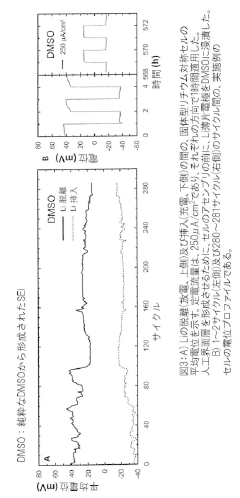
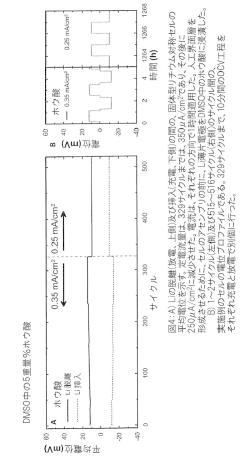
Solid-State Lithium-Sulfur Cells: Materials And Interfaces
AUG 22, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Li-S Battery Technology Background and Objectives
Lithium-sulfur (Li-S) batteries have emerged as a promising next-generation energy storage technology, offering theoretical energy densities up to 2600 Wh/kg, significantly surpassing the 387 Wh/kg limit of conventional lithium-ion batteries. This remarkable potential stems from sulfur's high theoretical capacity of 1675 mAh/g and its natural abundance, making it both economically viable and environmentally sustainable.
The development of Li-S battery technology can be traced back to the 1960s when the first conceptual designs were proposed. However, significant progress only began in the early 2000s when researchers started addressing the fundamental challenges inherent to the system. The evolution of this technology has been characterized by incremental improvements in addressing issues such as the shuttle effect, volume expansion, and poor conductivity of sulfur.
Current technological trends in Li-S batteries focus on three primary directions: cathode engineering to accommodate sulfur's volume changes, electrolyte optimization to mitigate polysulfide dissolution, and the development of protective layers for lithium metal anodes. The most recent and promising advancement is the integration of solid-state electrolytes to create all-solid-state Li-S batteries, which potentially eliminates many of the challenges associated with liquid electrolyte systems.
The solid-state approach represents a paradigm shift in Li-S technology, offering a pathway to overcome the persistent shuttle effect while enhancing safety and longevity. This direction aligns with broader industry trends toward solid-state battery technologies across various chemistries. The materials and interfaces in solid-state Li-S cells present unique challenges and opportunities that distinguish them from both conventional Li-S batteries and other solid-state systems.
The primary technical objectives for solid-state Li-S battery development include achieving stable interfaces between sulfur cathodes and solid electrolytes, developing solid electrolytes with sufficient ionic conductivity at room temperature, and ensuring compatibility with lithium metal anodes. Additionally, researchers aim to maintain the high energy density advantage of Li-S chemistry while addressing cycle life limitations.
Looking forward, the technology roadmap for solid-state Li-S batteries envisions commercial viability within the next decade, with initial applications in specialized high-energy-density requirements such as aerospace and defense, before potentially expanding to consumer electronics and electric vehicles. The achievement of these objectives would represent a significant breakthrough in energy storage technology, potentially enabling a new generation of lighter, more powerful, and more sustainable battery systems.
The development of Li-S battery technology can be traced back to the 1960s when the first conceptual designs were proposed. However, significant progress only began in the early 2000s when researchers started addressing the fundamental challenges inherent to the system. The evolution of this technology has been characterized by incremental improvements in addressing issues such as the shuttle effect, volume expansion, and poor conductivity of sulfur.
Current technological trends in Li-S batteries focus on three primary directions: cathode engineering to accommodate sulfur's volume changes, electrolyte optimization to mitigate polysulfide dissolution, and the development of protective layers for lithium metal anodes. The most recent and promising advancement is the integration of solid-state electrolytes to create all-solid-state Li-S batteries, which potentially eliminates many of the challenges associated with liquid electrolyte systems.
