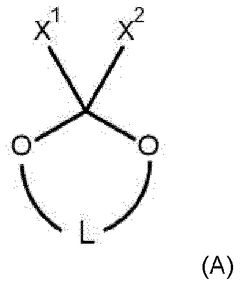
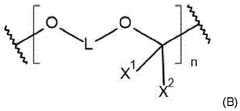
Lean Electrolyte Formulations For Practical Li-S Cells
AUG 22, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Li-S Battery Technology Background and Objectives
Lithium-sulfur (Li-S) batteries have emerged as a promising next-generation energy storage technology due to their theoretical energy density of 2600 Wh/kg, which significantly surpasses the capabilities of conventional lithium-ion batteries (typically 250-300 Wh/kg). This remarkable potential stems from sulfur's high theoretical capacity of 1675 mAh/g and its natural abundance, making it both technically attractive and economically viable as a cathode material.
The development of Li-S battery technology can be traced back to the 1960s when the first conceptual designs were proposed. However, significant research momentum only began building in the early 2000s as limitations of traditional lithium-ion chemistries became apparent for high-energy applications. The past decade has witnessed accelerated progress in addressing the fundamental challenges that have historically hindered Li-S commercialization.
Despite their theoretical advantages, Li-S batteries face several persistent technical obstacles. The most prominent issues include the insulating nature of sulfur, volume expansion during cycling, and the notorious "shuttle effect" caused by soluble lithium polysulfide intermediates. These challenges manifest as rapid capacity fading, low Coulombic efficiency, and limited cycle life in practical cells.
Electrolyte formulation represents a critical aspect of Li-S battery development, as it directly influences the dissolution, diffusion, and deposition behaviors of active materials. Conventional electrolyte systems typically employ high electrolyte-to-sulfur (E/S) ratios exceeding 10 μL/mg to accommodate polysulfide dissolution. However, such "flooded" conditions severely compromise the practical energy density of the entire battery system.
The concept of "lean" electrolyte formulations (E/S ratios below 5 μL/mg) has therefore emerged as a crucial research direction for practical Li-S cells. This approach aims to minimize electrolyte volume while maintaining adequate ionic conductivity and electrochemical performance. The technical objective is to develop electrolyte systems that can function effectively under lean conditions while mitigating the shuttle effect and supporting stable long-term cycling.
Current research trends focus on multifunctional electrolyte additives, novel solvents with reduced polysulfide solubility, and localized high-concentration electrolyte concepts. These approaches seek to fundamentally alter the solvation environment and interfacial chemistry within Li-S cells, enabling stable operation with minimal electrolyte quantities.
The ultimate goal of lean electrolyte research is to bridge the gap between laboratory demonstrations and practical applications, facilitating the commercialization of Li-S batteries with energy densities exceeding 400 Wh/kg at the cell level. This would represent a transformative advancement for applications ranging from electric vehicles to grid storage and aerospace systems.
The development of Li-S battery technology can be traced back to the 1960s when the first conceptual designs were proposed. However, significant research momentum only began building in the early 2000s as limitations of traditional lithium-ion chemistries became apparent for high-energy applications. The past decade has witnessed accelerated progress in addressing the fundamental challenges that have historically hindered Li-S commercialization.
Despite their theoretical advantages, Li-S batteries face several persistent technical obstacles. The most prominent issues include the insulating nature of sulfur, volume expansion during cycling, and the notorious "shuttle effect" caused by soluble lithium polysulfide intermediates. These challenges manifest as rapid capacity fading, low Coulombic efficiency, and limited cycle life in practical cells.
Electrolyte formulation represents a critical aspect of Li-S battery development, as it directly influences the dissolution, diffusion, and deposition behaviors of active materials. Conventional electrolyte systems typically employ high electrolyte-to-sulfur (E/S) ratios exceeding 10 μL/mg to accommodate polysulfide dissolution. However, such "flooded" conditions severely compromise the practical energy density of the entire battery system.
