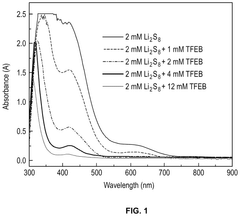
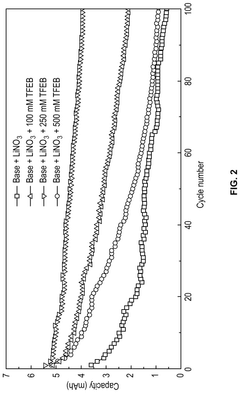
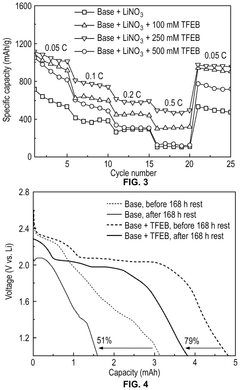
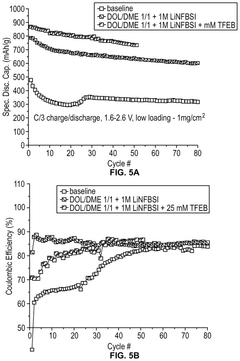
Electrolyte Additives That Improve Li-S Cycle Life
AUG 22, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Li-S Battery Fundamentals and Research Objectives
Lithium-sulfur (Li-S) batteries represent a promising next-generation energy storage technology due to their theoretical energy density of 2600 Wh/kg, which significantly surpasses that of conventional lithium-ion batteries (typically 250-300 Wh/kg). This exceptional energy density stems from sulfur's high theoretical capacity of 1675 mAh/g and its natural abundance, making Li-S batteries potentially more cost-effective and environmentally friendly than current commercial alternatives.
The fundamental working principle of Li-S batteries involves the conversion reaction between lithium and sulfur. During discharge, lithium ions react with sulfur to form various lithium polysulfides (Li₂Sₓ, 2≤x≤8), ultimately producing Li₂S. The reverse process occurs during charging. This multi-step conversion mechanism differs significantly from the intercalation chemistry of conventional lithium-ion batteries.
Despite their promising attributes, Li-S batteries face several critical challenges that have hindered their commercial viability. The most significant issue is their limited cycle life, typically deteriorating after 100-200 cycles under practical conditions. This rapid capacity fade stems from multiple interconnected mechanisms: the dissolution of lithium polysulfides in the electrolyte (known as the "shuttle effect"), volume expansion of sulfur (approximately 80%) during cycling, and lithium anode degradation due to side reactions with dissolved polysulfides.
Electrolyte additives have emerged as a crucial research direction for addressing these challenges. These additives can modify the electrolyte-electrode interfaces, suppress polysulfide dissolution, enhance ionic conductivity, and protect the lithium anode. The strategic design and implementation of effective additives represent a potentially cost-effective approach to improving Li-S battery performance without requiring complete redesign of cell components.
The primary research objectives in this field include: (1) developing electrolyte additives that can effectively suppress the polysulfide shuttle effect; (2) identifying additives that form stable solid electrolyte interphase (SEI) layers on the lithium anode; (3) creating additive combinations that simultaneously address multiple degradation mechanisms; and (4) ensuring that these additives remain compatible with other cell components and manufacturing processes.
Recent advances have demonstrated that certain classes of additives, including lithium nitrate (LiNO₃), fluorinated compounds, and various organic molecules, can significantly extend cycle life. However, a comprehensive understanding of additive mechanisms and their synergistic effects remains incomplete, presenting opportunities for further innovation and optimization in this rapidly evolving field.
The fundamental working principle of Li-S batteries involves the conversion reaction between lithium and sulfur. During discharge, lithium ions react with sulfur to form various lithium polysulfides (Li₂Sₓ, 2≤x≤8), ultimately producing Li₂S. The reverse process occurs during charging. This multi-step conversion mechanism differs significantly from the intercalation chemistry of conventional lithium-ion batteries.
