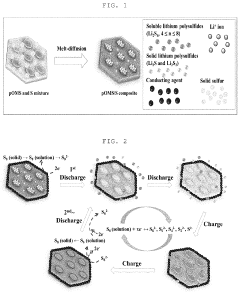
Conductive Host Materials For Sulfur Cathodes Comparison
AUG 22, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Conductive Host Materials Background and Objectives
Lithium-sulfur (Li-S) batteries have emerged as promising next-generation energy storage systems due to their high theoretical energy density (2600 Wh/kg), which significantly exceeds that of conventional lithium-ion batteries. The sulfur cathode, as a critical component, has attracted substantial research attention since the early 2000s when the potential of Li-S technology began to be seriously explored for commercial applications.
The development of conductive host materials for sulfur cathodes represents a fundamental research direction aimed at addressing the inherent limitations of sulfur in battery applications. Historically, sulfur's poor electrical conductivity (5×10^-30 S/cm at 25°C) has been a major obstacle, necessitating the incorporation of conductive materials to facilitate electron transport during electrochemical reactions.
Early research focused primarily on carbon-based materials such as activated carbon and carbon black as hosts for sulfur. The evolution progressed through various carbon architectures including carbon nanotubes (CNTs), graphene, and mesoporous carbon, each offering incremental improvements in conductivity and sulfur utilization. By 2010, hierarchical porous carbon structures emerged, providing both physical confinement for polysulfides and enhanced electrical pathways.
Beyond carbon-based materials, conductive polymers such as polyaniline, polypyrrole, and PEDOT:PSS have been investigated as alternative host materials, offering unique advantages in terms of flexibility and chemical interaction with sulfur species. More recently, metal-based conductive hosts including metal oxides, sulfides, and metal-organic frameworks (MOFs) have gained attention for their ability to chemically bind polysulfides while maintaining electrical conductivity.
The primary technical objectives in developing conductive host materials include: enhancing the overall conductivity of the sulfur cathode to improve rate capability; providing physical confinement to mitigate polysulfide dissolution; establishing chemical interactions to trap soluble polysulfides; accommodating volume expansion during cycling; and maintaining structural integrity over extended charge-discharge cycles.
Current research trends are moving toward multifunctional conductive hosts that simultaneously address multiple challenges. These include hybrid materials combining the high conductivity of carbon with the strong polysulfide adsorption capabilities of polar materials, as well as three-dimensional architectures designed to optimize ion transport pathways while maximizing active material loading.
The ultimate goal remains the development of conductive host materials that enable high-loading, long-cycle-life sulfur cathodes capable of delivering energy densities approaching the theoretical maximum, thus facilitating the commercial viability of Li-S batteries for applications ranging from portable electronics to electric vehicles and grid-scale energy storage.
The development of conductive host materials for sulfur cathodes represents a fundamental research direction aimed at addressing the inherent limitations of sulfur in battery applications. Historically, sulfur's poor electrical conductivity (5×10^-30 S/cm at 25°C) has been a major obstacle, necessitating the incorporation of conductive materials to facilitate electron transport during electrochemical reactions.
Early research focused primarily on carbon-based materials such as activated carbon and carbon black as hosts for sulfur. The evolution progressed through various carbon architectures including carbon nanotubes (CNTs), graphene, and mesoporous carbon, each offering incremental improvements in conductivity and sulfur utilization. By 2010, hierarchical porous carbon structures emerged, providing both physical confinement for polysulfides and enhanced electrical pathways.
Beyond carbon-based materials, conductive polymers such as polyaniline, polypyrrole, and PEDOT:PSS have been investigated as alternative host materials, offering unique advantages in terms of flexibility and chemical interaction with sulfur species. More recently, metal-based conductive hosts including metal oxides, sulfides, and metal-organic frameworks (MOFs) have gained attention for their ability to chemically bind polysulfides while maintaining electrical conductivity.
The primary technical objectives in developing conductive host materials include: enhancing the overall conductivity of the sulfur cathode to improve rate capability; providing physical confinement to mitigate polysulfide dissolution; establishing chemical interactions to trap soluble polysulfides; accommodating volume expansion during cycling; and maintaining structural integrity over extended charge-discharge cycles.
Current research trends are moving toward multifunctional conductive hosts that simultaneously address multiple challenges. These include hybrid materials combining the high conductivity of carbon with the strong polysulfide adsorption capabilities of polar materials, as well as three-dimensional architectures designed to optimize ion transport pathways while maximizing active material loading.