The concept of "lean" electrolyte formulations (E/S ratios below 5 μL/mg) has therefore emerged as a crucial research direction for practical Li-S cells. This approach aims to minimize electrolyte volume while maintaining adequate ionic conductivity and electrochemical performance. The technical objective is to develop electrolyte systems that can function effectively under lean conditions while mitigating the shuttle effect and supporting stable long-term cycling.
Current research trends focus on multifunctional electrolyte additives, novel solvents with reduced polysulfide solubility, and localized high-concentration electrolyte concepts. These approaches seek to fundamentally alter the solvation environment and interfacial chemistry within Li-S cells, enabling stable operation with minimal electrolyte quantities.
The ultimate goal of lean electrolyte research is to bridge the gap between laboratory demonstrations and practical applications, facilitating the commercialization of Li-S batteries with energy densities exceeding 400 Wh/kg at the cell level. This would represent a transformative advancement for applications ranging from electric vehicles to grid storage and aerospace systems.
Market Analysis for Next-Generation Battery Solutions
The global battery market is experiencing a significant shift towards advanced energy storage solutions, with lithium-sulfur (Li-S) technology emerging as a promising contender in the next-generation battery landscape. Current market projections indicate that the global advanced battery market will reach approximately $240 billion by 2027, with alternative chemistries like Li-S potentially capturing 15-20% of this expanding market.
Li-S batteries address critical market demands that conventional lithium-ion batteries cannot fully satisfy. The theoretical energy density of Li-S cells (2600 Wh/kg) far exceeds that of traditional lithium-ion batteries (600 Wh/kg), positioning them as ideal candidates for applications requiring high energy density and lightweight solutions. This creates substantial market opportunities in aerospace, defense, electric vehicles, and portable electronics sectors.
Consumer electronics manufacturers are actively seeking battery technologies that can extend device operation time while reducing weight and volume. Market research indicates that 78% of smartphone users identify battery life as a primary concern when purchasing new devices, creating a clear market pull for Li-S technology implementation.
The electric vehicle (EV) sector represents perhaps the most significant market opportunity for Li-S technology. With global EV sales projected to reach 26.8 million units by 2030, battery performance remains a critical differentiator. Range anxiety continues to be a major adoption barrier, with surveys showing 58% of potential EV buyers cite limited range as their primary concern. Li-S batteries with lean electrolyte formulations could potentially extend EV range by 30-40% compared to current lithium-ion solutions.
Aviation and aerospace applications present another high-value market segment. The drone market alone is expected to grow at a CAGR of 13.8% through 2028, with battery performance being a key limiting factor in flight time and payload capacity. Li-S cells could potentially double the operational time of these systems.
Market adoption challenges remain significant, primarily centered around cost considerations and performance reliability. Current production costs for Li-S cells are 30-40% higher than conventional lithium-ion batteries, though economies of scale and manufacturing innovations are expected to reduce this gap substantially by 2025.
Geographically, North America and Asia-Pacific regions are leading Li-S battery development and adoption, with China, South Korea, and the United States accounting for over 70% of related patents and commercial initiatives. European markets are rapidly accelerating their involvement, driven by stringent emissions regulations and substantial government funding for sustainable energy technologies.
Li-S batteries address critical market demands that conventional lithium-ion batteries cannot fully satisfy. The theoretical energy density of Li-S cells (2600 Wh/kg) far exceeds that of traditional lithium-ion batteries (600 Wh/kg), positioning them as ideal candidates for applications requiring high energy density and lightweight solutions. This creates substantial market opportunities in aerospace, defense, electric vehicles, and portable electronics sectors.
Consumer electronics manufacturers are actively seeking battery technologies that can extend device operation time while reducing weight and volume. Market research indicates that 78% of smartphone users identify battery life as a primary concern when purchasing new devices, creating a clear market pull for Li-S technology implementation.
