Binder Selection And Processing For Li-S Electrodes
AUG 22, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Li-S Battery Binder Evolution and Objectives
Lithium-sulfur (Li-S) batteries have emerged as promising candidates for next-generation energy storage systems due to their high theoretical energy density (2600 Wh/kg), which significantly surpasses that of conventional lithium-ion batteries. The evolution of binder technologies for Li-S electrodes represents a critical aspect of their development trajectory since the early 2000s when research on these systems began to accelerate.
Initially, traditional polyvinylidene fluoride (PVDF) binders dominated Li-S electrode formulations, borrowed directly from lithium-ion battery technology without specific optimization for sulfur's unique characteristics. This approach resulted in limited cycle life and capacity retention due to inadequate accommodation of the volume changes and polysulfide dissolution inherent to Li-S chemistry.
The mid-2010s marked a significant shift toward water-based binder systems, with carboxymethyl cellulose (CMC) and styrene-butadiene rubber (SBR) combinations gaining prominence. This transition was driven by both environmental considerations and the discovery that aqueous processing could enhance the distribution of conductive additives and active materials, leading to improved electrochemical performance.
A breakthrough occurred around 2015-2018 with the development of functional binders specifically designed to address the "shuttle effect" - the migration of polysulfides between electrodes that causes capacity fading. Polymers containing polar functional groups, such as polyethylene oxide (PEO), polyacrylic acid (PAA), and gelatin, demonstrated enhanced ability to chemically interact with polysulfides, effectively trapping them within the cathode structure.
Recent advancements have focused on multifunctional binder systems that simultaneously address multiple challenges in Li-S batteries. These include conductive polymers like polypyrrole (PPy) and polyaniline (PANI) that serve dual roles as binders and conductivity enhancers, as well as self-healing polymers that can accommodate the mechanical stress from volume changes during cycling.
The current technical objectives for Li-S battery binders center on several key parameters: mechanical stability to withstand 80% volume expansion during cycling; chemical functionality to interact with and immobilize polysulfides; electronic conductivity to reduce the need for carbon additives; and processability compatible with large-scale manufacturing techniques.
Looking forward, the field is moving toward bio-derived sustainable binders with enhanced functionality, as well as composite binder systems that can be tailored to specific cell designs and operating conditions. The ultimate goal remains developing binder technologies that enable Li-S batteries to achieve their theoretical performance metrics while maintaining stability over thousands of cycles, thus facilitating their commercial viability for applications ranging from electric vehicles to grid-scale energy storage.
Initially, traditional polyvinylidene fluoride (PVDF) binders dominated Li-S electrode formulations, borrowed directly from lithium-ion battery technology without specific optimization for sulfur's unique characteristics. This approach resulted in limited cycle life and capacity retention due to inadequate accommodation of the volume changes and polysulfide dissolution inherent to Li-S chemistry.
The mid-2010s marked a significant shift toward water-based binder systems, with carboxymethyl cellulose (CMC) and styrene-butadiene rubber (SBR) combinations gaining prominence. This transition was driven by both environmental considerations and the discovery that aqueous processing could enhance the distribution of conductive additives and active materials, leading to improved electrochemical performance.
A breakthrough occurred around 2015-2018 with the development of functional binders specifically designed to address the "shuttle effect" - the migration of polysulfides between electrodes that causes capacity fading. Polymers containing polar functional groups, such as polyethylene oxide (PEO), polyacrylic acid (PAA), and gelatin, demonstrated enhanced ability to chemically interact with polysulfides, effectively trapping them within the cathode structure.
Recent advancements have focused on multifunctional binder systems that simultaneously address multiple challenges in Li-S batteries. These include conductive polymers like polypyrrole (PPy) and polyaniline (PANI) that serve dual roles as binders and conductivity enhancers, as well as self-healing polymers that can accommodate the mechanical stress from volume changes during cycling.
The current technical objectives for Li-S battery binders center on several key parameters: mechanical stability to withstand 80% volume expansion during cycling; chemical functionality to interact with and immobilize polysulfides; electronic conductivity to reduce the need for carbon additives; and processability compatible with large-scale manufacturing techniques.