The solid-state approach represents a paradigm shift in Li-S technology, offering a pathway to overcome the persistent shuttle effect while enhancing safety and longevity. This direction aligns with broader industry trends toward solid-state battery technologies across various chemistries. The materials and interfaces in solid-state Li-S cells present unique challenges and opportunities that distinguish them from both conventional Li-S batteries and other solid-state systems.
The primary technical objectives for solid-state Li-S battery development include achieving stable interfaces between sulfur cathodes and solid electrolytes, developing solid electrolytes with sufficient ionic conductivity at room temperature, and ensuring compatibility with lithium metal anodes. Additionally, researchers aim to maintain the high energy density advantage of Li-S chemistry while addressing cycle life limitations.
Looking forward, the technology roadmap for solid-state Li-S batteries envisions commercial viability within the next decade, with initial applications in specialized high-energy-density requirements such as aerospace and defense, before potentially expanding to consumer electronics and electric vehicles. The achievement of these objectives would represent a significant breakthrough in energy storage technology, potentially enabling a new generation of lighter, more powerful, and more sustainable battery systems.
Market Analysis for Solid-State Li-S Batteries
The global market for solid-state lithium-sulfur (Li-S) batteries is experiencing significant growth potential, driven by increasing demand for high-energy-density storage solutions across multiple sectors. Current market projections indicate that the solid-state battery market could reach $8 billion by 2026, with Li-S technology potentially capturing a substantial portion of this emerging segment due to its theoretical energy density advantages over conventional lithium-ion batteries.
The automotive industry represents the primary market driver for solid-state Li-S batteries, as electric vehicle manufacturers seek solutions that offer greater range, faster charging capabilities, and enhanced safety profiles. Major automotive OEMs including Toyota, Volkswagen, and BMW have made substantial investments in solid-state battery technologies, recognizing the potential competitive advantage these systems could provide in next-generation electric vehicles.
Consumer electronics constitutes another significant market segment, where the demand for longer-lasting portable devices continues to grow. The theoretical energy density of Li-S batteries (approximately 2,600 Wh/kg) far exceeds that of current lithium-ion technologies (250-300 Wh/kg), making them particularly attractive for applications where weight and volume constraints are critical factors.
The aerospace and defense sectors also present substantial opportunities for solid-state Li-S batteries, particularly for drone technology, satellite systems, and other specialized applications requiring high energy density and operational safety. Market analysis indicates these sectors could represent early adoption pathways for premium-priced solid-state Li-S solutions before economies of scale enable broader commercial deployment.
Regional market distribution shows Asia-Pacific leading in manufacturing capacity development, with Japan, South Korea, and China making significant investments in solid-state battery production infrastructure. North America and Europe are focusing more heavily on research and development activities, with substantial government funding supporting innovation in this space.
Market barriers include high production costs, with current estimates suggesting solid-state Li-S batteries may initially cost 2-3 times more than conventional lithium-ion batteries. Manufacturing scalability remains challenging, particularly regarding the production of solid electrolytes with consistent quality and performance characteristics.
Consumer adoption will likely follow a staged approach, beginning with premium applications where performance advantages outweigh cost considerations, followed by broader market penetration as manufacturing processes mature and economies of scale develop. Market analysts project that solid-state Li-S batteries could begin capturing meaningful market share by 2025-2027, contingent upon successful resolution of current materials interface challenges and demonstration of cycle life improvements.
The automotive industry represents the primary market driver for solid-state Li-S batteries, as electric vehicle manufacturers seek solutions that offer greater range, faster charging capabilities, and enhanced safety profiles. Major automotive OEMs including Toyota, Volkswagen, and BMW have made substantial investments in solid-state battery technologies, recognizing the potential competitive advantage these systems could provide in next-generation electric vehicles.
Consumer electronics constitutes another significant market segment, where the demand for longer-lasting portable devices continues to grow. The theoretical energy density of Li-S batteries (approximately 2,600 Wh/kg) far exceeds that of current lithium-ion technologies (250-300 Wh/kg), making them particularly attractive for applications where weight and volume constraints are critical factors.
