Polysulfide Shuttle Mitigation: Materials And Coatings
AUG 22, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Polysulfide Shuttle Effect Background and Objectives
The polysulfide shuttle effect represents one of the most significant challenges in lithium-sulfur (Li-S) battery technology development. This phenomenon occurs during the charging and discharging cycles when soluble lithium polysulfide intermediates (Li2Sx, 4≤x≤8) dissolve in the electrolyte and migrate between electrodes. This migration creates a "shuttle" mechanism that leads to active material loss, self-discharge, reduced coulombic efficiency, and accelerated capacity fading.
The evolution of understanding this effect dates back to the early 1990s when researchers first identified the dissolution problem in sulfur cathodes. By the early 2000s, the term "shuttle effect" was formally established in scientific literature, and systematic investigations began to characterize its electrochemical implications. The past decade has witnessed exponential growth in research focused on mitigating this effect, reflecting the increasing interest in Li-S batteries as next-generation energy storage solutions.
The technical trajectory has evolved from simply acknowledging the problem to developing sophisticated materials and coating strategies specifically designed to suppress polysulfide dissolution and migration. Initial approaches focused on electrolyte modifications, followed by physical confinement strategies, and more recently, the development of functional materials with chemical polysulfide-trapping capabilities.
The primary objective of polysulfide shuttle mitigation research is to develop practical, scalable, and cost-effective solutions that can enable Li-S batteries to achieve their theoretical energy density of 2600 Wh/kg while maintaining stable cycling performance over thousands of cycles. This represents a five-fold increase over current lithium-ion technologies, potentially revolutionizing applications from electric vehicles to grid-scale storage.
Specific technical goals include: reducing polysulfide dissolution by at least 90% compared to conventional systems; extending cycle life to >1000 cycles with <0.05% capacity fade per cycle; improving coulombic efficiency to >99%; and accomplishing these improvements without significantly increasing weight, volume, or manufacturing complexity of the battery system.
The urgency of this research is underscored by global sustainability initiatives and the pressing need for high-energy-density storage solutions to support renewable energy integration and transportation electrification. Success in mitigating the shuttle effect would remove the primary barrier to commercial viability of Li-S technology, potentially triggering a paradigm shift in energy storage capabilities across multiple industries.
The evolution of understanding this effect dates back to the early 1990s when researchers first identified the dissolution problem in sulfur cathodes. By the early 2000s, the term "shuttle effect" was formally established in scientific literature, and systematic investigations began to characterize its electrochemical implications. The past decade has witnessed exponential growth in research focused on mitigating this effect, reflecting the increasing interest in Li-S batteries as next-generation energy storage solutions.
The technical trajectory has evolved from simply acknowledging the problem to developing sophisticated materials and coating strategies specifically designed to suppress polysulfide dissolution and migration. Initial approaches focused on electrolyte modifications, followed by physical confinement strategies, and more recently, the development of functional materials with chemical polysulfide-trapping capabilities.
The primary objective of polysulfide shuttle mitigation research is to develop practical, scalable, and cost-effective solutions that can enable Li-S batteries to achieve their theoretical energy density of 2600 Wh/kg while maintaining stable cycling performance over thousands of cycles. This represents a five-fold increase over current lithium-ion technologies, potentially revolutionizing applications from electric vehicles to grid-scale storage.
Specific technical goals include: reducing polysulfide dissolution by at least 90% compared to conventional systems; extending cycle life to >1000 cycles with <0.05% capacity fade per cycle; improving coulombic efficiency to >99%; and accomplishing these improvements without significantly increasing weight, volume, or manufacturing complexity of the battery system.
The urgency of this research is underscored by global sustainability initiatives and the pressing need for high-energy-density storage solutions to support renewable energy integration and transportation electrification. Success in mitigating the shuttle effect would remove the primary barrier to commercial viability of Li-S technology, potentially triggering a paradigm shift in energy storage capabilities across multiple industries.