Looking forward, the field is moving toward bio-derived sustainable binders with enhanced functionality, as well as composite binder systems that can be tailored to specific cell designs and operating conditions. The ultimate goal remains developing binder technologies that enable Li-S batteries to achieve their theoretical performance metrics while maintaining stability over thousands of cycles, thus facilitating their commercial viability for applications ranging from electric vehicles to grid-scale energy storage.
Market Analysis for Li-S Battery Technology
The lithium-sulfur (Li-S) battery market is experiencing significant growth potential due to its theoretical energy density advantages over conventional lithium-ion batteries. Current market projections indicate that the global Li-S battery market could reach $2.1 billion by 2030, with a compound annual growth rate of approximately 35% from 2023 to 2030. This growth is primarily driven by increasing demand for high-energy-density storage solutions in aerospace, defense, and electric vehicle applications.
The market demand for Li-S batteries is particularly strong in sectors requiring lightweight, high-capacity energy storage. The aerospace industry represents a key early adoption market, with companies like Airbus and Boeing exploring Li-S technology for satellite and drone applications. The electric vehicle segment presents the largest potential market, though commercial adoption remains limited due to cycle life challenges.
Regional analysis shows Asia-Pacific leading the market development, with China, South Korea, and Japan hosting the majority of research initiatives and manufacturing facilities. Europe follows closely, with significant research programs in the UK, Germany, and France. North America shows strong research activity but fewer commercialization efforts compared to Asia.
Market segmentation reveals three primary application categories: transportation (projected 45% market share), aerospace and defense (30%), and portable electronics (15%), with other applications comprising the remaining 10%. The transportation segment is expected to demonstrate the highest growth rate as battery performance improves.
Key market drivers include increasing demand for higher energy density batteries, the lower cost potential of sulfur as a cathode material compared to traditional transition metal oxides, and environmental benefits due to sulfur's abundance and lower toxicity. The push for sustainable battery technologies also supports market growth as sulfur represents a more environmentally friendly alternative to cobalt-based cathodes.
Market barriers primarily relate to technical challenges, particularly the binder selection and electrode processing issues that impact cycle life and performance stability. The "shuttle effect" caused by polysulfide dissolution remains a significant technical hurdle that directly affects market adoption rates. Current commercial Li-S batteries typically achieve only 200-300 cycles, whereas mainstream applications require 1,000+ cycles.
Investment in Li-S technology has accelerated, with venture capital funding exceeding $500 million in 2022 alone. Major battery manufacturers and automotive companies are establishing strategic partnerships with Li-S technology developers, indicating growing confidence in the technology's commercial potential.
The market demand for Li-S batteries is particularly strong in sectors requiring lightweight, high-capacity energy storage. The aerospace industry represents a key early adoption market, with companies like Airbus and Boeing exploring Li-S technology for satellite and drone applications. The electric vehicle segment presents the largest potential market, though commercial adoption remains limited due to cycle life challenges.
Regional analysis shows Asia-Pacific leading the market development, with China, South Korea, and Japan hosting the majority of research initiatives and manufacturing facilities. Europe follows closely, with significant research programs in the UK, Germany, and France. North America shows strong research activity but fewer commercialization efforts compared to Asia.
Market segmentation reveals three primary application categories: transportation (projected 45% market share), aerospace and defense (30%), and portable electronics (15%), with other applications comprising the remaining 10%. The transportation segment is expected to demonstrate the highest growth rate as battery performance improves.
Key market drivers include increasing demand for higher energy density batteries, the lower cost potential of sulfur as a cathode material compared to traditional transition metal oxides, and environmental benefits due to sulfur's abundance and lower toxicity. The push for sustainable battery technologies also supports market growth as sulfur represents a more environmentally friendly alternative to cobalt-based cathodes.
Market barriers primarily relate to technical challenges, particularly the binder selection and electrode processing issues that impact cycle life and performance stability. The "shuttle effect" caused by polysulfide dissolution remains a significant technical hurdle that directly affects market adoption rates. Current commercial Li-S batteries typically achieve only 200-300 cycles, whereas mainstream applications require 1,000+ cycles.
Investment in Li-S technology has accelerated, with venture capital funding exceeding $500 million in 2022 alone. Major battery manufacturers and automotive companies are establishing strategic partnerships with Li-S technology developers, indicating growing confidence in the technology's commercial potential.