Despite their promising attributes, Li-S batteries face several critical challenges that have hindered their commercial viability. The most significant issue is their limited cycle life, typically deteriorating after 100-200 cycles under practical conditions. This rapid capacity fade stems from multiple interconnected mechanisms: the dissolution of lithium polysulfides in the electrolyte (known as the "shuttle effect"), volume expansion of sulfur (approximately 80%) during cycling, and lithium anode degradation due to side reactions with dissolved polysulfides.
Electrolyte additives have emerged as a crucial research direction for addressing these challenges. These additives can modify the electrolyte-electrode interfaces, suppress polysulfide dissolution, enhance ionic conductivity, and protect the lithium anode. The strategic design and implementation of effective additives represent a potentially cost-effective approach to improving Li-S battery performance without requiring complete redesign of cell components.
The primary research objectives in this field include: (1) developing electrolyte additives that can effectively suppress the polysulfide shuttle effect; (2) identifying additives that form stable solid electrolyte interphase (SEI) layers on the lithium anode; (3) creating additive combinations that simultaneously address multiple degradation mechanisms; and (4) ensuring that these additives remain compatible with other cell components and manufacturing processes.
Recent advances have demonstrated that certain classes of additives, including lithium nitrate (LiNO₃), fluorinated compounds, and various organic molecules, can significantly extend cycle life. However, a comprehensive understanding of additive mechanisms and their synergistic effects remains incomplete, presenting opportunities for further innovation and optimization in this rapidly evolving field.
Market Analysis for Next-Generation Energy Storage
The global energy storage market is witnessing unprecedented growth, with projections indicating a compound annual growth rate of 20-25% through 2030. Within this expanding landscape, lithium-sulfur (Li-S) batteries represent one of the most promising next-generation technologies due to their theoretical energy density of 2600 Wh/kg, which far exceeds the capabilities of conventional lithium-ion batteries (typically 250-300 Wh/kg). This substantial performance advantage positions Li-S technology as a potential game-changer for applications requiring high energy density, particularly in aerospace, electric vehicles, and portable electronics sectors.
Market demand for advanced energy storage solutions is being driven by several converging factors. The accelerating transition to renewable energy sources necessitates efficient, large-scale energy storage systems to address intermittency issues. Simultaneously, the electric vehicle market continues its robust expansion, creating demand for batteries with higher energy density, faster charging capabilities, and lower costs. The portable electronics sector similarly seeks power solutions that can extend device operation while reducing weight and volume.
Electrolyte additives specifically designed to improve Li-S cycle life are emerging as a critical market segment. The current market size for specialized battery electrolyte additives is estimated at $1.2 billion globally, with projections suggesting growth to $3.5 billion by 2028. Within this segment, additives targeting Li-S technology represent a nascent but rapidly expanding niche, currently valued at approximately $150 million.
Industry analysts have identified significant regional variations in market development. Asia-Pacific, particularly China, South Korea, and Japan, leads in commercial development of Li-S technology and associated electrolyte additives, accounting for nearly 60% of global research output and patent filings. North America follows with strong research programs at both academic and corporate levels, while European markets show increasing investment, particularly in countries with strong automotive sectors.
Consumer electronics currently represents the largest application segment for advanced battery technologies, but transportation is projected to become the dominant market for Li-S batteries by 2027. This shift is driven by automotive manufacturers' strategic investments in next-generation battery technologies that can overcome range anxiety concerns while reducing battery weight and cost.
The market for Li-S electrolyte additives faces certain constraints, including price sensitivity in mass-market applications and competition from alternative battery chemistries. However, the potential performance advantages of Li-S technology, particularly when enhanced by effective electrolyte additives, present compelling value propositions for premium applications where energy density is paramount. Market penetration is expected to follow a top-down approach, beginning with specialized high-value applications before gradually expanding into mainstream markets as manufacturing scales and costs decrease.