Market Analysis for Li-S Battery Technologies
The lithium-sulfur (Li-S) battery market is experiencing significant growth potential due to the technology's theoretical energy density of 2600 Wh/kg, which far exceeds that of conventional lithium-ion batteries (typically 250-300 Wh/kg). This substantial energy density advantage positions Li-S batteries as a promising solution for applications requiring high energy storage capacity, particularly in electric vehicles, aerospace, and portable electronics sectors.
Current market projections indicate that the global Li-S battery market is expected to grow at a compound annual growth rate of approximately 35% between 2023 and 2030. This growth is primarily driven by increasing demand for higher energy density storage solutions and the push toward sustainable energy technologies. The market value, currently estimated at around $600 million, is projected to reach $2.5 billion by 2030.
The electric vehicle segment represents the largest potential market for Li-S batteries, accounting for nearly 45% of projected demand. This is attributed to the automotive industry's aggressive pursuit of longer-range electric vehicles to overcome consumer range anxiety. The aerospace and defense sectors follow closely, representing approximately 30% of the market, where weight reduction is a critical factor in operational efficiency.
Despite promising market prospects, commercial adoption faces significant barriers related to polysulfide shuttle effects. This technical challenge has created a specialized sub-market for polysulfide shuttle mitigation materials and coatings, estimated at $150 million currently and growing rapidly. Companies specializing in advanced materials for battery components are increasingly focusing R&D efforts on this specific challenge.
Regional analysis shows Asia-Pacific leading the market development with approximately 55% share, primarily due to strong manufacturing capabilities in China, South Korea, and Japan. North America follows with 25% market share, driven by substantial research investments and strategic government initiatives supporting advanced battery technologies.
Consumer electronics manufacturers are showing increasing interest in Li-S technology, particularly for applications requiring high energy density in limited space. This segment is expected to grow at 40% annually as polysulfide shuttle mitigation technologies mature and enable longer cycle life.
Market adoption is heavily dependent on overcoming the polysulfide shuttle effect, with industry surveys indicating that 78% of potential commercial users cite cycle life limitations as the primary barrier to adoption. This creates a direct market opportunity for effective materials and coatings that can mitigate this effect, with potential adopters willing to pay premium prices for solutions that demonstrably extend battery lifespan beyond 500 cycles.
Current market projections indicate that the global Li-S battery market is expected to grow at a compound annual growth rate of approximately 35% between 2023 and 2030. This growth is primarily driven by increasing demand for higher energy density storage solutions and the push toward sustainable energy technologies. The market value, currently estimated at around $600 million, is projected to reach $2.5 billion by 2030.
The electric vehicle segment represents the largest potential market for Li-S batteries, accounting for nearly 45% of projected demand. This is attributed to the automotive industry's aggressive pursuit of longer-range electric vehicles to overcome consumer range anxiety. The aerospace and defense sectors follow closely, representing approximately 30% of the market, where weight reduction is a critical factor in operational efficiency.
Despite promising market prospects, commercial adoption faces significant barriers related to polysulfide shuttle effects. This technical challenge has created a specialized sub-market for polysulfide shuttle mitigation materials and coatings, estimated at $150 million currently and growing rapidly. Companies specializing in advanced materials for battery components are increasingly focusing R&D efforts on this specific challenge.
Regional analysis shows Asia-Pacific leading the market development with approximately 55% share, primarily due to strong manufacturing capabilities in China, South Korea, and Japan. North America follows with 25% market share, driven by substantial research investments and strategic government initiatives supporting advanced battery technologies.
Consumer electronics manufacturers are showing increasing interest in Li-S technology, particularly for applications requiring high energy density in limited space. This segment is expected to grow at 40% annually as polysulfide shuttle mitigation technologies mature and enable longer cycle life.
Market adoption is heavily dependent on overcoming the polysulfide shuttle effect, with industry surveys indicating that 78% of potential commercial users cite cycle life limitations as the primary barrier to adoption. This creates a direct market opportunity for effective materials and coatings that can mitigate this effect, with potential adopters willing to pay premium prices for solutions that demonstrably extend battery lifespan beyond 500 cycles.