The ultimate goal remains the development of conductive host materials that enable high-loading, long-cycle-life sulfur cathodes capable of delivering energy densities approaching the theoretical maximum, thus facilitating the commercial viability of Li-S batteries for applications ranging from portable electronics to electric vehicles and grid-scale energy storage.
Market Analysis for Li-S Battery Applications
The lithium-sulfur (Li-S) battery market is experiencing significant growth potential due to the theoretical energy density advantages these batteries offer over conventional lithium-ion technologies. Current market projections indicate that the global Li-S battery market could reach $2.1 billion by 2026, with a compound annual growth rate of approximately 35% from 2021 to 2026. This growth is primarily driven by increasing demands for high-energy-density storage solutions in aerospace, defense, and electric vehicle applications.
The electric vehicle sector represents the largest potential market for Li-S batteries, with automotive manufacturers actively seeking alternatives to traditional lithium-ion batteries to extend driving range and reduce weight. Market research indicates that if Li-S batteries can achieve commercial viability with sufficient cycle life, they could capture up to 15% of the premium electric vehicle battery market by 2030.
Aerospace and defense applications constitute another significant market segment, where the lightweight properties of Li-S batteries provide crucial advantages. The drone and unmanned aerial vehicle (UAV) market particularly values the high gravimetric energy density that Li-S technology offers, with potential market penetration estimated at 25% by 2028 if current technical challenges are overcome.
Consumer electronics manufacturers are also showing increasing interest in Li-S technology, especially for applications requiring high energy density in limited space. However, this market segment remains cautious due to cycle life limitations, with adoption contingent upon significant improvements in battery longevity.
Market barriers for Li-S battery commercialization primarily revolve around the conductive host materials for sulfur cathodes. The market currently favors carbon-based host materials due to their cost-effectiveness and established supply chains, but emerging materials like metal oxides and MOFs (Metal-Organic Frameworks) are gaining attention for their superior performance characteristics.
Regional market analysis shows Asia-Pacific leading Li-S battery development and potential adoption, with China, South Korea, and Japan hosting major research initiatives and manufacturing capabilities. North America follows closely, with significant research funding and startup activity, while Europe demonstrates strong interest particularly in the automotive sector.
The market dynamics are heavily influenced by the performance-to-cost ratio of different conductive host materials. Carbon-based materials currently dominate due to lower production costs, but as manufacturing scales increase for advanced materials, the market is expected to shift toward higher-performance options that enable longer cycle life and higher practical energy densities.
The electric vehicle sector represents the largest potential market for Li-S batteries, with automotive manufacturers actively seeking alternatives to traditional lithium-ion batteries to extend driving range and reduce weight. Market research indicates that if Li-S batteries can achieve commercial viability with sufficient cycle life, they could capture up to 15% of the premium electric vehicle battery market by 2030.
Aerospace and defense applications constitute another significant market segment, where the lightweight properties of Li-S batteries provide crucial advantages. The drone and unmanned aerial vehicle (UAV) market particularly values the high gravimetric energy density that Li-S technology offers, with potential market penetration estimated at 25% by 2028 if current technical challenges are overcome.
Consumer electronics manufacturers are also showing increasing interest in Li-S technology, especially for applications requiring high energy density in limited space. However, this market segment remains cautious due to cycle life limitations, with adoption contingent upon significant improvements in battery longevity.
Market barriers for Li-S battery commercialization primarily revolve around the conductive host materials for sulfur cathodes. The market currently favors carbon-based host materials due to their cost-effectiveness and established supply chains, but emerging materials like metal oxides and MOFs (Metal-Organic Frameworks) are gaining attention for their superior performance characteristics.
Regional market analysis shows Asia-Pacific leading Li-S battery development and potential adoption, with China, South Korea, and Japan hosting major research initiatives and manufacturing capabilities. North America follows closely, with significant research funding and startup activity, while Europe demonstrates strong interest particularly in the automotive sector.
The market dynamics are heavily influenced by the performance-to-cost ratio of different conductive host materials. Carbon-based materials currently dominate due to lower production costs, but as manufacturing scales increase for advanced materials, the market is expected to shift toward higher-performance options that enable longer cycle life and higher practical energy densities.
Current Status and Challenges of Sulfur Cathode Materials
Lithium-sulfur (Li-S) batteries have emerged as promising candidates for next-generation energy storage systems due to their high theoretical energy density (2600 Wh/kg) and the natural abundance of sulfur. However, the commercialization of Li-S batteries faces significant challenges, primarily related to the cathode materials.