The electric vehicle (EV) sector represents perhaps the most significant market opportunity for Li-S technology. With global EV sales projected to reach 26.8 million units by 2030, battery performance remains a critical differentiator. Range anxiety continues to be a major adoption barrier, with surveys showing 58% of potential EV buyers cite limited range as their primary concern. Li-S batteries with lean electrolyte formulations could potentially extend EV range by 30-40% compared to current lithium-ion solutions.
Aviation and aerospace applications present another high-value market segment. The drone market alone is expected to grow at a CAGR of 13.8% through 2028, with battery performance being a key limiting factor in flight time and payload capacity. Li-S cells could potentially double the operational time of these systems.
Market adoption challenges remain significant, primarily centered around cost considerations and performance reliability. Current production costs for Li-S cells are 30-40% higher than conventional lithium-ion batteries, though economies of scale and manufacturing innovations are expected to reduce this gap substantially by 2025.
Geographically, North America and Asia-Pacific regions are leading Li-S battery development and adoption, with China, South Korea, and the United States accounting for over 70% of related patents and commercial initiatives. European markets are rapidly accelerating their involvement, driven by stringent emissions regulations and substantial government funding for sustainable energy technologies.
Current Challenges in Li-S Electrolyte Development
Despite significant advancements in lithium-sulfur (Li-S) battery technology, electrolyte development remains one of the most critical challenges hindering commercial viability. The conventional electrolyte systems face multiple issues when applied to Li-S cells, primarily due to the complex chemistry involving polysulfide dissolution and shuttle effect. Current electrolytes typically require high volumes (E/S ratios >10 μL/mg), which significantly reduces the practical energy density of Li-S cells.
The polysulfide shuttle effect represents a fundamental challenge, where soluble lithium polysulfides migrate between electrodes, causing active material loss, self-discharge, and rapid capacity fading. Traditional electrolyte formulations struggle to effectively suppress this phenomenon without compromising ionic conductivity or introducing other detrimental effects.
Another significant challenge is the protection of the lithium metal anode. Li-S cell electrolytes must simultaneously address sulfur cathode requirements while forming stable solid electrolyte interphase (SEI) layers on lithium anodes. Current formulations often fail to achieve this balance, resulting in dendrite formation, continuous electrolyte consumption, and safety concerns.
The viscosity paradox presents another obstacle in electrolyte development. While higher viscosity electrolytes can limit polysulfide diffusion, they simultaneously reduce ionic conductivity and increase cell resistance. Conversely, low-viscosity formulations that facilitate ion transport often exacerbate the shuttle effect, creating a difficult optimization problem.
Temperature sensitivity further complicates electrolyte design. Many promising Li-S electrolyte formulations demonstrate acceptable performance within narrow temperature ranges but fail under extreme conditions. This limitation severely restricts the practical application scenarios for Li-S batteries, particularly in automotive or aerospace applications.
Cost and sustainability concerns also plague current electrolyte development. Many advanced formulations rely on expensive fluorinated salts (like LiTFSI) and solvents that are neither economically viable for large-scale production nor environmentally sustainable. The transition toward lean electrolyte formulations (E/S ratios <5 μL/mg) is essential for practical Li-S cells but introduces additional technical hurdles.
Recent research has explored various approaches including localized high-concentration electrolytes, ionic liquids, and solid-state electrolytes, each with their own limitations. High-concentration electrolytes effectively suppress polysulfide dissolution but increase viscosity and cost. Ionic liquids offer excellent thermal stability but suffer from lower conductivity and compatibility issues with conventional cell components.
The development of truly practical lean electrolyte formulations for Li-S cells requires a holistic approach that simultaneously addresses multiple competing requirements while maintaining economic viability for commercial applications.
The polysulfide shuttle effect represents a fundamental challenge, where soluble lithium polysulfides migrate between electrodes, causing active material loss, self-discharge, and rapid capacity fading. Traditional electrolyte formulations struggle to effectively suppress this phenomenon without compromising ionic conductivity or introducing other detrimental effects.