Current Challenges in Polysulfide Shuttle Mitigation
The polysulfide shuttle effect remains one of the most significant challenges hindering the commercial viability of lithium-sulfur (Li-S) batteries. This phenomenon occurs when soluble lithium polysulfide intermediates (Li2Sx, 4≤x≤8) dissolve in the electrolyte during cycling, migrate between electrodes, and participate in parasitic reactions. These shuttling polysulfides cause rapid capacity fading, low Coulombic efficiency, self-discharge, and shortened battery lifespan.
Current mitigation strategies face several critical limitations. Physical confinement approaches using porous carbon materials demonstrate insufficient polysulfide retention during long-term cycling. The non-polar nature of most carbon hosts results in weak interactions with polar polysulfides, allowing gradual leakage despite initial confinement. Additionally, the high porosity necessary for sulfur loading often compromises mechanical stability during volume expansion.
Chemical binding strategies utilizing metal oxides, sulfides, and nitrides show promising polysulfide adsorption capabilities but introduce electrical conductivity issues. The trade-off between strong chemical binding and efficient electron transfer remains unresolved. Many materials with excellent binding properties exhibit poor conductivity, necessitating additional conductive additives that reduce energy density and complicate manufacturing processes.
Electrolyte modification approaches using additives or alternative solvent systems face scalability challenges. While certain additives effectively suppress the shuttle effect in laboratory settings, their long-term stability, compatibility with other battery components, and cost-effectiveness at industrial scales remain questionable. Furthermore, many effective additives negatively impact ionic conductivity, creating a performance dilemma.
Protective coating technologies for separators and electrodes struggle with uniformity and durability issues. Current coating methods often result in uneven coverage, creating vulnerable spots for polysulfide penetration. Additionally, many coatings degrade or delaminate during extended cycling, particularly under the stress of volume changes inherent to Li-S chemistry.
The development of multifunctional materials that simultaneously address multiple aspects of the shuttle effect represents another significant challenge. Materials must ideally combine strong polysulfide adsorption, high electrical conductivity, catalytic activity for polysulfide conversion, and mechanical stability—a combination rarely achieved in current solutions.
Scale-up and manufacturing considerations further complicate mitigation efforts. Many laboratory-scale solutions employ complex synthesis procedures, expensive materials, or processes incompatible with existing battery manufacturing infrastructure. The gap between promising research results and commercially viable solutions remains substantial, with few approaches demonstrating both technical effectiveness and economic feasibility at production scales.
Current mitigation strategies face several critical limitations. Physical confinement approaches using porous carbon materials demonstrate insufficient polysulfide retention during long-term cycling. The non-polar nature of most carbon hosts results in weak interactions with polar polysulfides, allowing gradual leakage despite initial confinement. Additionally, the high porosity necessary for sulfur loading often compromises mechanical stability during volume expansion.
Chemical binding strategies utilizing metal oxides, sulfides, and nitrides show promising polysulfide adsorption capabilities but introduce electrical conductivity issues. The trade-off between strong chemical binding and efficient electron transfer remains unresolved. Many materials with excellent binding properties exhibit poor conductivity, necessitating additional conductive additives that reduce energy density and complicate manufacturing processes.
Electrolyte modification approaches using additives or alternative solvent systems face scalability challenges. While certain additives effectively suppress the shuttle effect in laboratory settings, their long-term stability, compatibility with other battery components, and cost-effectiveness at industrial scales remain questionable. Furthermore, many effective additives negatively impact ionic conductivity, creating a performance dilemma.
Protective coating technologies for separators and electrodes struggle with uniformity and durability issues. Current coating methods often result in uneven coverage, creating vulnerable spots for polysulfide penetration. Additionally, many coatings degrade or delaminate during extended cycling, particularly under the stress of volume changes inherent to Li-S chemistry.