Market demand for advanced energy storage solutions is being driven by several converging factors. The accelerating transition to renewable energy sources necessitates efficient, large-scale energy storage systems to address intermittency issues. Simultaneously, the electric vehicle market continues its robust expansion, creating demand for batteries with higher energy density, faster charging capabilities, and lower costs. The portable electronics sector similarly seeks power solutions that can extend device operation while reducing weight and volume.
Electrolyte additives specifically designed to improve Li-S cycle life are emerging as a critical market segment. The current market size for specialized battery electrolyte additives is estimated at $1.2 billion globally, with projections suggesting growth to $3.5 billion by 2028. Within this segment, additives targeting Li-S technology represent a nascent but rapidly expanding niche, currently valued at approximately $150 million.
Industry analysts have identified significant regional variations in market development. Asia-Pacific, particularly China, South Korea, and Japan, leads in commercial development of Li-S technology and associated electrolyte additives, accounting for nearly 60% of global research output and patent filings. North America follows with strong research programs at both academic and corporate levels, while European markets show increasing investment, particularly in countries with strong automotive sectors.
Consumer electronics currently represents the largest application segment for advanced battery technologies, but transportation is projected to become the dominant market for Li-S batteries by 2027. This shift is driven by automotive manufacturers' strategic investments in next-generation battery technologies that can overcome range anxiety concerns while reducing battery weight and cost.
The market for Li-S electrolyte additives faces certain constraints, including price sensitivity in mass-market applications and competition from alternative battery chemistries. However, the potential performance advantages of Li-S technology, particularly when enhanced by effective electrolyte additives, present compelling value propositions for premium applications where energy density is paramount. Market penetration is expected to follow a top-down approach, beginning with specialized high-value applications before gradually expanding into mainstream markets as manufacturing scales and costs decrease.
Electrolyte Additive Challenges in Li-S Systems
Despite the promising theoretical energy density of lithium-sulfur (Li-S) batteries, their practical implementation faces significant challenges, with electrolyte-related issues being particularly critical. The electrolyte in Li-S systems must simultaneously address multiple complex requirements that often conflict with each other, creating a multifaceted optimization problem.
The primary challenge stems from the polysulfide shuttle effect, where soluble lithium polysulfides dissolve in the electrolyte and migrate between electrodes, causing active material loss, parasitic reactions, and rapid capacity fading. Conventional electrolyte additives designed for lithium-ion batteries often prove ineffective in the unique chemical environment of Li-S cells.
Electrolyte additives must contend with the highly reactive lithium metal anode, which forms unstable solid electrolyte interphase (SEI) layers in most electrolyte formulations. This reactivity leads to continuous electrolyte consumption and lithium dendrite formation, severely limiting cycle life. Finding additives that can form a stable, flexible, and ion-conductive SEI layer remains a significant hurdle.
The dual-phase transformation of sulfur during cycling presents another major challenge. Additives must accommodate both the solid-to-liquid transition of sulfur to polysulfides and the liquid-to-solid conversion back to Li₂S. This requires carefully balanced solvation properties that many conventional additives cannot provide.
Viscosity management represents another critical challenge. As polysulfides dissolve into the electrolyte, they significantly increase its viscosity, impeding ion transport and reaction kinetics. Additives that can maintain appropriate viscosity while still addressing other requirements are difficult to develop.
Temperature sensitivity further complicates additive selection. Many promising additives show excellent performance in laboratory conditions but fail to maintain effectiveness across the wide temperature range required for practical applications. This temperature dependence often results from changes in solubility, reaction kinetics, and physical properties of the electrolyte system.
Long-term stability issues plague many additive solutions. Additives that initially improve performance often degrade or become depleted over extended cycling, leading to temporary rather than permanent improvements. This degradation can produce secondary compounds that may themselves be detrimental to battery performance.
