarchical porous structures with rational design of pore size distribution have demonstrated superior performance by accommodating sulfur volumetric changes while maintaining structural integrity throughout repeated charge-discharge cycles.
The integration of two-dimensional materials such as graphene and MXenes has opened new avenues for electrode design, offering exceptional mechanical properties and unique surface chemistry that can be leveraged for polysulfide adsorption. These materials, when properly functionalized, can serve dual roles as sulfur hosts in cathodes and protective layers for lithium anodes, addressing multiple failure mechanisms simultaneously.
Advanced polymer science has contributed substantially to the creation of functional binders and separators that mitigate polysulfide shuttling effects. These polymeric materials, often incorporating polar functional groups, can effectively trap dissolved polysulfides through chemical interactions, preventing their migration to the lithium anode. Composite materials combining the advantages of different components have emerged as promising candidates for cathode structures, offering enhanced mechanical stability and electrochemical performance.
Surface modification techniques have evolved to create protective interfaces on both electrodes, with particular emphasis on the lithium metal anode. These modifications typically involve the application of artificial solid electrolyte interphase (SEI) layers composed of inorganic compounds, polymers, or hybrid materials. Such protective layers serve as physical barriers against polysulfide attack while maintaining sufficient ionic conductivity for lithium transport.
Electrolyte engineering has witnessed significant progress with the development of localized high-concentration electrolytes and ionic liquids that suppress polysulfide dissolution. These advanced electrolyte formulations often incorporate additives that contribute to stable SEI formation on the lithium anode, thereby enhancing cycling stability and coulombic efficiency.
Nanoscale engineering approaches have enabled precise control over electrode architectures, resulting in optimized sulfur utilization and reduced volume expansion during cycling. Hierarchical porous structures with rational design of pore size distribution have demonstrated superior performance by accommodating sulfur volumetric changes while maintaining structural integrity throughout repeated charge-discharge cycles.
The integration of two-dimensional materials such as graphene and MXenes has opened new avenues for electrode design, offering exceptional mechanical properties and unique surface chemistry that can be leveraged for polysulfide adsorption. These materials, when properly functionalized, can serve dual roles as sulfur hosts in cathodes and protective layers for lithium anodes, addressing multiple failure mechanisms simultaneously.
Environmental Impact and Sustainability of Li-S Technology
Lithium-Sulfur (Li-S) batteries represent a promising alternative to conventional lithium-ion technologies, offering higher theoretical energy densities and potentially lower costs. However, the environmental implications of this technology must be thoroughly assessed to ensure its sustainability in the long term.
The environmental advantages of Li-S batteries are significant when compared to traditional lithium-ion batteries. Sulfur, the primary cathode material, is abundant in nature and often available as a byproduct of petroleum refining processes, making it an environmentally preferable alternative to cobalt and nickel used in conventional batteries. This abundance translates to reduced mining impacts and lower ecological footprints associated with raw material extraction.
Carbon footprint analyses of Li-S battery production indicate potential reductions in greenhouse gas emissions compared to conventional lithium-ion technologies. This advantage stems primarily from the simplified cathode production process and reduced energy requirements during manufacturing. However, these benefits can only be fully realized when anode protection techniques are optimized to extend battery lifespan, as premature failure negates the environmental advantages through increased replacement frequency.
The end-of-life management of Li-S batteries presents both challenges and opportunities. Sulfur components are generally less toxic and more recyclable than materials in conventional batteries, potentially facilitating more efficient recycling processes. Advanced anode protection techniques that incorporate environmentally benign materials further enhance the recyclability profile of these systems.
Water usage and pollution risks associated with Li-S battery production require careful consideration. While sulfur processing typically demands less water than cobalt or nickel refining, the potential for sulfur compounds to form acidic byproducts necessitates robust waste management protocols. Anode protection techniques utilizing water-soluble components must be evaluated for their potential environmental impact during production and disposal phases.
The sustainability of Li-S technology is intrinsically linked to the effectiveness of anode protection techniques. Extended cycling capabilities directly reduce the environmental burden by decreasing the frequency of battery replacement and associated manufacturing impacts. Research indicates that each doubling of cycle life can reduce lifetime environmental impact by approximately 50%, highlighting the critical importance of advanced anode protection strategies in achieving sustainability goals.
Regulatory frameworks worldwide are increasingly incorporating life-cycle assessment requirements for battery technologies. Li-S systems with optimized anode protection may offer competitive advantages in markets with stringent environmental regulations, potentially accelerating commercial adoption while simultaneously reducing ecological impacts across the technology's life cycle.
The environmental advantages of Li-S batteries are significant when compared to traditional lithium-ion batteries. Sulfur, the primary cathode material, is abundant in nature and often available as a byproduct of petroleum refining processes, making it an environmentally preferable alternative to cobalt and nickel used in conventional batteries. This abundance translates to reduced mining impacts and lower ecological footprints associated with raw material extraction.
Carbon footprint analyses of Li-S battery production indicate potential reductions in greenhouse gas emissions compared to conventional lithium-ion technologies. This advantage stems primarily from the simplified cathode production process and reduced energy requirements during manufacturing. However, these benefits can only be fully realized when anode protection techniques are optimized to extend battery lifespan, as premature failure negates the environmental advantages through increased replacement frequency.
The end-of-life management of Li-S batteries presents both challenges and opportunities. Sulfur components are generally less toxic and more recyclable than materials in conventional batteries, potentially facilitating more efficient recycling processes. Advanced anode protection techniques that incorporate environmentally benign materials further enhance the recyclability profile of these systems.
Water usage and pollution risks associated with Li-S battery production require careful consideration. While sulfur processing typically demands less water than cobalt or nickel refining, the potential for sulfur compounds to form acidic byproducts necessitates robust waste management protocols. Anode protection techniques utilizing water-soluble components must be evaluated for their potential environmental impact during production and disposal phases.
The sustainability of Li-S technology is intrinsically linked to the effectiveness of anode protection techniques. Extended cycling capabilities directly reduce the environmental burden by decreasing the frequency of battery replacement and associated manufacturing impacts. Research indicates that each doubling of cycle life can reduce lifetime environmental impact by approximately 50%, highlighting the critical importance of advanced anode protection strategies in achieving sustainability goals.
Regulatory frameworks worldwide are increasingly incorporating life-cycle assessment requirements for battery technologies. Li-S systems with optimized anode protection may offer competitive advantages in markets with stringent environmental regulations, potentially accelerating commercial adoption while simultaneously reducing ecological impacts across the technology's life cycle.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!