The aerospace and defense sectors also present substantial opportunities for solid-state Li-S batteries, particularly for drone technology, satellite systems, and other specialized applications requiring high energy density and operational safety. Market analysis indicates these sectors could represent early adoption pathways for premium-priced solid-state Li-S solutions before economies of scale enable broader commercial deployment.
Regional market distribution shows Asia-Pacific leading in manufacturing capacity development, with Japan, South Korea, and China making significant investments in solid-state battery production infrastructure. North America and Europe are focusing more heavily on research and development activities, with substantial government funding supporting innovation in this space.
Market barriers include high production costs, with current estimates suggesting solid-state Li-S batteries may initially cost 2-3 times more than conventional lithium-ion batteries. Manufacturing scalability remains challenging, particularly regarding the production of solid electrolytes with consistent quality and performance characteristics.
Consumer adoption will likely follow a staged approach, beginning with premium applications where performance advantages outweigh cost considerations, followed by broader market penetration as manufacturing processes mature and economies of scale develop. Market analysts project that solid-state Li-S batteries could begin capturing meaningful market share by 2025-2027, contingent upon successful resolution of current materials interface challenges and demonstration of cycle life improvements.
Current Challenges in Li-S Cell Development
Despite the promising theoretical energy density of lithium-sulfur (Li-S) batteries, their commercial deployment faces significant challenges. The primary obstacle remains the polysulfide shuttle effect, where soluble lithium polysulfides formed during discharge migrate between electrodes, causing capacity fading and reduced coulombic efficiency. This phenomenon is particularly problematic in conventional liquid electrolyte systems, leading researchers to explore solid-state configurations.
The insulating nature of sulfur presents another major hurdle, with its electrical conductivity of approximately 5×10^-30 S/cm severely limiting electron transport during electrochemical reactions. This necessitates the incorporation of conductive additives, which unfortunately reduces the overall energy density of the cell by adding inactive weight.
Volume expansion during cycling constitutes a critical challenge, as sulfur undergoes substantial volumetric changes (up to 80%) during lithiation/delithiation processes. In solid-state configurations, this expansion creates mechanical stress at interfaces, leading to contact loss between active materials and solid electrolytes, ultimately resulting in capacity degradation and cell failure.
Interface stability between lithium metal anodes and solid electrolytes remains problematic. The high reactivity of lithium metal with most solid electrolyte materials leads to the formation of resistive interphases that impede ion transport. Additionally, lithium dendrite growth through solid electrolytes continues to threaten cell safety and longevity.
The development of suitable solid electrolytes for Li-S systems presents unique challenges. The ideal solid electrolyte must exhibit high lithium-ion conductivity (>10^-4 S/cm at room temperature), while simultaneously preventing polysulfide migration. Current solid electrolyte materials struggle to balance these requirements effectively.
Manufacturing scalability poses significant barriers to commercialization. Traditional solid-state battery fabrication techniques often involve high-temperature sintering processes incompatible with sulfur's low melting point (115°C). Alternative low-temperature processing methods frequently result in high interfacial resistance and poor mechanical integrity.
The cost factor cannot be overlooked, as current solid-state Li-S cell prototypes utilize expensive materials and complex manufacturing processes. For commercial viability, production costs must be substantially reduced while maintaining performance advantages over conventional lithium-ion technologies.
Analytical limitations further complicate development efforts, as characterizing the complex interfaces and reaction mechanisms within solid-state Li-S cells requires sophisticated in-situ and operando techniques that are not widely accessible to all research groups.
The insulating nature of sulfur presents another major hurdle, with its electrical conductivity of approximately 5×10^-30 S/cm severely limiting electron transport during electrochemical reactions. This necessitates the incorporation of conductive additives, which unfortunately reduces the overall energy density of the cell by adding inactive weight.