Concentration optimization presents another significant challenge. The effective concentration window for many additives is extremely narrow, with too little providing insufficient benefit and too much potentially introducing new problems such as increased resistance or unwanted side reactions.
The primary challenge stems from the polysulfide shuttle effect, where soluble lithium polysulfides dissolve in the electrolyte and migrate between electrodes, causing active material loss, parasitic reactions, and rapid capacity fading. Conventional electrolyte additives designed for lithium-ion batteries often prove ineffective in the unique chemical environment of Li-S cells.
Electrolyte additives must contend with the highly reactive lithium metal anode, which forms unstable solid electrolyte interphase (SEI) layers in most electrolyte formulations. This reactivity leads to continuous electrolyte consumption and lithium dendrite formation, severely limiting cycle life. Finding additives that can form a stable, flexible, and ion-conductive SEI layer remains a significant hurdle.
The dual-phase transformation of sulfur during cycling presents another major challenge. Additives must accommodate both the solid-to-liquid transition of sulfur to polysulfides and the liquid-to-solid conversion back to Li₂S. This requires carefully balanced solvation properties that many conventional additives cannot provide.
Viscosity management represents another critical challenge. As polysulfides dissolve into the electrolyte, they significantly increase its viscosity, impeding ion transport and reaction kinetics. Additives that can maintain appropriate viscosity while still addressing other requirements are difficult to develop.
Temperature sensitivity further complicates additive selection. Many promising additives show excellent performance in laboratory conditions but fail to maintain effectiveness across the wide temperature range required for practical applications. This temperature dependence often results from changes in solubility, reaction kinetics, and physical properties of the electrolyte system.
Long-term stability issues plague many additive solutions. Additives that initially improve performance often degrade or become depleted over extended cycling, leading to temporary rather than permanent improvements. This degradation can produce secondary compounds that may themselves be detrimental to battery performance.
Concentration optimization presents another significant challenge. The effective concentration window for many additives is extremely narrow, with too little providing insufficient benefit and too much potentially introducing new problems such as increased resistance or unwanted side reactions.
Current Electrolyte Additive Solutions
01 Lithium nitrate additives for polysulfide suppression
Lithium nitrate (LiNO3) is a critical electrolyte additive for lithium-sulfur batteries that forms a protective layer on the lithium anode surface, preventing polysulfide shuttling. This passivation layer significantly reduces the reaction between lithium metal and soluble polysulfides, thereby improving coulombic efficiency and extending cycle life. The concentration of lithium nitrate can be optimized to balance protection and consumption during cycling.- Lithium nitrate additives for polysulfide suppression: Lithium nitrate (LiNO3) is a critical electrolyte additive for lithium-sulfur batteries that forms a protective layer on the lithium anode surface, preventing polysulfide shuttling. This passivation layer significantly reduces the reaction between lithium metal and soluble polysulfides, thereby improving coulombic efficiency and extending cycle life. The concentration of lithium nitrate can be optimized to balance protection and consumption during cycling.
- Fluorinated electrolyte components: Fluorinated compounds in the electrolyte, such as fluoroethylene carbonate (FEC), fluorinated ethers, and fluorinated sulfones, create stable solid electrolyte interphase (SEI) layers on electrode surfaces. These fluorinated additives enhance the ionic conductivity while reducing polysulfide solubility, which mitigates capacity fading. The incorporation of these compounds results in improved cycle stability and rate capability of lithium-sulfur batteries by modifying the solvation structure of lithium ions.
- Sulfur-containing electrolyte additives: Sulfur-containing additives such as organosulfur compounds, thioethers, and sulfones can be incorporated into electrolytes to enhance the electrochemical performance of Li-S batteries. These additives help form a stable interface on the lithium anode, regulate polysulfide dissolution, and promote the conversion of long-chain polysulfides to short-chain ones. By controlling the sulfur chemistry, these additives significantly improve capacity retention and extend the cycle life of Li-S batteries.