Another significant challenge is the protection of the lithium metal anode. Li-S cell electrolytes must simultaneously address sulfur cathode requirements while forming stable solid electrolyte interphase (SEI) layers on lithium anodes. Current formulations often fail to achieve this balance, resulting in dendrite formation, continuous electrolyte consumption, and safety concerns.
The viscosity paradox presents another obstacle in electrolyte development. While higher viscosity electrolytes can limit polysulfide diffusion, they simultaneously reduce ionic conductivity and increase cell resistance. Conversely, low-viscosity formulations that facilitate ion transport often exacerbate the shuttle effect, creating a difficult optimization problem.
Temperature sensitivity further complicates electrolyte design. Many promising Li-S electrolyte formulations demonstrate acceptable performance within narrow temperature ranges but fail under extreme conditions. This limitation severely restricts the practical application scenarios for Li-S batteries, particularly in automotive or aerospace applications.
Cost and sustainability concerns also plague current electrolyte development. Many advanced formulations rely on expensive fluorinated salts (like LiTFSI) and solvents that are neither economically viable for large-scale production nor environmentally sustainable. The transition toward lean electrolyte formulations (E/S ratios <5 μL/mg) is essential for practical Li-S cells but introduces additional technical hurdles.
Recent research has explored various approaches including localized high-concentration electrolytes, ionic liquids, and solid-state electrolytes, each with their own limitations. High-concentration electrolytes effectively suppress polysulfide dissolution but increase viscosity and cost. Ionic liquids offer excellent thermal stability but suffer from lower conductivity and compatibility issues with conventional cell components.
The development of truly practical lean electrolyte formulations for Li-S cells requires a holistic approach that simultaneously addresses multiple competing requirements while maintaining economic viability for commercial applications.
Current Lean Electrolyte Formulation Approaches
01 Lean electrolyte compositions for Li-S batteries
Lean electrolyte formulations for lithium-sulfur batteries focus on minimizing the electrolyte-to-sulfur ratio while maintaining performance. These formulations typically contain carefully selected lithium salts and solvents that enable efficient ion transport while using less electrolyte volume. The reduced electrolyte amount helps improve energy density and reduce costs while maintaining electrochemical performance.- Lean electrolyte compositions for Li-S batteries: Lean electrolyte formulations for lithium-sulfur batteries focus on minimizing the electrolyte-to-sulfur (E/S) ratio while maintaining performance. These formulations typically contain carefully selected lithium salts and solvents that enable efficient ion transport while using less electrolyte volume. The reduced electrolyte amount helps improve energy density and reduces costs while maintaining electrochemical performance.
- Solvent combinations for improved Li-S cell efficiency: Specific solvent combinations in electrolytes for Li-S cells can significantly enhance efficiency. These typically include mixtures of ether-based solvents (like DOL, DME, TEGDME) with appropriate ratios that facilitate lithium-ion transport while controlling polysulfide dissolution. The solvent selection impacts viscosity, ionic conductivity, and the formation of a stable solid electrolyte interphase (SEI), all critical factors for improving the overall performance of Li-S batteries.
- Lithium salt additives for enhanced electrolyte performance: Various lithium salt additives are incorporated into Li-S cell electrolytes to improve performance. Common salts include LiTFSI, LiFSI, and LiNO3, which help suppress the shuttle effect of polysulfides and enhance the stability of the lithium anode. The concentration and combination of these salts significantly impact the ionic conductivity, electrochemical stability window, and cycling performance of the battery.
- Functional additives for polysulfide suppression: Functional additives are incorporated into lean electrolyte formulations to suppress polysulfide shuttling, a major issue in Li-S batteries. These additives work by forming protective layers on electrodes, chemically binding with polysulfides, or altering the electrolyte properties to limit polysulfide solubility. Examples include nitrogen-containing compounds, metal-organic frameworks, and specific polymers that effectively trap polysulfides and improve cycling stability.