The development of multifunctional materials that simultaneously address multiple aspects of the shuttle effect represents another significant challenge. Materials must ideally combine strong polysulfide adsorption, high electrical conductivity, catalytic activity for polysulfide conversion, and mechanical stability—a combination rarely achieved in current solutions.
Scale-up and manufacturing considerations further complicate mitigation efforts. Many laboratory-scale solutions employ complex synthesis procedures, expensive materials, or processes incompatible with existing battery manufacturing infrastructure. The gap between promising research results and commercially viable solutions remains substantial, with few approaches demonstrating both technical effectiveness and economic feasibility at production scales.
State-of-the-Art Materials and Coating Solutions
01 Use of additives to suppress polysulfide shuttle effect
Various additives can be incorporated into lithium-sulfur batteries to suppress the polysulfide shuttle effect. These additives work by adsorbing polysulfides, forming chemical bonds with them, or creating physical barriers to prevent their migration. Examples include certain polymers, metal oxides, and functional compounds that can effectively trap polysulfides and reduce their dissolution into the electrolyte, thereby mitigating capacity fade and improving battery performance.- Use of additives to suppress polysulfide shuttle effect: Various additives can be incorporated into lithium-sulfur batteries to suppress the polysulfide shuttle effect. These additives work by trapping or binding polysulfides, preventing their migration between electrodes. Common additives include polymers, metal oxides, and carbon-based materials that can physically or chemically interact with polysulfides to limit their dissolution and diffusion in the electrolyte, thereby improving battery cycling stability and efficiency.
- Electrolyte modifications for polysulfide shuttle mitigation: Modifying the electrolyte composition is an effective approach to mitigate the polysulfide shuttle effect. This includes using electrolyte additives, adjusting salt concentrations, incorporating ionic liquids, or developing solid-state or gel electrolytes. These modifications aim to reduce polysulfide solubility or mobility within the electrolyte system, thereby suppressing the shuttle mechanism and enhancing the electrochemical performance of lithium-sulfur batteries.
- Cathode structure engineering to contain polysulfides: Engineering the cathode structure is crucial for containing polysulfides and preventing the shuttle effect. This approach involves designing porous structures, using conductive frameworks, or creating physical barriers within the cathode to trap polysulfides. Advanced cathode architectures can effectively confine polysulfides within the cathode region during cycling, reducing their dissolution into the electrolyte and mitigating the shuttle effect.
- Separator modifications and interlayer strategies: Modified separators and interlayers placed between the cathode and anode can effectively block polysulfide migration. These components can be functionalized with materials that have strong affinity for polysulfides or designed with specific pore structures that physically prevent polysulfide passage. Such barrier strategies help maintain electrode integrity and prevent capacity fading caused by the shuttle effect in lithium-sulfur batteries.
- Chemical bonding and encapsulation techniques: Chemical bonding and encapsulation techniques involve creating strong chemical interactions between sulfur species and host materials or completely encapsulating sulfur within protective shells. These approaches aim to prevent polysulfide formation or release during battery operation. By chemically binding sulfur or physically encapsulating it within conductive matrices, the dissolution of polysulfides into the electrolyte is significantly reduced, effectively suppressing the shuttle effect.