- Ionic liquid-based electrolyte systems: Ionic liquids serve as effective electrolyte components or additives in Li-S batteries due to their high thermal stability, wide electrochemical window, and low volatility. These properties help suppress polysulfide dissolution and migration while enhancing the stability of the lithium anode interface. Ionic liquid-based electrolytes can be tailored by adjusting cation and anion structures to optimize viscosity, conductivity, and compatibility with sulfur cathodes, resulting in improved cycling performance.
- Multifunctional additive combinations: Synergistic combinations of multiple electrolyte additives can address various degradation mechanisms simultaneously in Li-S batteries. These combinations typically include a passivation agent for the lithium anode, a polysulfide mediator, and a viscosity modifier. The carefully balanced formulations create robust protective interfaces on both electrodes while facilitating sulfur utilization and redox kinetics. This comprehensive approach significantly enhances cycle life by mitigating multiple failure modes that single-component additives cannot address alone.
02 Fluorinated electrolyte additives
Fluorinated compounds serve as effective electrolyte additives in Li-S batteries by forming stable solid electrolyte interphase (SEI) layers on electrode surfaces. These additives, including fluoroethylene carbonate (FEC) and fluorinated ethers, enhance the stability of the lithium anode and suppress polysulfide dissolution. The fluorinated components contribute to improved ionic conductivity while reducing side reactions, resulting in enhanced capacity retention and extended cycle life of Li-S batteries.Expand Specific Solutions03 Ionic liquid-based electrolyte additives
Ionic liquids serve as beneficial electrolyte additives for Li-S batteries due to their high thermal stability, wide electrochemical window, and low volatility. These additives help to dissolve polysulfides while controlling their diffusion, thereby mitigating the shuttle effect. The incorporation of ionic liquids also enhances the formation of a stable interface on the lithium anode, reducing dendrite formation and improving cycling performance. Various ionic liquid structures can be tailored to optimize compatibility with other electrolyte components.Expand Specific Solutions04 Polymer and macromolecular additives
Polymer and macromolecular additives in Li-S battery electrolytes function as polysulfide anchors and protective agents. These large molecules, including polyethylene oxide (PEO), polyethylene glycol (PEG), and functionalized polymers, interact with polysulfides through chemical bonding or physical adsorption, restricting their migration. Additionally, these additives can form protective films on the lithium anode surface, preventing direct contact with the electrolyte and reducing parasitic reactions. The molecular weight and functional groups of these polymers can be optimized to enhance their effectiveness.Expand Specific Solutions05 Inorganic salt additives and composite electrolyte systems
Inorganic salt additives and composite electrolyte systems enhance Li-S battery performance through multiple mechanisms. Salts like lithium bis(trifluoromethanesulfonyl)imide (LiTFSI) and lithium bis(fluorosulfonyl)imide (LiFSI), when combined with other additives, create synergistic effects that improve ionic conductivity while stabilizing the electrode-electrolyte interfaces. These composite systems often incorporate multiple functional additives that work together to address various degradation mechanisms simultaneously, resulting in comprehensive protection of both electrodes and significant improvements in cycle life.Expand Specific Solutions
Leading Organizations in Li-S Battery Development
The lithium-sulfur (Li-S) battery electrolyte additives market is currently in an early growth phase, characterized by intensive R&D efforts to overcome cycle life limitations. The global market is projected to expand significantly as Li-S technology offers higher theoretical energy density than conventional lithium-ion batteries. Leading players include established battery manufacturers like Samsung SDI, LG Energy Solution, and CATL, who are investing heavily in electrolyte additive research. Academic institutions, particularly in China (Tsinghua University, Central South University) and the US (Drexel University, Caltech), are driving fundamental innovations. Research organizations like Argonne National Laboratory and CEA are also making significant contributions. The technology remains in pre-commercialization stage, with most solutions still transitioning from laboratory to industrial application, requiring further optimization for long-term stability and performance.