The insulating nature of sulfur (5×10^-30 S/cm) represents a fundamental obstacle, severely limiting electron transport during electrochemical reactions. This poor conductivity leads to low sulfur utilization and reduced rate capability in practical applications. Additionally, the volume expansion of sulfur during lithiation (approximately 80%) causes mechanical instability and electrode degradation over cycling.
Perhaps the most notorious challenge is the "shuttle effect," where soluble lithium polysulfide intermediates (Li2Sx, 4≤x≤8) dissolve in the electrolyte and shuttle between electrodes. This phenomenon results in active material loss, parasitic reactions with the lithium anode, and rapid capacity fading. Current research indicates that after just 100 cycles, many Li-S batteries retain less than 80% of their initial capacity.
The development of conductive host materials has become a critical research direction to address these challenges. Carbon-based materials, including graphene, carbon nanotubes, and porous carbon, currently dominate the field due to their excellent electrical conductivity and adjustable pore structures. However, their non-polar nature limits their ability to chemically interact with polar polysulfides, resulting in inadequate containment of the shuttle effect.
Metal oxides and sulfides have gained attention for their strong chemical affinity toward polysulfides, but their relatively low conductivity often necessitates hybridization with carbon materials. Recent advances in metal-organic frameworks (MOFs) and conductive polymers show promise in balancing conductivity and polysulfide adsorption capabilities.
Geographically, research in this field is concentrated in East Asia (particularly China, South Korea, and Japan), North America, and Europe. Chinese institutions lead in publication volume, while pioneering work from Stanford University, Pacific Northwest National Laboratory, and the University of Waterloo has established fundamental design principles for conductive host materials.
The sulfur loading in current laboratory-scale cathodes typically remains below 5 mg/cm², significantly lower than the 6-8 mg/cm² required for commercial viability. Additionally, most reported performance metrics are obtained at low current densities that do not reflect real-world operating conditions, highlighting the gap between laboratory achievements and practical applications.
The insulating nature of sulfur (5×10^-30 S/cm) represents a fundamental obstacle, severely limiting electron transport during electrochemical reactions. This poor conductivity leads to low sulfur utilization and reduced rate capability in practical applications. Additionally, the volume expansion of sulfur during lithiation (approximately 80%) causes mechanical instability and electrode degradation over cycling.
Perhaps the most notorious challenge is the "shuttle effect," where soluble lithium polysulfide intermediates (Li2Sx, 4≤x≤8) dissolve in the electrolyte and shuttle between electrodes. This phenomenon results in active material loss, parasitic reactions with the lithium anode, and rapid capacity fading. Current research indicates that after just 100 cycles, many Li-S batteries retain less than 80% of their initial capacity.
The development of conductive host materials has become a critical research direction to address these challenges. Carbon-based materials, including graphene, carbon nanotubes, and porous carbon, currently dominate the field due to their excellent electrical conductivity and adjustable pore structures. However, their non-polar nature limits their ability to chemically interact with polar polysulfides, resulting in inadequate containment of the shuttle effect.
Metal oxides and sulfides have gained attention for their strong chemical affinity toward polysulfides, but their relatively low conductivity often necessitates hybridization with carbon materials. Recent advances in metal-organic frameworks (MOFs) and conductive polymers show promise in balancing conductivity and polysulfide adsorption capabilities.
Geographically, research in this field is concentrated in East Asia (particularly China, South Korea, and Japan), North America, and Europe. Chinese institutions lead in publication volume, while pioneering work from Stanford University, Pacific Northwest National Laboratory, and the University of Waterloo has established fundamental design principles for conductive host materials.
The sulfur loading in current laboratory-scale cathodes typically remains below 5 mg/cm², significantly lower than the 6-8 mg/cm² required for commercial viability. Additionally, most reported performance metrics are obtained at low current densities that do not reflect real-world operating conditions, highlighting the gap between laboratory achievements and practical applications.