Current Binder Technologies and Challenges
The binder selection for lithium-sulfur (Li-S) batteries represents a critical component that significantly impacts electrode performance, stability, and overall battery efficiency. Currently, the most widely used binders in Li-S electrodes include polyvinylidene fluoride (PVDF), polyethylene oxide (PEO), carboxymethyl cellulose (CMC), and styrene-butadiene rubber (SBR). PVDF has been the traditional choice due to its excellent electrochemical stability and strong adhesion properties, but it requires toxic N-methyl-2-pyrrolidone (NMP) as a solvent, raising environmental and cost concerns.
Water-soluble binders like CMC and SBR have gained attention as environmentally friendly alternatives that enable aqueous electrode processing. These binders offer advantages in terms of reduced processing costs and environmental impact. Additionally, modified natural polymers such as gelatin and alginate have shown promise due to their biodegradability and ability to form strong hydrogen bonds with active materials.
Despite these advancements, current binder technologies face significant challenges in Li-S battery applications. The most pressing issue is the "polysulfide shuttle effect," where soluble lithium polysulfides dissolve in the electrolyte and migrate between electrodes, causing capacity fading and reduced cycle life. Conventional binders lack the functional groups necessary to effectively trap these polysulfides.
Another major challenge is the substantial volume expansion (up to 80%) that sulfur undergoes during cycling. This expansion creates mechanical stress that can lead to electrode pulverization and delamination from the current collector. Most existing binders cannot accommodate this extreme volume change while maintaining structural integrity and electrical connectivity throughout the electrode.
The electrical conductivity limitations present another obstacle. Sulfur and its discharge products are inherently insulating, requiring conductive additives in the electrode formulation. However, traditional binders are electrically insulating, necessitating higher proportions of conductive additives that reduce the overall energy density of the battery.
Processing challenges also persist, particularly in achieving uniform dispersion of sulfur, carbon, and binder components. The hydrophobic nature of carbon materials often conflicts with hydrophilic binders, leading to agglomeration and non-uniform electrode structures that compromise performance and cycle life.
Recent research has focused on developing multifunctional binders that can simultaneously address multiple challenges. These include conductive polymers like PEDOT:PSS and polyaniline that improve electrical conductivity while binding electrode components, and polar binders with functional groups designed specifically to interact with and trap polysulfides through chemical bonding.
Water-soluble binders like CMC and SBR have gained attention as environmentally friendly alternatives that enable aqueous electrode processing. These binders offer advantages in terms of reduced processing costs and environmental impact. Additionally, modified natural polymers such as gelatin and alginate have shown promise due to their biodegradability and ability to form strong hydrogen bonds with active materials.
Despite these advancements, current binder technologies face significant challenges in Li-S battery applications. The most pressing issue is the "polysulfide shuttle effect," where soluble lithium polysulfides dissolve in the electrolyte and migrate between electrodes, causing capacity fading and reduced cycle life. Conventional binders lack the functional groups necessary to effectively trap these polysulfides.
Another major challenge is the substantial volume expansion (up to 80%) that sulfur undergoes during cycling. This expansion creates mechanical stress that can lead to electrode pulverization and delamination from the current collector. Most existing binders cannot accommodate this extreme volume change while maintaining structural integrity and electrical connectivity throughout the electrode.
The electrical conductivity limitations present another obstacle. Sulfur and its discharge products are inherently insulating, requiring conductive additives in the electrode formulation. However, traditional binders are electrically insulating, necessitating higher proportions of conductive additives that reduce the overall energy density of the battery.
Processing challenges also persist, particularly in achieving uniform dispersion of sulfur, carbon, and binder components. The hydrophobic nature of carbon materials often conflicts with hydrophilic binders, leading to agglomeration and non-uniform electrode structures that compromise performance and cycle life.
Recent research has focused on developing multifunctional binders that can simultaneously address multiple challenges. These include conductive polymers like PEDOT:PSS and polyaniline that improve electrical conductivity while binding electrode components, and polar binders with functional groups designed specifically to interact with and trap polysulfides through chemical bonding.