02 Modified electrolyte compositions for polysulfide shuttle mitigation
Specialized electrolyte formulations can significantly reduce the polysulfide shuttle effect in lithium-sulfur batteries. These formulations may include electrolyte additives, alternative solvents, or modified salt concentrations that limit polysulfide solubility or mobility. By optimizing the electrolyte composition, the dissolution and migration of polysulfides between electrodes can be suppressed, leading to improved cycling stability and coulombic efficiency of the battery.Expand Specific Solutions03 Structured cathode designs to contain polysulfides
Advanced cathode architectures can be designed to physically confine polysulfides and prevent their diffusion. These designs may incorporate porous structures, encapsulation techniques, or hierarchical frameworks that effectively trap polysulfides within the cathode region. By creating physical barriers or chemical binding sites within the cathode structure, the migration of polysulfides to the anode can be significantly reduced, mitigating the shuttle effect and improving battery performance.Expand Specific Solutions04 Protective coatings and interlayers for polysulfide confinement
Specialized coatings and interlayers can be applied to battery components to block polysulfide migration. These protective layers may be applied to separators, electrodes, or other battery components to create selective barriers that allow lithium ion transport while blocking polysulfide movement. Materials used for these coatings include polymers, carbon-based materials, and inorganic compounds that can effectively intercept polysulfides before they participate in the shuttle mechanism.Expand Specific Solutions05 Novel binder systems for improved polysulfide retention
Advanced binder systems can enhance the retention of active materials and polysulfides within the cathode structure. These binders may have functional groups that chemically interact with polysulfides or physical properties that improve the structural integrity of the electrode during cycling. By using binders with strong adhesion properties and chemical affinity for polysulfides, the dissolution and migration of sulfur species can be reduced, thereby mitigating the shuttle effect and extending battery life.Expand Specific Solutions
Leading Companies and Research Institutions in Li-S Battery Field
The polysulfide shuttle mitigation market is currently in a growth phase, driven by increasing demand for high-performance lithium-sulfur batteries. The global market size is expanding rapidly as energy storage technologies gain prominence, with projections indicating significant growth over the next decade. Technologically, the field shows moderate maturity with ongoing innovation. Leading players include PolyPlus Battery Co., which specializes in protected lithium electrode technology, and major corporations like Dow Global Technologies and Johnson Matthey developing advanced materials solutions. Academic institutions such as Shanghai Jiao Tong University and University of British Columbia are contributing fundamental research, while government entities like the US Government provide funding support. The competitive landscape features a mix of specialized startups, established chemical companies, and research institutions working on novel coating materials and electrolyte additives.
Dow Global Technologies LLC
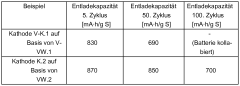
Technical Solution: Dow Global Technologies has developed a comprehensive materials solution for polysulfide shuttle mitigation in lithium-sulfur batteries through their advanced polymer technology platform. Their approach centers on functionalized polymeric materials that serve as both physical barriers and chemical traps for polysulfides. The company has created specialized fluoropolymer-based separator coatings with tailored porosity and surface chemistry that selectively block polysulfide migration while maintaining high lithium-ion conductivity. Additionally, Dow has developed cathode binders with specific functional groups that form strong chemical bonds with polysulfides, effectively immobilizing them within the cathode structure. Their solution also includes electrolyte additives based on modified siloxane compounds that form protective films on lithium metal surfaces, preventing side reactions with dissolved polysulfides. This integrated materials approach has demonstrated significant improvements in cycling stability, with test cells maintaining over 85% capacity retention after 300 cycles.
Strengths: Comprehensive materials solution addressing multiple aspects of polysulfide shuttling; leverages Dow's extensive polymer expertise; materials compatible with existing battery manufacturing processes. Weaknesses: May require precise formulation control for optimal performance; potential trade-offs between polysulfide trapping efficiency and overall battery power performance; long-term stability under extreme temperature conditions still being evaluated.
Honeywell International Technologies Ltd.
Technical Solution: Honeywell has developed an innovative materials approach to address polysulfide shuttling in lithium-sulfur batteries through their advanced materials science capabilities. Their solution incorporates specially engineered fluorinated polymers as functional separator coatings that create a selective barrier against polysulfide migration. These coatings feature precisely controlled pore structures and surface chemistry that allow lithium-ion transport while effectively blocking larger polysulfide molecules. Additionally, Honeywell has created proprietary cathode additives based on modified carbon structures with tailored surface functional groups that chemically bind with polysulfides, preventing their dissolution into the electrolyte. Their technology also includes specialized electrolyte formulations containing flame-retardant additives that simultaneously enhance safety and suppress polysulfide shuttling through the formation of stable interfacial layers. This integrated approach has shown promising results in laboratory testing, with prototype cells demonstrating significantly improved cycle life compared to conventional lithium-sulfur designs.