Comparative Analysis of Current Conductive Host Solutions
01 Carbon-based conductive host materials
Carbon-based materials serve as effective conductive hosts for sulfur cathodes due to their high electrical conductivity and large surface area. These materials include graphene, carbon nanotubes, porous carbon, and carbon fibers which can encapsulate sulfur particles, prevent polysulfide dissolution, and maintain electrical contact throughout charge-discharge cycles. The porous structure of carbon-based hosts allows for sulfur expansion during cycling while maintaining structural integrity and electronic pathways.- Carbon-based conductive host materials: Carbon-based materials serve as effective conductive hosts for sulfur cathodes due to their high electrical conductivity and large surface area. These materials include graphene, carbon nanotubes, porous carbon, and carbon fibers. The carbon structure provides pathways for electron transport while physically confining sulfur and polysulfides, preventing their dissolution into the electrolyte. This enhances the overall conductivity of the cathode and improves the cycling stability of lithium-sulfur batteries.
- Metal oxide/sulfide conductive frameworks: Metal oxides and sulfides can be incorporated into sulfur cathodes as conductive host materials. These compounds, including titanium dioxide, manganese dioxide, and molybdenum disulfide, provide strong chemical interactions with polysulfides, effectively trapping them within the cathode structure. Additionally, some metal compounds offer catalytic effects that facilitate the conversion of polysulfides during cycling, enhancing the conductivity and electrochemical performance of the sulfur cathode.
- Polymer-based conductive matrices: Conductive polymers can be used as host materials for sulfur cathodes to improve electrical conductivity while providing flexibility and structural stability. Polymers such as polyaniline, polypyrrole, and PEDOT:PSS create a conductive network that facilitates electron transport throughout the cathode. These polymer matrices can also be designed to interact with polysulfides through functional groups, reducing their shuttle effect and enhancing the overall performance of lithium-sulfur batteries.
- Composite and hybrid conductive structures: Hybrid and composite structures combining multiple conductive materials can create synergistic effects for sulfur cathodes. These structures typically integrate carbon materials with metal compounds or conductive polymers to simultaneously address multiple challenges in lithium-sulfur batteries. The composite architecture provides enhanced electrical conductivity, mechanical stability, and polysulfide trapping capabilities. Examples include carbon-metal oxide composites, polymer-carbon hybrids, and multi-layered structures that effectively improve the conductivity and cycling performance.
- Nanostructured conductive frameworks: Nanostructured materials with tailored architectures serve as advanced conductive hosts for sulfur cathodes. These include hollow spheres, yolk-shell structures, and three-dimensional interconnected networks that provide high surface area and abundant reaction sites. The nanoscale design enables efficient electron transport pathways while creating physical confinement for sulfur and its discharge products. Additionally, the controlled porosity in these nanostructures facilitates electrolyte penetration and ion transport, significantly enhancing the conductivity and rate capability of sulfur cathodes.
02 Metal oxide/sulfide composite hosts
Metal oxides and sulfides can be incorporated into host materials to enhance the conductivity and performance of sulfur cathodes. These compounds, such as titanium dioxide, manganese dioxide, and molybdenum disulfide, can form composite structures with carbon materials to create dual-functional hosts. The metal compounds provide chemical adsorption sites for polysulfides while the carbon component ensures electrical conductivity. This combination effectively addresses the shuttle effect while maintaining high electronic conductivity throughout the electrode.Expand Specific Solutions03 Conductive polymers as sulfur hosts
Conductive polymers offer unique advantages as host materials for sulfur cathodes, including flexibility, processability, and tunable conductivity. Polymers such as polyaniline, polypyrrole, and PEDOT:PSS can be synthesized with various morphologies to encapsulate sulfur while providing electronic pathways. These polymers can be further modified with functional groups that chemically interact with polysulfides, reducing their dissolution into the electrolyte while maintaining good electronic contact with sulfur particles throughout cycling.Expand Specific Solutions04 3D interconnected network structures
Three-dimensional interconnected network structures provide continuous electron transport pathways and sufficient space for sulfur loading and expansion. These architectures can be created using various materials including carbon aerogels, metal-organic frameworks, and hierarchical porous structures. The 3D networks maintain structural integrity during cycling while offering high surface area for sulfur deposition and multiple conductive pathways. This design effectively addresses both the conductivity issues and volume expansion challenges in sulfur cathodes.Expand Specific Solutions05 Metal-doped carbon composites
Incorporating metal nanoparticles or ions into carbon matrices creates highly conductive host materials with enhanced interaction with sulfur and polysulfides. Metals such as nickel, cobalt, iron, and platinum can be dispersed throughout carbon structures to form catalytic sites that facilitate redox reactions while improving overall conductivity. These metal-doped carbon composites combine the high conductivity of metals with the lightweight, porous nature of carbon materials, resulting in sulfur cathodes with improved cycling stability and rate capability.Expand Specific Solutions
Leading Companies and Research Institutions in Sulfur Cathodes
The lithium-sulfur battery market is currently in its early growth phase, with conductive host materials for sulfur cathodes representing a critical technological frontier. Market size is projected to expand significantly as these batteries offer theoretical energy densities far exceeding conventional lithium-ion systems. The competitive landscape features established players like LG Energy Solution, Toyota, Nissan, and Bosch investing heavily in R&D alongside specialized companies such as PolyPlus Battery and Conamix. Academic institutions including Tsinghua University, Central South University, and Cornell University are driving fundamental research breakthroughs. The technology remains in pre-commercialization stages with challenges in cycle life and sulfur utilization efficiency, though recent carbon-based host material innovations from companies like Honeycomb Battery show promising advances toward market-viable solutions.