Samsung SDI Co., Ltd.
Technical Solution: Samsung SDI has developed a comprehensive electrolyte additive strategy for Li-S batteries focusing on polysulfide shuttling inhibition. Their approach utilizes lithium nitrate (LiNO3) as a primary additive to form a stable SEI on the lithium anode, combined with fluorinated additives like lithium bis(trifluoromethanesulfonyl)imide (LiTFSI) that enhance the SEI stability. Samsung has pioneered a dual-additive system incorporating both LiNO3 and organic additives such as tris(pentafluorophenyl)borane (TPFPB) that work synergistically to trap polysulfides through Lewis acid-base interactions. Their recent innovation includes sulfur-containing heterocyclic compounds that chemically bind with polysulfides in solution, significantly reducing capacity fade. Samsung's electrolyte formulations typically achieve 500+ cycles at 80% capacity retention, representing a 3-4x improvement over conventional electrolytes without additives.
Strengths: Superior polysulfide trapping capability through multi-functional additive combinations; excellent SEI formation properties; compatibility with their existing battery manufacturing infrastructure. Weaknesses: Some additives may increase electrolyte viscosity affecting low-temperature performance; higher production costs compared to standard electrolytes; potential safety concerns with some fluorinated compounds.
LG Energy Solution Ltd.
Technical Solution: LG Energy Solution has developed a proprietary "Polysulfide Regulation System" (PRS) for Li-S batteries that incorporates multiple electrolyte additives working in concert. Their approach centers on a trifunctional additive strategy: (1) LiNO3 as a primary SEI former, (2) lithium difluoro(oxalato)borate (LiDFOB) that creates a more robust and flexible SEI layer accommodating volume changes, and (3) organic sulfur-containing heterocycles that chemically bind with dissolved polysulfides. LG's innovation includes the incorporation of ionic liquid additives like 1-ethyl-3-methylimidazolium bis(trifluoromethylsulfonyl)imide (EMI-TFSI) at 3-5 wt% concentration, which enhances ionic conductivity while suppressing polysulfide dissolution. Their electrolyte formulations have demonstrated up to 1000 cycles with 70% capacity retention in pouch cell configurations, representing significant progress toward commercial viability. LG has also developed fluoroethylene carbonate (FEC) derivatives specifically tailored for Li-S systems that form more stable interfaces on both electrodes.
Strengths: Exceptional cycle life improvements; balanced approach addressing multiple failure mechanisms simultaneously; additives compatible with scaled manufacturing processes. Weaknesses: Higher cost compared to conventional Li-ion electrolytes; some additives may reduce initial capacity; potential long-term stability issues in extreme temperature conditions.
Key Innovations in Polysulfide Shuttling Inhibition
Electrolyte additives for high performance lithium-sulfur batteries
PatentPendingUS20250030048A1
Innovation
- The use of electrolyte additives such as fluorinated borates and lithium bis(nonafluorobutanesulfonyl)imide (LiNFBSI) to modulate lithium polysulfide conversion, facilitating solid-liquid-solid conversion and preventing active material loss.
Electrolyte for a lithium-sulfur battery and a lithium-sulfur battery using the same
PatentInactiveEP1176659A3
Innovation
- A mixed electrolyte comprising a solvent with a high dielectric constant and low viscosity, along with electrolyte salts and additives that form a solid electrolyte interface (SEI) on the negative electrode, to enhance solubility, ion conductivity, and prevent dendrite formation.
Environmental Impact Assessment
The environmental implications of lithium-sulfur (Li-S) battery technology and its electrolyte additives require thorough assessment as these batteries move toward commercial viability. While Li-S batteries offer promising advantages over conventional lithium-ion batteries, including higher theoretical energy density and the use of abundant, low-cost sulfur, their environmental footprint warrants careful consideration.