Strengths: Multifunctional approach addressing both polysulfide shuttling and safety concerns; leverages Honeywell's expertise in specialty chemicals and advanced materials; potential for integration with existing battery manufacturing processes. Weaknesses: May increase overall battery cost due to specialized materials; optimization required for different operating conditions and battery designs; long-term stability and performance in large-format cells still being validated.
Key Patents and Innovations in Shuttle Effect Suppression
Composite materials, their production and their use in electrical cells
PatentWO2012164443A1
Innovation
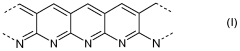
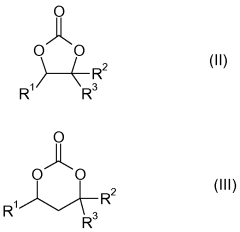
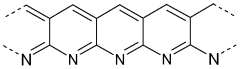
- A composite material is developed comprising a reaction product of an organic polymer, sulfur, and carbon with high sp2 hybridized carbon atoms, combined with a perfluorinated or partially fluorinated polymer, which forms a stable cathode material that reduces polysulfide migration and enhances energy density.
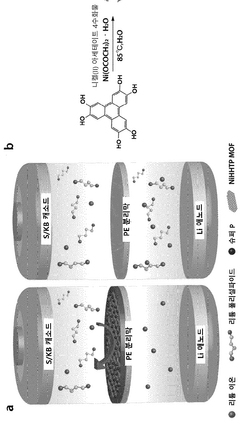
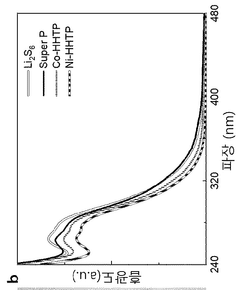
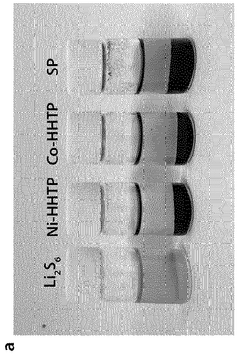
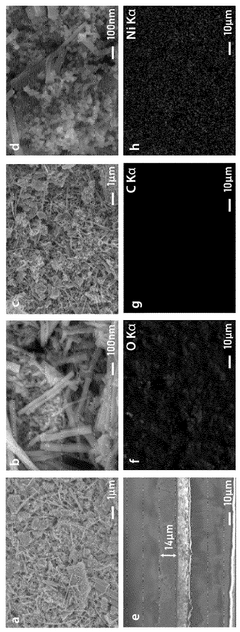
Separator coating material, comprising conductive metal-organic framework, for secondary battery, and lithium-sulfur secondary battery comprising same
PatentWO2025009921A1
Innovation
- A separator coating material containing a conductive metal-organic framework, such as Ni3(2,3,6,7,10,11-hexahydroxytriphenylene)2, combined with carbon conductive materials like carbon black and a binder, enhances lithium polysulfide adsorption and prevents polysulfide dissolution, improving battery performance and lifespan.
Environmental Impact and Sustainability Assessment
The environmental impact of lithium-sulfur (Li-S) battery technology, particularly in relation to polysulfide shuttle mitigation strategies, presents both challenges and opportunities for sustainable energy storage solutions. Current materials and coatings used to address the shuttle effect must be evaluated not only for their technical efficacy but also for their environmental footprint throughout the entire lifecycle.
Traditional shuttle mitigation approaches often rely on heavy metals, fluorinated compounds, and complex synthetic materials that pose significant environmental concerns. For instance, many metal oxide-based additives contain cobalt, nickel, or manganese, which involve environmentally damaging mining practices and generate toxic waste during production. Similarly, fluorinated electrolyte additives contribute to persistent environmental pollutants that resist natural degradation.