Volume expansion during cycling constitutes a critical challenge, as sulfur undergoes substantial volumetric changes (up to 80%) during lithiation/delithiation processes. In solid-state configurations, this expansion creates mechanical stress at interfaces, leading to contact loss between active materials and solid electrolytes, ultimately resulting in capacity degradation and cell failure.
Interface stability between lithium metal anodes and solid electrolytes remains problematic. The high reactivity of lithium metal with most solid electrolyte materials leads to the formation of resistive interphases that impede ion transport. Additionally, lithium dendrite growth through solid electrolytes continues to threaten cell safety and longevity.
The development of suitable solid electrolytes for Li-S systems presents unique challenges. The ideal solid electrolyte must exhibit high lithium-ion conductivity (>10^-4 S/cm at room temperature), while simultaneously preventing polysulfide migration. Current solid electrolyte materials struggle to balance these requirements effectively.
Manufacturing scalability poses significant barriers to commercialization. Traditional solid-state battery fabrication techniques often involve high-temperature sintering processes incompatible with sulfur's low melting point (115°C). Alternative low-temperature processing methods frequently result in high interfacial resistance and poor mechanical integrity.
The cost factor cannot be overlooked, as current solid-state Li-S cell prototypes utilize expensive materials and complex manufacturing processes. For commercial viability, production costs must be substantially reduced while maintaining performance advantages over conventional lithium-ion technologies.
Analytical limitations further complicate development efforts, as characterizing the complex interfaces and reaction mechanisms within solid-state Li-S cells requires sophisticated in-situ and operando techniques that are not widely accessible to all research groups.
Current Material Solutions for Li-S Interfaces
01 Solid electrolyte materials for lithium-sulfur batteries
Various solid electrolyte materials can be used in lithium-sulfur batteries to improve performance and safety. These include polymer electrolytes, ceramic electrolytes, and composite electrolytes that combine different materials to achieve optimal ionic conductivity while preventing lithium dendrite growth and polysulfide shuttling. Solid electrolytes help overcome challenges associated with conventional liquid electrolytes, such as electrolyte leakage and flammability issues.- Solid electrolyte materials for lithium-sulfur batteries: Various solid electrolyte materials can be used in lithium-sulfur batteries to improve performance and safety. These materials include polymer electrolytes, ceramic electrolytes, and composite electrolytes that combine different materials to achieve optimal properties. Solid electrolytes help prevent polysulfide shuttling, a common issue in lithium-sulfur batteries, and enhance the overall stability and cycle life of the battery.
- Interface engineering between electrodes and electrolytes: Interface engineering is crucial for solid-state lithium-sulfur batteries to ensure good contact between the electrodes and the solid electrolyte. Various approaches include surface coatings, buffer layers, and interface modifiers that can reduce interfacial resistance and improve ion transport across the interfaces. These techniques help address issues such as volume changes during cycling and chemical incompatibilities between components.
- Sulfur cathode modifications and composites: Modifications to the sulfur cathode can significantly improve the performance of solid-state lithium-sulfur batteries. These include creating sulfur-carbon composites, using conductive additives, and developing porous structures to accommodate volume changes during cycling. Such modifications help increase the utilization of active material, enhance conductivity, and improve the overall energy density of the battery.
- Lithium anode protection strategies: Protecting the lithium metal anode is essential for the stability and safety of solid-state lithium-sulfur batteries. Various strategies include using protective layers, artificial SEI (solid electrolyte interphase) formation, and lithium alloys to prevent dendrite formation and side reactions. These approaches help extend battery life and improve safety by preventing short circuits and degradation of the anode.
- Novel cell designs and architectures: Innovative cell designs and architectures can address the challenges of solid-state lithium-sulfur batteries. These include 3D structures, sandwich configurations, and integrated designs that optimize ion transport pathways and mechanical stability. Novel cell architectures help accommodate volume changes, improve energy density, and enhance the overall performance and manufacturability of solid-state lithium-sulfur batteries.