LG Energy Solution Ltd.
Technical Solution: LG Energy Solution has developed a sophisticated approach to conductive host materials for sulfur cathodes centered on their "dual-phase conductive network" technology. This innovative solution combines graphene-based materials with conductive polymers to create a host structure that addresses multiple challenges in lithium-sulfur battery chemistry. The graphene component, typically reduced graphene oxide (rGO) with a sheet-like morphology, provides exceptional electrical conductivity (>10^3 S/cm) and high surface area (>1000 m²/g) for sulfur deposition[2]. LG's proprietary polymer component, based on modified polypyrrole derivatives, creates strong chemical interactions with polysulfides while maintaining good ionic conductivity. Their research demonstrates that these composite hosts can achieve sulfur utilization rates exceeding 85% with capacity retention of >80% after 500 cycles at practical loading levels (>5 mg/cm²)[4]. LG has further enhanced these materials with nitrogen and sulfur co-doping of the carbon framework, creating additional polar sites that strengthen polysulfide adsorption without compromising electrical conductivity[8].
Strengths: Excellent balance of electrical conductivity and polysulfide trapping; proven scalability for mass production; compatible with existing manufacturing infrastructure; strong cycle life performance. Weaknesses: Higher material costs compared to simple carbon hosts; requires precise control of synthesis conditions; some designs may have lower volumetric energy density.
Tsinghua University
Technical Solution: Tsinghua University has developed advanced conductive host materials for sulfur cathodes focusing on hierarchical porous carbon structures. Their approach involves synthesizing nitrogen-doped mesoporous carbon materials with controlled pore architectures that effectively trap polysulfides while maintaining high electrical conductivity. The research team has demonstrated carbon hosts with specific surface areas exceeding 1200 m²/g and pore volumes of 2.5 cm³/g, creating an interconnected conductive network that facilitates both electron and ion transport[1]. Their latest innovations include graphene-based 3D frameworks with covalently bonded sulfur atoms, achieving sulfur loading capacities of up to 80 wt% while maintaining structural integrity during cycling[3]. The university has also pioneered polar-functionalized carbon materials that form strong chemical bonds with lithium polysulfides, significantly reducing the shuttle effect that typically plagues lithium-sulfur batteries[5].
Strengths: Superior polysulfide trapping capability through precisely engineered pore structures; excellent electrical conductivity; high sulfur loading capacity; innovative surface functionalization techniques. Weaknesses: Complex and potentially costly synthesis procedures; challenges in scaling production to industrial levels; some designs may sacrifice energy density for improved cycle stability.
Key Technical Innovations in Host Material Design
Porous inorganic insulator-sulfur composite, and lithium-sulfur battery comprising the same
PatentActiveUS20200044239A1
Innovation
- A porous inorganic insulator-sulfur composite is developed, where sulfur is supported in the pores of a non-conductive porous inorganic material like silica or titania, eliminating the need for a carbon body and metal oxide, enhancing interaction with sulfur and maintaining it within the cathodic reaction zone.
Carbon and metal oxide composite cathode for batteries
PatentWO2024081819A3
Innovation
- Development of a novel composite cathode material combining resorcinol-formaldehyde carbon (RFC) with metals and/or metal oxides as a host structure for sulfur in lithium-sulfur batteries.
- Integration of elemental sulfur with a dual-functional host material that can provide both physical confinement and chemical interaction with sulfur/polysulfides.
- Creation of a cathode material system that incorporates additional conductive carbon and binder to optimize the overall electrochemical performance of lithium-sulfur batteries.