Recent advancements in bio-derived materials for polysulfide adsorption represent a promising shift toward more sustainable alternatives. Cellulose-based separators, lignin-derived carbon materials, and chitosan-based coatings demonstrate comparable performance to synthetic counterparts while significantly reducing environmental impact. These materials offer biodegradability advantages and can be sourced from renewable feedstocks or agricultural waste streams.
Life cycle assessment (LCA) studies indicate that the environmental benefits of Li-S batteries can only be fully realized when shuttle mitigation strategies incorporate sustainability principles. The carbon footprint of manufacturing specialized coatings and materials must be balanced against the extended cycle life they provide. Research suggests that a Li-S battery with effective but environmentally problematic shuttle inhibitors may have a higher overall environmental impact than one with slightly less effective but more sustainable solutions.
Water consumption represents another critical environmental consideration, particularly for hydrothermal synthesis methods commonly used in preparing functional materials for sulfur cathodes. Advanced coating techniques like atomic layer deposition offer precision but often require substantial energy inputs and specialized precursors with their own environmental implications.
Recycling and end-of-life management present unique challenges for Li-S batteries with specialized coatings. The diversity of materials used in shuttle mitigation strategies can complicate separation processes and reduce recovery rates of valuable components. Designing materials with recyclability in mind—such as thermally reversible polymers or easily separable composite structures—represents an emerging focus area for sustainable battery development.
Regulatory frameworks worldwide are increasingly emphasizing reduced environmental impact in battery technologies, with particular attention to hazardous substance restrictions and recycling requirements. Future shuttle mitigation strategies must align with these evolving standards while maintaining performance targets, driving innovation toward inherently sustainable materials and processes that address both technical and environmental challenges simultaneously.
Traditional shuttle mitigation approaches often rely on heavy metals, fluorinated compounds, and complex synthetic materials that pose significant environmental concerns. For instance, many metal oxide-based additives contain cobalt, nickel, or manganese, which involve environmentally damaging mining practices and generate toxic waste during production. Similarly, fluorinated electrolyte additives contribute to persistent environmental pollutants that resist natural degradation.
Recent advancements in bio-derived materials for polysulfide adsorption represent a promising shift toward more sustainable alternatives. Cellulose-based separators, lignin-derived carbon materials, and chitosan-based coatings demonstrate comparable performance to synthetic counterparts while significantly reducing environmental impact. These materials offer biodegradability advantages and can be sourced from renewable feedstocks or agricultural waste streams.
Life cycle assessment (LCA) studies indicate that the environmental benefits of Li-S batteries can only be fully realized when shuttle mitigation strategies incorporate sustainability principles. The carbon footprint of manufacturing specialized coatings and materials must be balanced against the extended cycle life they provide. Research suggests that a Li-S battery with effective but environmentally problematic shuttle inhibitors may have a higher overall environmental impact than one with slightly less effective but more sustainable solutions.
Water consumption represents another critical environmental consideration, particularly for hydrothermal synthesis methods commonly used in preparing functional materials for sulfur cathodes. Advanced coating techniques like atomic layer deposition offer precision but often require substantial energy inputs and specialized precursors with their own environmental implications.
Recycling and end-of-life management present unique challenges for Li-S batteries with specialized coatings. The diversity of materials used in shuttle mitigation strategies can complicate separation processes and reduce recovery rates of valuable components. Designing materials with recyclability in mind—such as thermally reversible polymers or easily separable composite structures—represents an emerging focus area for sustainable battery development.
Regulatory frameworks worldwide are increasingly emphasizing reduced environmental impact in battery technologies, with particular attention to hazardous substance restrictions and recycling requirements. Future shuttle mitigation strategies must align with these evolving standards while maintaining performance targets, driving innovation toward inherently sustainable materials and processes that address both technical and environmental challenges simultaneously.
Scalability and Manufacturing Considerations
The scalability of polysulfide shuttle mitigation technologies represents a critical factor in the commercial viability of lithium-sulfur batteries. Current laboratory-scale solutions for polysulfide shuttling often employ complex materials and coating processes that present significant challenges when transitioning to mass production environments. These challenges must be systematically addressed to enable widespread adoption of lithium-sulfur battery technology.