02 Interface engineering in solid-state lithium-sulfur batteries
Interface engineering is crucial for solid-state lithium-sulfur batteries to address issues at the electrode-electrolyte interfaces. This includes modifying the interfaces between the lithium anode and solid electrolyte, as well as between the sulfur cathode and solid electrolyte. Various coating materials, buffer layers, and interface modification techniques are employed to reduce interfacial resistance, enhance ion transport, and improve the overall electrochemical performance of the battery.Expand Specific Solutions03 Cathode materials and structures for solid-state lithium-sulfur batteries
Advanced cathode materials and structures are developed for solid-state lithium-sulfur batteries to improve sulfur utilization and prevent polysulfide dissolution. These include carbon-based materials, conductive polymers, and metal oxides that can host sulfur and facilitate electron/ion transport. Hierarchical porous structures, core-shell configurations, and nanocomposites are designed to accommodate volume changes during cycling and enhance the electrochemical performance of the sulfur cathode.Expand Specific Solutions04 Anode protection strategies for solid-state lithium-sulfur batteries
Various strategies are employed to protect the lithium metal anode in solid-state lithium-sulfur batteries. These include artificial solid electrolyte interphase layers, protective coatings, and buffer layers that prevent direct contact between lithium metal and the solid electrolyte. These protection strategies aim to suppress lithium dendrite growth, reduce side reactions, and enhance the cycling stability and safety of the battery.Expand Specific Solutions05 Manufacturing methods for solid-state lithium-sulfur batteries
Various manufacturing methods are developed for solid-state lithium-sulfur batteries to address challenges in cell assembly and scale-up. These include dry and wet processing techniques for electrode preparation, solid electrolyte synthesis and processing methods, and cell assembly approaches that ensure good contact between components. Advanced manufacturing techniques aim to reduce interfacial resistance, enhance component integration, and improve the overall performance and reliability of solid-state lithium-sulfur batteries.Expand Specific Solutions
Key Industry Players in Solid-State Li-S Research
The solid-state lithium-sulfur battery market is currently in an early growth phase, characterized by intensive R&D activities and limited commercial deployment. With a projected market size reaching $6-8 billion by 2030, this technology promises significantly higher energy density than conventional lithium-ion batteries. Leading automotive manufacturers including Toyota, Nissan, Honda, and Rivian are actively developing this technology, while specialized companies like Sion Power and Honeycomb Battery Co. are pioneering commercial applications. Research institutions such as Forschungszentrum Jülich and Cornell University are addressing fundamental challenges in materials interfaces. Major chemical companies including LG Chem, Arkema, and Global Graphene Group are developing advanced materials to overcome conductivity and stability issues that currently limit widespread adoption.
Toyota Motor Corp.
Technical Solution: Toyota has pioneered advanced solid-state lithium-sulfur battery technology through its integrated materials approach. Their technology employs a sulfide-based solid electrolyte with high ionic conductivity (>10^-3 S/cm at room temperature) combined with a specialized lithium metal anode protection layer. Toyota's design incorporates a hierarchical carbon-sulfur composite cathode structure that maximizes active material utilization while minimizing volume expansion during cycling. The company has developed proprietary interface engineering techniques that reduce contact resistance between the solid electrolyte and electrodes, addressing one of the key challenges in solid-state battery performance. Their research demonstrates stable cycling with minimal capacity fade over 300+ cycles and operation across a wider temperature range (-20°C to 60°C) than conventional lithium-sulfur cells with liquid electrolytes. Toyota has filed numerous patents on their solid-state lithium-sulfur technology, particularly focusing on interface stabilization methods and manufacturing processes suitable for automotive-scale production.
Strengths: Extensive experience in battery integration for automotive applications; strong intellectual property portfolio in solid-state technology; vertical integration capabilities from materials to vehicle systems. Weaknesses: Higher production costs compared to conventional lithium-ion batteries; challenges with scaling manufacturing to meet automotive volume requirements; interface stability issues during extreme temperature operation.