Current Binder Selection Methodologies
01 Polymer-based binders for Li-S electrodes
Various polymer-based binders can be used in Li-S electrodes to improve electrode performance. These polymers help maintain the structural integrity of the electrode during cycling, accommodate volume changes, and enhance the adhesion between active materials and current collectors. Common polymer binders include PVDF (polyvinylidene fluoride), polyethylene oxide (PEO), and other synthetic polymers that can effectively bind sulfur and conductive additives while maintaining good ionic conductivity.- Polymer-based binders for Li-S electrodes: Various polymer-based binders can be used in Li-S electrodes to improve electrode performance. These polymers help maintain the structural integrity of the electrode during cycling, accommodate volume changes, and enhance the adhesion between active materials and current collectors. Common polymer binders include PVDF (polyvinylidene fluoride), CMC (carboxymethyl cellulose), and water-soluble polymers that can form strong bonds with sulfur particles, improving cycle life and capacity retention.
- Conductive binders for enhanced electrochemical performance: Conductive binders combine the adhesive properties of traditional binders with electronic conductivity, addressing the poor conductivity of sulfur in Li-S batteries. These specialized binders create conductive networks throughout the electrode, facilitating electron transport and improving sulfur utilization. Examples include conductive polymers like PEDOT:PSS, polyaniline, and carbon-based composite binders. The use of conductive binders can significantly enhance rate capability, cycling stability, and overall electrochemical performance of Li-S batteries.
- Functional binders with polysulfide-trapping capabilities: Functional binders designed specifically for Li-S batteries contain chemical groups that can interact with and trap lithium polysulfides, addressing the shuttle effect that causes capacity fading. These binders feature polar functional groups, such as amines, carboxyl, or hydroxyl groups, that form chemical bonds with polysulfides, preventing their dissolution into the electrolyte. By immobilizing polysulfides within the cathode structure, these functional binders improve coulombic efficiency, cycling stability, and extend the battery lifespan.
- Composite and hybrid binder systems: Composite and hybrid binder systems combine multiple materials to achieve synergistic effects in Li-S electrodes. These systems typically incorporate two or more components, such as polymers with different functionalities, polymer-inorganic hybrids, or polymer-carbon composites. The combination allows for simultaneous improvement in mechanical properties, conductivity, and polysulfide retention. Hybrid binders can be tailored to address multiple challenges in Li-S batteries, resulting in enhanced capacity, rate performance, and cycling stability compared to single-component binders.
- Binder-free and self-standing electrode designs: Binder-free and self-standing electrode designs eliminate traditional binders altogether, addressing limitations such as added weight, reduced conductivity, and limited active material loading. These approaches include 3D carbon frameworks, carbon nanotubes, or graphene-based architectures that can host sulfur without requiring additional binders. Self-standing electrodes offer advantages including higher sulfur loading, improved electron/ion transport, and better accommodation of volume changes during cycling, leading to enhanced energy density and electrochemical performance in Li-S batteries.
02 Water-soluble and eco-friendly binders
Water-soluble and environmentally friendly binders offer advantages for Li-S electrode fabrication, including reduced use of toxic organic solvents and improved sustainability. These binders, such as carboxymethyl cellulose (CMC), sodium alginate, and other natural polymer derivatives, can form strong hydrogen bonds with sulfur particles and conductive additives. They typically provide good mechanical properties and electrochemical stability while enabling more environmentally friendly manufacturing processes.Expand Specific Solutions03 Functional binders with polysulfide trapping capabilities
Specialized functional binders can be designed with chemical groups that interact with and trap lithium polysulfides, addressing the shuttle effect in Li-S batteries. These binders contain polar functional groups, such as amide, amino, or hydroxyl groups, that can form chemical bonds with polysulfides and prevent their dissolution into the electrolyte. By immobilizing polysulfides within the cathode structure, these functional binders significantly improve cycling stability and coulombic efficiency of Li-S batteries.Expand Specific Solutions04 Conductive binders for enhanced electrode performance
Conductive binders combine the adhesive properties of traditional binders with electronic conductivity, eliminating or reducing the need for additional conductive additives in Li-S electrodes. These binders typically incorporate conductive polymers like PEDOT:PSS, polyaniline, or polypyrrole, or are composite materials with conductive nanoparticles. By improving the electronic connectivity throughout the electrode, conductive binders enhance sulfur utilization, rate capability, and overall electrochemical performance of Li-S batteries.Expand Specific Solutions05 Cross-linked and self-healing binder systems
Cross-linked and self-healing binder systems provide enhanced mechanical stability and adaptability to the volume changes during cycling of Li-S batteries. These advanced binder systems utilize cross-linking agents or contain dynamic bonds that can break and reform during electrode expansion and contraction. The three-dimensional network structure of cross-linked binders helps maintain electrode integrity, while self-healing properties allow the binder to recover from mechanical damage, leading to improved cycle life and capacity retention in Li-S batteries.Expand Specific Solutions
Key Industry Players in Li-S Battery Materials
The lithium-sulfur (Li-S) battery electrode binder technology landscape is currently in an early growth phase, with significant research momentum but limited commercial deployment. The market is projected to expand substantially as Li-S batteries offer theoretical energy densities up to five times higher than conventional lithium-ion batteries. Key players include major battery manufacturers like LG Energy Solution, LG Chem, and CATL (Ningde Amperex Technology), who are investing heavily in R&D to overcome technical challenges. Academic institutions such as Swiss Federal Institute of Technology, Nanyang Technological University, and Sun Yat-Sen University are advancing fundamental research, while specialized companies like SABIC and DuPont are developing novel binder materials. The technology remains at mid-maturity level, with challenges in cycle stability and sulfur utilization efficiency still requiring innovative binder solutions before widespread commercialization.