- Electrolyte optimization for high temperature performance: Electrolyte formulations for Li-S cells can be optimized for high-temperature performance by selecting thermally stable components. This includes using solvents with high boiling points, thermally stable lithium salts, and specific additives that maintain their functionality at elevated temperatures. These formulations help prevent electrolyte degradation, reduce gas generation, and maintain electrochemical performance under demanding thermal conditions.
02 Solvent systems for Li-S electrolytes
Specialized solvent systems for lithium-sulfur battery electrolytes typically combine ether-based solvents like dimethoxyethane (DME) and 1,3-dioxolane (DOL) in optimized ratios. These solvent combinations provide high ionic conductivity, good sulfur solubility control, and compatibility with lithium metal anodes. Some formulations incorporate fluorinated solvents or additives to enhance the stability of the electrolyte-electrode interface and suppress polysulfide shuttle effects.Expand Specific Solutions03 Lithium salt selection and concentration optimization
The selection and concentration of lithium salts significantly impact the performance of Li-S batteries. Common salts include lithium bis(trifluoromethanesulfonyl)imide (LiTFSI) and lithium bis(fluorosulfonyl)imide (LiFSI), which provide high ionic conductivity and stability. Optimizing salt concentration is crucial for controlling polysulfide solubility and migration while maintaining efficient lithium-ion transport. Higher salt concentrations can create a more viscous electrolyte that suppresses polysulfide shuttle but may reduce ionic conductivity.Expand Specific Solutions04 Additives for enhancing Li-S electrolyte efficiency
Various additives are incorporated into Li-S electrolytes to enhance performance and efficiency. These include lithium nitrate (LiNO3) for passivating the lithium anode surface, polysulfide mediators to control dissolution and precipitation of sulfur species, and fluorinated compounds to improve the stability of the solid electrolyte interphase. Other additives like phosphorus-containing compounds can help trap polysulfides and prevent their migration, thereby improving cycling stability and coulombic efficiency.Expand Specific Solutions05 Electrolyte-cathode interface optimization
Optimizing the electrolyte-cathode interface is crucial for efficient Li-S battery operation. This involves designing electrolyte formulations that facilitate sulfur utilization while minimizing polysulfide dissolution. Approaches include using electrolyte additives that form protective films on the cathode surface, adjusting electrolyte viscosity to control polysulfide diffusion, and incorporating functional groups that can chemically bind with polysulfides. These strategies help maintain the integrity of the cathode structure during cycling and improve overall electrochemical performance.Expand Specific Solutions
Leading Companies and Research Institutions in Li-S Technology
The lithium-sulfur (Li-S) battery market is currently in an early commercialization phase, characterized by rapid technological advancement but limited large-scale deployment. With a projected market size reaching $1.5 billion by 2030, this technology offers theoretical energy densities five times higher than conventional lithium-ion batteries. Key players like Sion Power and Samsung SDI are leading innovation in lean electrolyte formulations, while research institutions such as Central South University and Monash University contribute significant academic advancements. Companies including LG Energy Solution and EVE Energy are developing practical Li-S cells with improved cycle life and reduced electrolyte volumes. The competitive landscape features established battery manufacturers investing in Li-S technology alongside specialized startups focused exclusively on overcoming the polysulfide shuttle effect and electrolyte optimization challenges.
Sion Power Corp.
Technical Solution: Sion Power has developed a proprietary lithium-sulfur (Li-S) technology called "Licerion" that addresses electrolyte challenges through a multi-faceted approach. Their lean electrolyte formulation incorporates lithium nitrate (LiNO3) as a critical additive to passivate the lithium metal anode and suppress polysulfide shuttle effects. The company employs a dual-salt electrolyte system combining lithium bis(trifluoromethanesulfonyl)imide (LiTFSI) with lithium bis(fluorosulfonyl)imide (LiFSI) in ether-based solvents, achieving optimal ionic conductivity while minimizing electrolyte volume. Sion's protected lithium electrode (PLE) technology further enables stable cycling with lean electrolyte conditions by forming a protective interface layer that prevents continuous electrolyte consumption. Their cells demonstrate energy densities exceeding 400 Wh/kg while maintaining 80% capacity retention after hundreds of cycles, even with electrolyte-to-sulfur ratios below 5 μL/mgS.