Nissan Motor Co., Ltd.
Technical Solution: Nissan has developed a proprietary solid-state lithium-sulfur battery technology that builds on their extensive experience in electric vehicle battery systems. Their approach centers on a hybrid solid electrolyte system that combines the benefits of sulfide-based and oxide-based materials to achieve both high ionic conductivity and mechanical stability. Nissan's cathode design incorporates a carbon-sulfur composite structure with engineered interfaces that maximize active material utilization while minimizing polysulfide dissolution. A key innovation in their technology is the implementation of a gradient interface between the solid electrolyte and electrodes, which reduces contact resistance and improves cycling stability. Their lithium metal anode protection strategy employs a multi-layer approach that effectively suppresses dendrite formation while maintaining high coulombic efficiency. Laboratory testing has demonstrated energy densities of approximately 450 Wh/kg with stable cycling performance over 250+ cycles. Nissan has integrated this technology into their broader electric vehicle strategy, with plans for commercial implementation in specialized applications before scaling to passenger vehicles. Their manufacturing approach focuses on processes compatible with existing production infrastructure to accelerate commercialization.
Strengths: Deep integration with automotive requirements and testing protocols; extensive experience with battery system design and thermal management; established supply chain relationships. Weaknesses: Challenges with achieving consistent interface properties at scale; higher material costs compared to conventional lithium-ion batteries; limited high-rate performance compared to liquid electrolyte systems.
Critical Patents in Solid-State Electrolyte Materials
Solid state lithium-sulfur battery and manufacturing method thereof
PatentPendingJP2024031545A
Innovation
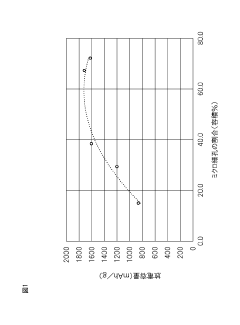
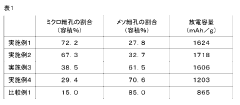
- A solid lithium-sulfur battery design with a positive electrode layer containing porous carbon with a specific ratio of micropores (2 nm or less) and mesopores (2-50 nm) and sulfur supported in these pores, with a volume ratio of micropores to total pores ranging from 25.0% to 73.0%, optimized for solid electrolytes.
Interface layer between lithium metal and solid electrolyte
PatentActiveJP2019125578A
Innovation
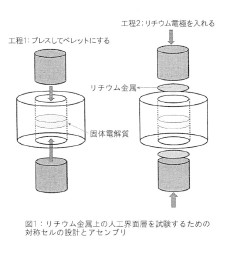
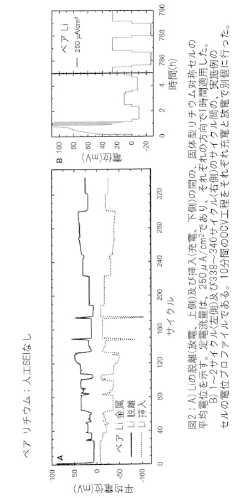
- An artificial interfacial layer formed by reacting lithium metal with an acid in a non-aqueous solvent creates a lithium-ion conducting but electronically non-conducting layer on the lithium metal surface, preventing electrolyte decomposition and stabilizing the electrode.
Environmental Impact and Sustainability Assessment
The environmental impact of solid-state lithium-sulfur (Li-S) battery technology represents a critical dimension in evaluating its viability as a next-generation energy storage solution. Compared to conventional lithium-ion batteries, solid-state Li-S cells offer significant sustainability advantages through their utilization of sulfur—an abundant, low-cost, and environmentally benign material—as the cathode active material. This approach substantially reduces dependence on critical raw materials such as cobalt and nickel, which face supply constraints and ethical mining concerns.