LG Energy Solution Ltd.
Technical Solution: LG Energy Solution has developed advanced polymer-based binder systems specifically engineered for lithium-sulfur (Li-S) electrodes. Their approach focuses on using modified polyvinylidene fluoride (PVDF) derivatives with functional groups that can effectively trap polysulfides through chemical bonding. The company has implemented a dual-binder strategy combining PVDF with water-soluble polymers like polyethylene oxide (PEO) to create a three-dimensional network structure that accommodates sulfur volumetric expansion while maintaining electrode integrity. Their proprietary binder formulation includes conductive additives that enhance the overall electronic conductivity of the sulfur cathode, addressing one of the key challenges in Li-S battery technology. LG Energy Solution's processing technique involves a specialized slurry preparation method that ensures homogeneous distribution of active materials and optimized electrode microstructure.
Strengths: Superior polysulfide trapping capability through chemical bonding mechanisms; excellent accommodation of volume changes during cycling; enhanced electronic conductivity of the electrode. Weaknesses: Higher manufacturing complexity due to dual-binder system; potential increased costs compared to conventional binders; processing parameters require precise control for optimal performance.
Dalian Institute of Chemical Physics Chinese Academy of Sci
Technical Solution: Dalian Institute of Chemical Physics has pioneered innovative binder solutions for Li-S electrodes focusing on amphiphilic polymer architectures. Their research has developed a series of functionalized binders containing both polar groups (to interact with lithium polysulfides) and non-polar segments (to enhance processability). A standout approach involves using modified polyacrylonitrile (PAN) binders with sulfur chemically bonded to the polymer backbone, creating a "sulfurized PAN" composite that significantly reduces polysulfide dissolution. Their processing methodology employs a controlled thermal treatment that induces partial cyclization of the polymer structure, creating a conductive framework while simultaneously trapping sulfur within the molecular architecture. The institute has also developed water-based binder systems incorporating graphene oxide and cellulose derivatives, offering environmentally friendly alternatives to traditional NMP-based processing while providing excellent adhesion properties and ionic conductivity pathways.
Strengths: Exceptional polysulfide retention through chemical bonding; environmentally friendly water-based processing options; dual functionality as both binder and active material host. Weaknesses: Complex synthesis procedures may limit large-scale production; thermal treatment processes require precise control; potential trade-off between mechanical properties and electrochemical performance.
Critical Patents in Li-S Electrode Binder Technology
Binder for lithium-sulfur electrode, positive electrode including the same, and lithium-sulfur secondary battery including the same
PatentPendingUS20240170675A1
Innovation
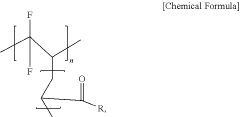
- A modified polyvinylidene fluoride (PVdF)-based binder with functional groups like carboxylic or carbonyl groups is used, which is soluble in organic solvents, interacting with lithium polysulfide to suppress shuttle reactions while maintaining binding characteristics, allowing for high loading electrodes with reduced binder content.
Binder for positive electrode of lithium-sulfur secondary battery and method for preparing positive electrode using same
PatentActiveUS20200176775A1
Innovation
- A water-soluble acrylic polymer binder is used for the positive electrode, comprising an acrylic monomer polymerized unit, a non-acrylic monomer polymerized unit, and a redox monomer polymerized unit, which provides high adsorption force, low solubility in electrolyte, and inhibits sulfur sublimation, enhancing electrode stability and cycle characteristics.