Material selection for scalable manufacturing requires careful consideration of cost, availability, and processing compatibility. While carbon-based materials like graphene and carbon nanotubes demonstrate excellent polysulfide trapping capabilities, their production costs remain prohibitively high for large-scale applications. Alternative materials such as metal oxides and metal-organic frameworks offer promising performance but face similar economic constraints. Manufacturers must balance performance requirements against material costs to achieve commercially viable solutions.
Process integration presents another significant hurdle in scaling polysulfide mitigation technologies. Coating processes developed in laboratory settings often utilize specialized equipment and precise environmental controls that are difficult to replicate in high-throughput manufacturing lines. Techniques such as atomic layer deposition provide excellent coating uniformity but operate at speeds incompatible with mass production requirements. Industry adoption will require adaptation of these processes to existing battery manufacturing infrastructure or development of novel approaches specifically designed for scalability.
Quality control methodologies must evolve to accommodate the unique characteristics of polysulfide mitigation materials and coatings. Current inspection techniques may be insufficient to detect defects in functional coatings that could lead to premature battery failure. Development of inline monitoring systems capable of verifying coating integrity and functional performance represents a critical need for manufacturing scale-up.
Environmental and safety considerations also impact manufacturing scalability. Many advanced materials for polysulfide mitigation involve potentially hazardous precursors or processing conditions. Regulatory compliance and worker safety protocols must be established for large-scale production facilities, potentially limiting the range of viable technical solutions. Sustainable manufacturing approaches that minimize environmental impact while maintaining performance will likely gain competitive advantage in the marketplace.
Cost modeling indicates that material costs currently dominate the economics of polysulfide mitigation technologies. As manufacturing scales increase, process optimization and economies of scale may shift this balance, making previously uneconomical approaches viable. Strategic partnerships between material suppliers, equipment manufacturers, and battery producers will be essential to drive the necessary innovations in manufacturing technology.
Material selection for scalable manufacturing requires careful consideration of cost, availability, and processing compatibility. While carbon-based materials like graphene and carbon nanotubes demonstrate excellent polysulfide trapping capabilities, their production costs remain prohibitively high for large-scale applications. Alternative materials such as metal oxides and metal-organic frameworks offer promising performance but face similar economic constraints. Manufacturers must balance performance requirements against material costs to achieve commercially viable solutions.
Process integration presents another significant hurdle in scaling polysulfide mitigation technologies. Coating processes developed in laboratory settings often utilize specialized equipment and precise environmental controls that are difficult to replicate in high-throughput manufacturing lines. Techniques such as atomic layer deposition provide excellent coating uniformity but operate at speeds incompatible with mass production requirements. Industry adoption will require adaptation of these processes to existing battery manufacturing infrastructure or development of novel approaches specifically designed for scalability.
Quality control methodologies must evolve to accommodate the unique characteristics of polysulfide mitigation materials and coatings. Current inspection techniques may be insufficient to detect defects in functional coatings that could lead to premature battery failure. Development of inline monitoring systems capable of verifying coating integrity and functional performance represents a critical need for manufacturing scale-up.
Environmental and safety considerations also impact manufacturing scalability. Many advanced materials for polysulfide mitigation involve potentially hazardous precursors or processing conditions. Regulatory compliance and worker safety protocols must be established for large-scale production facilities, potentially limiting the range of viable technical solutions. Sustainable manufacturing approaches that minimize environmental impact while maintaining performance will likely gain competitive advantage in the marketplace.
Cost modeling indicates that material costs currently dominate the economics of polysulfide mitigation technologies. As manufacturing scales increase, process optimization and economies of scale may shift this balance, making previously uneconomical approaches viable. Strategic partnerships between material suppliers, equipment manufacturers, and battery producers will be essential to drive the necessary innovations in manufacturing technology.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!