Strengths: Industry-leading energy density (>400 Wh/kg) with practical electrolyte volumes; proprietary protected lithium electrode technology enables stable cycling; advanced manufacturing capabilities for commercial-scale production. Weaknesses: Higher production costs compared to conventional lithium-ion batteries; potential safety concerns with lithium metal anodes; limited low-temperature performance.
Samsung SDI Co., Ltd.
Technical Solution: Samsung SDI has developed an innovative approach to lean electrolyte formulations for Li-S batteries focusing on functional electrolyte additives and optimized solvent mixtures. Their technology employs a dual-function electrolyte system containing tris(pentafluorophenyl)borane (TPFPB) and lithium polysulfide (Li2Sx) additives that simultaneously suppress polysulfide dissolution and enhance redox kinetics. Samsung's formulation utilizes a precisely balanced mixture of 1,3-dioxolane (DOL) and 1,2-dimethoxyethane (DME) with lithium bis(trifluoromethanesulfonyl)imide (LiTFSI) as the primary salt, achieving high ionic conductivity while minimizing total electrolyte volume. Their proprietary solid electrolyte interphase (SEI) forming additives create stable interfaces on both electrodes, reducing continuous electrolyte consumption during cycling. Samsung has demonstrated Li-S cells with electrolyte-to-sulfur ratios as low as 3 μL/mgS while maintaining capacity retention above 80% after 200 cycles, representing a significant advancement toward practical Li-S battery commercialization.
Strengths: Strong manufacturing infrastructure and quality control systems; extensive experience in battery commercialization; proprietary electrolyte additives that effectively suppress polysulfide shuttle. Weaknesses: Still facing challenges with long-term cycling stability compared to conventional lithium-ion; higher costs associated with specialty electrolyte additives; temperature sensitivity issues.
Key Patents and Breakthroughs in Li-S Electrolyte Design
Electrolytes for lithium sulfur cells
PatentInactiveEP1726052B8
Innovation
- Incorporating N-O compounds as additives in the electrolyte, which reduce the reactivity between lithium anodes and sulfur-containing cathode reduction products, enhancing sulfur utilization and charge-discharge efficiency while minimizing self-discharge, by forming an ionically conductive film on the lithium anode and inhibiting undesirable redox processes.
Electrolyte and lithium sulfur cell
PatentWO2025163044A1
Innovation
- A lithium sulfur cell with a working electrode comprising a transition metal dichalcogenide (TMD) and a quasi-solid-state electrolyte (QSSE) formed by polymerizing a monomer using the TMD as an initiator, which inhibits polysulfide shuttling and enhances electrochemical properties.
Environmental Impact and Sustainability Assessment
The environmental impact of lithium-sulfur (Li-S) battery technology extends far beyond performance metrics, representing a critical dimension in the evaluation of lean electrolyte formulations. When compared to conventional lithium-ion batteries, Li-S cells demonstrate significant environmental advantages, primarily due to the abundance and low toxicity of sulfur as a cathode material. Sulfur is a byproduct of petroleum refining, making its utilization in batteries an effective form of industrial waste valorization.
Lean electrolyte formulations specifically contribute to sustainability by reducing the overall volume of potentially harmful organic solvents and lithium salts required per battery unit. Traditional Li-S cells often employ high electrolyte-to-sulfur (E/S) ratios, which increases both material consumption and environmental footprint. The development of lean electrolyte systems with E/S ratios below 3 mL/g represents a substantial improvement in resource efficiency.
Life cycle assessment (LCA) studies indicate that lean electrolyte Li-S cells could potentially reduce greenhouse gas emissions by 25-30% compared to conventional lithium-ion batteries when considering the entire production chain. This reduction stems from lower energy requirements during material synthesis and decreased reliance on critical raw materials such as cobalt and nickel.