Life cycle assessment (LCA) studies indicate that solid-state Li-S cells potentially have a 25-30% lower carbon footprint during manufacturing compared to conventional lithium-ion batteries. This reduction stems primarily from the simplified cathode production process and the elimination of toxic organic electrolytes. The solid electrolytes employed in these systems, while energy-intensive to produce in current manufacturing paradigms, offer opportunities for process optimization that could further reduce environmental impacts.
Water consumption represents another key environmental consideration. Preliminary analyses suggest that solid-state Li-S cell production may require 15-20% less water than conventional battery manufacturing, primarily due to differences in electrode preparation and the absence of liquid electrolyte processing steps. However, certain solid electrolyte synthesis routes, particularly those involving solution-based methods, remain water-intensive and require further optimization.
End-of-life management presents both challenges and opportunities for solid-state Li-S technology. The absence of toxic liquid electrolytes simplifies recycling processes and reduces hazardous waste management requirements. Additionally, the high theoretical value of recoverable lithium and the relatively simple material composition enhance economic recycling feasibility. Research indicates potential recovery rates exceeding 90% for lithium and 95% for sulfur from spent cells using hydrometallurgical processes.
Resource efficiency constitutes a fundamental sustainability advantage of Li-S chemistry. Global sulfur reserves are abundant, with significant quantities available as byproducts from petroleum refining and natural gas processing. This availability contrasts sharply with the geopolitically concentrated and supply-constrained nature of cobalt and nickel resources used in conventional lithium-ion batteries.
Safety improvements inherent to solid-state designs—including reduced fire risk and elimination of volatile organic compounds—translate to decreased environmental hazards throughout the product lifecycle. These enhancements minimize the potential for acute environmental contamination events while simultaneously reducing the need for complex safety systems that add to material and energy footprints.
Life cycle assessment (LCA) studies indicate that solid-state Li-S cells potentially have a 25-30% lower carbon footprint during manufacturing compared to conventional lithium-ion batteries. This reduction stems primarily from the simplified cathode production process and the elimination of toxic organic electrolytes. The solid electrolytes employed in these systems, while energy-intensive to produce in current manufacturing paradigms, offer opportunities for process optimization that could further reduce environmental impacts.
Water consumption represents another key environmental consideration. Preliminary analyses suggest that solid-state Li-S cell production may require 15-20% less water than conventional battery manufacturing, primarily due to differences in electrode preparation and the absence of liquid electrolyte processing steps. However, certain solid electrolyte synthesis routes, particularly those involving solution-based methods, remain water-intensive and require further optimization.
End-of-life management presents both challenges and opportunities for solid-state Li-S technology. The absence of toxic liquid electrolytes simplifies recycling processes and reduces hazardous waste management requirements. Additionally, the high theoretical value of recoverable lithium and the relatively simple material composition enhance economic recycling feasibility. Research indicates potential recovery rates exceeding 90% for lithium and 95% for sulfur from spent cells using hydrometallurgical processes.
Resource efficiency constitutes a fundamental sustainability advantage of Li-S chemistry. Global sulfur reserves are abundant, with significant quantities available as byproducts from petroleum refining and natural gas processing. This availability contrasts sharply with the geopolitically concentrated and supply-constrained nature of cobalt and nickel resources used in conventional lithium-ion batteries.
Safety improvements inherent to solid-state designs—including reduced fire risk and elimination of volatile organic compounds—translate to decreased environmental hazards throughout the product lifecycle. These enhancements minimize the potential for acute environmental contamination events while simultaneously reducing the need for complex safety systems that add to material and energy footprints.
Safety Standards and Testing Protocols
Safety standards and testing protocols for solid-state lithium-sulfur (Li-S) cells represent a critical framework for ensuring the safe development, manufacturing, and deployment of this emerging battery technology. Unlike conventional lithium-ion batteries, solid-state Li-S cells present unique safety considerations due to their distinct materials, interfaces, and potential failure modes. Current safety standards primarily focus on traditional battery chemistries, necessitating adaptation and development of specialized protocols for solid-state Li-S technology.