Environmental Impact of Li-S Binder Materials
The environmental impact of binder materials in Li-S batteries represents a critical consideration as these energy storage systems move toward commercialization. Traditional binder materials used in lithium-ion batteries, such as polyvinylidene fluoride (PVDF), pose significant environmental concerns due to their non-biodegradable nature and the requirement for toxic N-methyl-2-pyrrolidone (NMP) as a solvent during electrode processing.
Water-based binder alternatives for Li-S electrodes, including carboxymethyl cellulose (CMC), styrene-butadiene rubber (SBR), and polyacrylic acid (PAA), offer substantial environmental advantages. These aqueous processing routes eliminate the need for NMP, reducing volatile organic compound (VOC) emissions by approximately 95% compared to PVDF-based manufacturing processes. Additionally, the energy consumption during electrode drying decreases by up to 40% when using water-based binders, contributing to a lower carbon footprint.
Biodegradable binders derived from natural polymers such as alginate, chitosan, and cellulose derivatives present promising environmental credentials for Li-S battery systems. Life cycle assessment (LCA) studies indicate that alginate-based binders can reduce the environmental impact by 30-45% across multiple impact categories including global warming potential, ecotoxicity, and resource depletion when compared to synthetic alternatives.
The end-of-life management of Li-S batteries is significantly influenced by binder selection. Recyclability assessments demonstrate that electrodes manufactured with water-soluble binders facilitate more efficient material recovery processes. Specifically, CMC and gelatin-based binders enable up to 85% recovery of active sulfur material through simple water-based separation techniques, whereas PVDF-bound electrodes typically achieve only 50-60% recovery rates using more aggressive chemical treatments.
Manufacturing scalability also affects the environmental profile of binder materials. Recent industrial trials indicate that water-based processing can reduce production energy requirements by 25-35% while decreasing hazardous waste generation by over 70%. However, challenges remain regarding longer drying times and potential metal current collector corrosion during aqueous processing, which may partially offset environmental benefits through increased production complexity.
Regulatory frameworks increasingly emphasize reduced environmental impact in battery manufacturing. The European Battery Directive and similar regulations in Asia and North America are progressively restricting the use of toxic solvents and non-recyclable components, creating market pressure for environmentally benign binder systems. This regulatory landscape is accelerating research into green binders for Li-S technology, with projected adoption of eco-friendly alternatives expected to reach 60% of commercial Li-S systems by 2030.
Water-based binder alternatives for Li-S electrodes, including carboxymethyl cellulose (CMC), styrene-butadiene rubber (SBR), and polyacrylic acid (PAA), offer substantial environmental advantages. These aqueous processing routes eliminate the need for NMP, reducing volatile organic compound (VOC) emissions by approximately 95% compared to PVDF-based manufacturing processes. Additionally, the energy consumption during electrode drying decreases by up to 40% when using water-based binders, contributing to a lower carbon footprint.
Biodegradable binders derived from natural polymers such as alginate, chitosan, and cellulose derivatives present promising environmental credentials for Li-S battery systems. Life cycle assessment (LCA) studies indicate that alginate-based binders can reduce the environmental impact by 30-45% across multiple impact categories including global warming potential, ecotoxicity, and resource depletion when compared to synthetic alternatives.
The end-of-life management of Li-S batteries is significantly influenced by binder selection. Recyclability assessments demonstrate that electrodes manufactured with water-soluble binders facilitate more efficient material recovery processes. Specifically, CMC and gelatin-based binders enable up to 85% recovery of active sulfur material through simple water-based separation techniques, whereas PVDF-bound electrodes typically achieve only 50-60% recovery rates using more aggressive chemical treatments.
Manufacturing scalability also affects the environmental profile of binder materials. Recent industrial trials indicate that water-based processing can reduce production energy requirements by 25-35% while decreasing hazardous waste generation by over 70%. However, challenges remain regarding longer drying times and potential metal current collector corrosion during aqueous processing, which may partially offset environmental benefits through increased production complexity.