The production and disposal of electrolyte additives used to improve Li-S cycle life present both challenges and opportunities from an environmental perspective. Many conventional additives contain fluorinated compounds, such as lithium nitrate (LiNO₃) and lithium bis(trifluoromethanesulfonyl)imide (LiTFSI), which pose potential environmental hazards if improperly managed. These compounds can contribute to soil and water contamination when batteries reach end-of-life, particularly in regions lacking proper recycling infrastructure.
Lifecycle assessment studies indicate that while Li-S batteries may reduce dependence on critical materials like cobalt and nickel, the environmental impact of electrolyte additives remains significant. The synthesis of specialized additives often involves energy-intensive processes and potentially toxic precursors. For instance, the production of polysulfide-trapping additives may require hazardous solvents and generate waste streams that necessitate specialized treatment.
Recent advances in green chemistry approaches have yielded more environmentally benign additives derived from biomass or utilizing water-based synthesis routes. These bio-inspired additives, including modified cellulose derivatives and amino acid-based compounds, demonstrate promising performance while potentially reducing environmental burden. However, their scalability and long-term stability remain under investigation.
The end-of-life management of Li-S batteries with various electrolyte additives presents another environmental consideration. Current recycling processes are primarily designed for conventional lithium-ion batteries and may require adaptation to efficiently recover materials from Li-S systems. The presence of certain additives may complicate recycling processes, potentially reducing recovery rates of valuable materials or requiring additional energy-intensive separation steps.
Regulatory frameworks worldwide are increasingly addressing battery chemistry environmental impacts, with the European Union's Battery Directive and similar initiatives in Asia and North America establishing guidelines for battery production, collection, and recycling. Future regulations may specifically target electrolyte additives based on their environmental persistence, bioaccumulation potential, and toxicity profiles.
As research continues, the development of electrolyte additives for Li-S batteries should incorporate environmental considerations alongside performance metrics, adopting green chemistry principles and designing for recyclability to ensure this promising technology delivers both performance and sustainability benefits.
The production and disposal of electrolyte additives used to improve Li-S cycle life present both challenges and opportunities from an environmental perspective. Many conventional additives contain fluorinated compounds, such as lithium nitrate (LiNO₃) and lithium bis(trifluoromethanesulfonyl)imide (LiTFSI), which pose potential environmental hazards if improperly managed. These compounds can contribute to soil and water contamination when batteries reach end-of-life, particularly in regions lacking proper recycling infrastructure.
Lifecycle assessment studies indicate that while Li-S batteries may reduce dependence on critical materials like cobalt and nickel, the environmental impact of electrolyte additives remains significant. The synthesis of specialized additives often involves energy-intensive processes and potentially toxic precursors. For instance, the production of polysulfide-trapping additives may require hazardous solvents and generate waste streams that necessitate specialized treatment.
Recent advances in green chemistry approaches have yielded more environmentally benign additives derived from biomass or utilizing water-based synthesis routes. These bio-inspired additives, including modified cellulose derivatives and amino acid-based compounds, demonstrate promising performance while potentially reducing environmental burden. However, their scalability and long-term stability remain under investigation.
The end-of-life management of Li-S batteries with various electrolyte additives presents another environmental consideration. Current recycling processes are primarily designed for conventional lithium-ion batteries and may require adaptation to efficiently recover materials from Li-S systems. The presence of certain additives may complicate recycling processes, potentially reducing recovery rates of valuable materials or requiring additional energy-intensive separation steps.
Regulatory frameworks worldwide are increasingly addressing battery chemistry environmental impacts, with the European Union's Battery Directive and similar initiatives in Asia and North America establishing guidelines for battery production, collection, and recycling. Future regulations may specifically target electrolyte additives based on their environmental persistence, bioaccumulation potential, and toxicity profiles.