The thermal stability testing of solid-state Li-S cells requires particular attention, as the sulfur cathode and lithium metal anode interactions with solid electrolytes create distinctive thermal behaviors. Standard tests include thermal runaway assessments, with modified parameters to account for the higher thermal stability thresholds typically observed in solid-state configurations. Differential scanning calorimetry (DSC) and accelerating rate calorimetry (ARC) have been adapted specifically for solid-state Li-S interfaces to detect potential exothermic reactions between components.
Mechanical integrity testing protocols have evolved to address the unique challenges of solid electrolytes in Li-S cells. These include indentation tests to evaluate electrolyte fracture resistance, and compression testing to assess interface stability under mechanical stress. The formation and propagation of lithium dendrites through solid electrolytes represents a particular safety concern, requiring specialized cycling protocols under controlled mechanical pressure conditions.
Electrical safety standards for solid-state Li-S cells focus on short-circuit prevention and overcharge protection. The UL 1642 and IEC 62133 standards have been modified to accommodate the different voltage profiles and failure mechanisms of Li-S chemistry. Additionally, specialized protocols have been developed to evaluate the stability of the solid electrolyte-electrode interfaces during extended cycling and under extreme conditions.
Environmental testing protocols assess cell performance and safety across a broader temperature range than conventional lithium-ion batteries, reflecting the potential advantages of solid-state Li-S technology in extreme environments. These include low-temperature performance down to -40°C and high-temperature stability up to 100°C, significantly expanding the safety testing envelope compared to liquid-electrolyte systems.
Harmonization efforts between international standards organizations including IEEE, UL, IEC, and ISO are currently underway to establish consistent global safety requirements for solid-state battery technologies, with specific provisions for Li-S chemistry. These collaborative initiatives aim to accelerate commercial adoption while ensuring rigorous safety verification across the entire product lifecycle, from materials selection through recycling and disposal.
The thermal stability testing of solid-state Li-S cells requires particular attention, as the sulfur cathode and lithium metal anode interactions with solid electrolytes create distinctive thermal behaviors. Standard tests include thermal runaway assessments, with modified parameters to account for the higher thermal stability thresholds typically observed in solid-state configurations. Differential scanning calorimetry (DSC) and accelerating rate calorimetry (ARC) have been adapted specifically for solid-state Li-S interfaces to detect potential exothermic reactions between components.
Mechanical integrity testing protocols have evolved to address the unique challenges of solid electrolytes in Li-S cells. These include indentation tests to evaluate electrolyte fracture resistance, and compression testing to assess interface stability under mechanical stress. The formation and propagation of lithium dendrites through solid electrolytes represents a particular safety concern, requiring specialized cycling protocols under controlled mechanical pressure conditions.
Electrical safety standards for solid-state Li-S cells focus on short-circuit prevention and overcharge protection. The UL 1642 and IEC 62133 standards have been modified to accommodate the different voltage profiles and failure mechanisms of Li-S chemistry. Additionally, specialized protocols have been developed to evaluate the stability of the solid electrolyte-electrode interfaces during extended cycling and under extreme conditions.
Environmental testing protocols assess cell performance and safety across a broader temperature range than conventional lithium-ion batteries, reflecting the potential advantages of solid-state Li-S technology in extreme environments. These include low-temperature performance down to -40°C and high-temperature stability up to 100°C, significantly expanding the safety testing envelope compared to liquid-electrolyte systems.
Harmonization efforts between international standards organizations including IEEE, UL, IEC, and ISO are currently underway to establish consistent global safety requirements for solid-state battery technologies, with specific provisions for Li-S chemistry. These collaborative initiatives aim to accelerate commercial adoption while ensuring rigorous safety verification across the entire product lifecycle, from materials selection through recycling and disposal.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!