Regulatory frameworks increasingly emphasize reduced environmental impact in battery manufacturing. The European Battery Directive and similar regulations in Asia and North America are progressively restricting the use of toxic solvents and non-recyclable components, creating market pressure for environmentally benign binder systems. This regulatory landscape is accelerating research into green binders for Li-S technology, with projected adoption of eco-friendly alternatives expected to reach 60% of commercial Li-S systems by 2030.
Scalability and Manufacturing Considerations
The scalability of lithium-sulfur (Li-S) battery technology from laboratory to industrial production presents significant challenges that must be addressed for commercial viability. Current laboratory-scale electrode preparation methods often involve manual processes and small batch production that cannot be directly transferred to mass manufacturing environments. The selection of appropriate binders and processing techniques becomes increasingly critical when considering large-scale production requirements.
Industrial manufacturing of Li-S electrodes demands binder systems that can maintain consistent performance across high-volume production. Water-based binder systems such as CMC/SBR combinations offer advantages in terms of environmental impact and processing cost, but require careful optimization to prevent sulfur dissolution and maintain electrode integrity during aqueous processing. Conversely, PVDF-based systems in NMP solvents provide excellent adhesion properties but present environmental and cost challenges at scale.
Processing parameters must be standardized for reproducible electrode quality in mass production. Mixing time, speed, and sequence significantly impact the homogeneity of electrode slurries, with longer mixing times potentially causing sulfur particle agglomeration or carbon network disruption. Temperature control during mixing and coating processes becomes more challenging at industrial scale, yet remains critical for maintaining consistent rheological properties of the slurry.
Coating thickness uniformity presents another scaling challenge, as variations can lead to inconsistent electrochemical performance across large-area electrodes. Advanced coating technologies such as slot-die coating or comma coating may offer better thickness control compared to traditional doctor blade methods when scaled to production widths. Drying protocols must also be optimized to prevent binder migration and ensure uniform distribution throughout the electrode structure.
The economic viability of Li-S technology depends heavily on developing manufacturing processes compatible with existing lithium-ion battery production infrastructure. Binders that enable processing conditions similar to those used in conventional lithium-ion manufacturing will accelerate industrial adoption. Recent advances in solvent recovery systems may mitigate environmental concerns associated with NMP-based processing, potentially expanding viable binder options.
Equipment modifications may be necessary to accommodate the unique properties of sulfur-carbon-binder composites, particularly addressing challenges related to the low density and high volume of carbon additives required in Li-S electrodes. Calendering parameters must be carefully controlled to achieve target porosity without damaging the delicate carbon network structure essential for sulfur utilization and polysulfide containment.
Industrial manufacturing of Li-S electrodes demands binder systems that can maintain consistent performance across high-volume production. Water-based binder systems such as CMC/SBR combinations offer advantages in terms of environmental impact and processing cost, but require careful optimization to prevent sulfur dissolution and maintain electrode integrity during aqueous processing. Conversely, PVDF-based systems in NMP solvents provide excellent adhesion properties but present environmental and cost challenges at scale.
Processing parameters must be standardized for reproducible electrode quality in mass production. Mixing time, speed, and sequence significantly impact the homogeneity of electrode slurries, with longer mixing times potentially causing sulfur particle agglomeration or carbon network disruption. Temperature control during mixing and coating processes becomes more challenging at industrial scale, yet remains critical for maintaining consistent rheological properties of the slurry.
Coating thickness uniformity presents another scaling challenge, as variations can lead to inconsistent electrochemical performance across large-area electrodes. Advanced coating technologies such as slot-die coating or comma coating may offer better thickness control compared to traditional doctor blade methods when scaled to production widths. Drying protocols must also be optimized to prevent binder migration and ensure uniform distribution throughout the electrode structure.
The economic viability of Li-S technology depends heavily on developing manufacturing processes compatible with existing lithium-ion battery production infrastructure. Binders that enable processing conditions similar to those used in conventional lithium-ion manufacturing will accelerate industrial adoption. Recent advances in solvent recovery systems may mitigate environmental concerns associated with NMP-based processing, potentially expanding viable binder options.
Equipment modifications may be necessary to accommodate the unique properties of sulfur-carbon-binder composites, particularly addressing challenges related to the low density and high volume of carbon additives required in Li-S electrodes. Calendering parameters must be carefully controlled to achieve target porosity without damaging the delicate carbon network structure essential for sulfur utilization and polysulfide containment.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!