Water consumption and land use impacts also favor lean electrolyte Li-S technology. The manufacturing process requires approximately 40% less water than conventional lithium-ion battery production, primarily due to simplified cathode preparation processes and reduced solvent needs. Additionally, sulfur extraction has a significantly smaller land footprint compared to lithium, cobalt, or nickel mining operations.
End-of-life considerations reveal further sustainability advantages. The simpler chemistry of lean electrolyte Li-S cells facilitates more straightforward recycling processes. Current research indicates recovery rates of up to 95% for sulfur and 85% for lithium from spent Li-S cells, substantially higher than recovery rates for conventional lithium-ion batteries.
However, challenges remain in the environmental profile of lean electrolyte formulations. Some advanced electrolyte additives, particularly fluorinated compounds used to stabilize the lithium metal anode, present potential environmental hazards if released. Research into green chemistry alternatives for these additives represents an important frontier for further improving the sustainability credentials of Li-S technology.
Regulatory frameworks worldwide are increasingly emphasizing battery sustainability, with the European Battery Directive and similar legislation in Asia and North America establishing recycling targets and material declaration requirements. Lean electrolyte Li-S cells are well-positioned to meet and exceed these emerging standards, potentially offering manufacturers compliance advantages in stringent regulatory environments.
Lean electrolyte formulations specifically contribute to sustainability by reducing the overall volume of potentially harmful organic solvents and lithium salts required per battery unit. Traditional Li-S cells often employ high electrolyte-to-sulfur (E/S) ratios, which increases both material consumption and environmental footprint. The development of lean electrolyte systems with E/S ratios below 3 mL/g represents a substantial improvement in resource efficiency.
Life cycle assessment (LCA) studies indicate that lean electrolyte Li-S cells could potentially reduce greenhouse gas emissions by 25-30% compared to conventional lithium-ion batteries when considering the entire production chain. This reduction stems from lower energy requirements during material synthesis and decreased reliance on critical raw materials such as cobalt and nickel.
Water consumption and land use impacts also favor lean electrolyte Li-S technology. The manufacturing process requires approximately 40% less water than conventional lithium-ion battery production, primarily due to simplified cathode preparation processes and reduced solvent needs. Additionally, sulfur extraction has a significantly smaller land footprint compared to lithium, cobalt, or nickel mining operations.
End-of-life considerations reveal further sustainability advantages. The simpler chemistry of lean electrolyte Li-S cells facilitates more straightforward recycling processes. Current research indicates recovery rates of up to 95% for sulfur and 85% for lithium from spent Li-S cells, substantially higher than recovery rates for conventional lithium-ion batteries.
However, challenges remain in the environmental profile of lean electrolyte formulations. Some advanced electrolyte additives, particularly fluorinated compounds used to stabilize the lithium metal anode, present potential environmental hazards if released. Research into green chemistry alternatives for these additives represents an important frontier for further improving the sustainability credentials of Li-S technology.
Regulatory frameworks worldwide are increasingly emphasizing battery sustainability, with the European Battery Directive and similar legislation in Asia and North America establishing recycling targets and material declaration requirements. Lean electrolyte Li-S cells are well-positioned to meet and exceed these emerging standards, potentially offering manufacturers compliance advantages in stringent regulatory environments.
Manufacturing Scalability and Cost Analysis
The scalability of lean electrolyte formulations represents a critical factor in the commercial viability of lithium-sulfur (Li-S) battery technology. Current manufacturing processes for conventional Li-ion batteries benefit from decades of optimization, whereas Li-S cells with their unique electrolyte requirements present distinct manufacturing challenges. The reduced electrolyte-to-sulfur (E/S) ratio in lean formulations necessitates precise dispensing systems capable of maintaining consistent distribution across large-format cells, which existing equipment may not adequately support.