As research continues, the development of electrolyte additives for Li-S batteries should incorporate environmental considerations alongside performance metrics, adopting green chemistry principles and designing for recyclability to ensure this promising technology delivers both performance and sustainability benefits.
Scalability and Manufacturing Considerations
The scalability of electrolyte additives for lithium-sulfur batteries presents significant challenges when transitioning from laboratory-scale research to commercial production. Current manufacturing processes must be adapted to incorporate these specialized additives while maintaining consistent quality and performance. The integration of additives such as lithium nitrate (LiNO₃), polysulfides, and various organic compounds requires precise control over mixing ratios, temperature conditions, and reaction environments.
Manufacturing considerations include the stability of additives during large-scale production processes. Many effective additives demonstrate excellent performance in controlled laboratory environments but may degrade or lose efficacy when subjected to industrial manufacturing conditions. Temperature sensitivity, shelf life, and compatibility with existing battery production equipment are critical factors that must be addressed before widespread implementation.
Cost implications represent another crucial aspect of scalability. While certain additives show remarkable improvements in cycle life, their economic viability depends on raw material availability, synthesis complexity, and required concentration levels. For instance, fluorinated additives that effectively passivate lithium metal surfaces often involve expensive precursors and complex synthesis routes, potentially limiting their commercial application despite technical benefits.
Supply chain considerations must also be evaluated when selecting additives for large-scale production. Sustainable sourcing of raw materials, particularly for rare or specialized compounds, may present bottlenecks in manufacturing scale-up. Additives derived from abundant resources with straightforward synthesis pathways offer advantages for mass production scenarios, even if they provide somewhat lower performance improvements.
Regulatory compliance adds another layer of complexity to manufacturing scale-up. New chemical additives must meet safety standards and environmental regulations across different markets. Toxicity profiles, environmental persistence, and disposal considerations all impact the feasibility of commercial implementation. Additives containing volatile organic compounds or heavy metals face particularly stringent regulatory hurdles despite potential electrochemical benefits.
Process integration represents the final critical consideration for scalability. The introduction of additives must be seamlessly incorporated into existing battery manufacturing workflows without requiring extensive equipment modifications or additional processing steps. Ideally, additives should be compatible with standard mixing and electrolyte preparation processes to minimize disruption to established production lines and avoid significant capital investments.
Manufacturing considerations include the stability of additives during large-scale production processes. Many effective additives demonstrate excellent performance in controlled laboratory environments but may degrade or lose efficacy when subjected to industrial manufacturing conditions. Temperature sensitivity, shelf life, and compatibility with existing battery production equipment are critical factors that must be addressed before widespread implementation.
Cost implications represent another crucial aspect of scalability. While certain additives show remarkable improvements in cycle life, their economic viability depends on raw material availability, synthesis complexity, and required concentration levels. For instance, fluorinated additives that effectively passivate lithium metal surfaces often involve expensive precursors and complex synthesis routes, potentially limiting their commercial application despite technical benefits.
Supply chain considerations must also be evaluated when selecting additives for large-scale production. Sustainable sourcing of raw materials, particularly for rare or specialized compounds, may present bottlenecks in manufacturing scale-up. Additives derived from abundant resources with straightforward synthesis pathways offer advantages for mass production scenarios, even if they provide somewhat lower performance improvements.
Regulatory compliance adds another layer of complexity to manufacturing scale-up. New chemical additives must meet safety standards and environmental regulations across different markets. Toxicity profiles, environmental persistence, and disposal considerations all impact the feasibility of commercial implementation. Additives containing volatile organic compounds or heavy metals face particularly stringent regulatory hurdles despite potential electrochemical benefits.
Process integration represents the final critical consideration for scalability. The introduction of additives must be seamlessly incorporated into existing battery manufacturing workflows without requiring extensive equipment modifications or additional processing steps. Ideally, additives should be compatible with standard mixing and electrolyte preparation processes to minimize disruption to established production lines and avoid significant capital investments.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!