Analysis of manufacturing costs reveals that electrolyte components constitute approximately 25-30% of the total material costs in Li-S cells. Traditional Li-S cells with high E/S ratios (>10 μL/mg) significantly inflate production expenses, whereas lean formulations (E/S ratios <5 μL/mg) could reduce electrolyte-related costs by 50-60%. This cost reduction potential makes lean electrolyte formulations particularly attractive for large-scale implementation, potentially bringing Li-S cell costs below $100/kWh, a critical threshold for widespread adoption.
The transition to lean electrolyte systems requires substantial modifications to existing battery production lines. Key manufacturing considerations include the need for enhanced mixing technologies to ensure homogeneous distribution of electrolyte additives, specialized filling equipment capable of precise low-volume dispensing, and modified formation protocols to accommodate the unique electrochemical behavior of lean electrolyte systems. These adaptations require capital investment but offer long-term cost benefits through reduced material usage.
Quality control processes present another significant challenge. Lean electrolyte systems have narrower performance tolerances, necessitating more sophisticated in-line monitoring systems to detect variations in electrolyte composition and distribution. Manufacturers must develop new testing protocols specifically designed to evaluate the performance and safety characteristics of lean electrolyte Li-S cells, as traditional Li-ion testing methodologies may not adequately capture their unique failure modes.
Supply chain considerations also impact manufacturing scalability. The specialized salts and solvents used in Li-S electrolytes currently have limited production capacity. Scaling to gigawatt-hour production levels would require significant expansion of the chemical supply chain, particularly for lithium bis(trifluoromethanesulfonyl)imide (LiTFSI) and lithium nitrate (LiNO₃), which are essential components in most Li-S electrolyte formulations. Diversification of suppliers and development of alternative materials could mitigate these supply chain risks.
Environmental and safety considerations in manufacturing must also be addressed. While lean electrolyte formulations reduce the volume of potentially hazardous materials, they often contain highly concentrated solutions that may present different handling challenges. Manufacturing facilities must implement appropriate containment systems and worker protection measures, particularly given the flammability concerns associated with many electrolyte solvents.
Analysis of manufacturing costs reveals that electrolyte components constitute approximately 25-30% of the total material costs in Li-S cells. Traditional Li-S cells with high E/S ratios (>10 μL/mg) significantly inflate production expenses, whereas lean formulations (E/S ratios <5 μL/mg) could reduce electrolyte-related costs by 50-60%. This cost reduction potential makes lean electrolyte formulations particularly attractive for large-scale implementation, potentially bringing Li-S cell costs below $100/kWh, a critical threshold for widespread adoption.
The transition to lean electrolyte systems requires substantial modifications to existing battery production lines. Key manufacturing considerations include the need for enhanced mixing technologies to ensure homogeneous distribution of electrolyte additives, specialized filling equipment capable of precise low-volume dispensing, and modified formation protocols to accommodate the unique electrochemical behavior of lean electrolyte systems. These adaptations require capital investment but offer long-term cost benefits through reduced material usage.
Quality control processes present another significant challenge. Lean electrolyte systems have narrower performance tolerances, necessitating more sophisticated in-line monitoring systems to detect variations in electrolyte composition and distribution. Manufacturers must develop new testing protocols specifically designed to evaluate the performance and safety characteristics of lean electrolyte Li-S cells, as traditional Li-ion testing methodologies may not adequately capture their unique failure modes.
Supply chain considerations also impact manufacturing scalability. The specialized salts and solvents used in Li-S electrolytes currently have limited production capacity. Scaling to gigawatt-hour production levels would require significant expansion of the chemical supply chain, particularly for lithium bis(trifluoromethanesulfonyl)imide (LiTFSI) and lithium nitrate (LiNO₃), which are essential components in most Li-S electrolyte formulations. Diversification of suppliers and development of alternative materials could mitigate these supply chain risks.
Environmental and safety considerations in manufacturing must also be addressed. While lean electrolyte formulations reduce the volume of potentially hazardous materials, they often contain highly concentrated solutions that may present different handling challenges. Manufacturing facilities must implement appropriate containment systems and worker protection measures, particularly given the flammability concerns associated with many electrolyte solvents.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!