Material Cost and Scalability Assessment
The economic viability of conductive host materials for sulfur cathodes represents a critical factor in their commercial adoption. Carbon-based materials, particularly activated carbon and carbon black, offer significant cost advantages with prices ranging from $1-5/kg, making them the most economically accessible options for large-scale applications. These materials benefit from established industrial production processes and widespread availability, enabling immediate scalability.
In contrast, graphene and carbon nanotubes present substantially higher cost barriers, with current market prices between $50-200/kg for industrial-grade graphene and $100-500/kg for multi-walled carbon nanotubes. Single-walled carbon nanotubes remain prohibitively expensive at $1,000-10,000/kg, limiting their practical application despite superior conductivity properties. However, ongoing manufacturing innovations are gradually reducing these costs, with projections suggesting potential price reductions of 30-50% within the next five years.
Metal-based conductive hosts, including metal oxides and metal sulfides, occupy a middle ground in the cost spectrum ($20-100/kg) but face challenges in scalable synthesis processes. The complex production methods often involve multiple steps, precise temperature control, and expensive precursors, creating barriers to mass production. Additionally, some metal-based materials require rare elements that may face supply chain constraints under high-volume manufacturing scenarios.
Scalability assessment reveals that carbon black and activated carbon benefit from mature production technologies capable of multi-ton annual outputs. These established manufacturing processes can be readily integrated into existing battery production lines with minimal modification. Graphene production has seen significant advances through chemical vapor deposition and liquid-phase exfoliation techniques, though challenges in maintaining consistent quality at scale persist.
Metal-organic frameworks and conductive polymers face the most significant scalability challenges, with current production capabilities limited to laboratory or small pilot scales. The intricate synthesis procedures and quality control requirements present substantial hurdles for industrial implementation. Recent developments in continuous flow synthesis and automated production systems show promise for addressing these limitations, potentially enabling cost-effective scaling within the next decade.
When evaluating total implementation costs, considerations must extend beyond raw material expenses to include processing requirements, equipment compatibility, and yield rates. Carbon-based materials generally require less specialized handling equipment, whereas metal-based alternatives often necessitate additional processing steps and more sophisticated manufacturing infrastructure, increasing their effective implementation cost by 20-40% compared to nominal material prices.
In contrast, graphene and carbon nanotubes present substantially higher cost barriers, with current market prices between $50-200/kg for industrial-grade graphene and $100-500/kg for multi-walled carbon nanotubes. Single-walled carbon nanotubes remain prohibitively expensive at $1,000-10,000/kg, limiting their practical application despite superior conductivity properties. However, ongoing manufacturing innovations are gradually reducing these costs, with projections suggesting potential price reductions of 30-50% within the next five years.
Metal-based conductive hosts, including metal oxides and metal sulfides, occupy a middle ground in the cost spectrum ($20-100/kg) but face challenges in scalable synthesis processes. The complex production methods often involve multiple steps, precise temperature control, and expensive precursors, creating barriers to mass production. Additionally, some metal-based materials require rare elements that may face supply chain constraints under high-volume manufacturing scenarios.
Scalability assessment reveals that carbon black and activated carbon benefit from mature production technologies capable of multi-ton annual outputs. These established manufacturing processes can be readily integrated into existing battery production lines with minimal modification. Graphene production has seen significant advances through chemical vapor deposition and liquid-phase exfoliation techniques, though challenges in maintaining consistent quality at scale persist.
Metal-organic frameworks and conductive polymers face the most significant scalability challenges, with current production capabilities limited to laboratory or small pilot scales. The intricate synthesis procedures and quality control requirements present substantial hurdles for industrial implementation. Recent developments in continuous flow synthesis and automated production systems show promise for addressing these limitations, potentially enabling cost-effective scaling within the next decade.
When evaluating total implementation costs, considerations must extend beyond raw material expenses to include processing requirements, equipment compatibility, and yield rates. Carbon-based materials generally require less specialized handling equipment, whereas metal-based alternatives often necessitate additional processing steps and more sophisticated manufacturing infrastructure, increasing their effective implementation cost by 20-40% compared to nominal material prices.
Environmental Impact and Sustainability Considerations
The environmental impact of lithium-sulfur (Li-S) batteries is increasingly becoming a critical consideration in their development and deployment. When comparing conductive host materials for sulfur cathodes, sustainability aspects must be thoroughly evaluated alongside performance metrics. Carbon-based materials, while offering excellent conductivity, present varying environmental footprints depending on their synthesis methods. Carbon nanotubes and graphene production often involve energy-intensive processes and potentially hazardous chemicals, raising concerns about their life-cycle environmental impact.
Metal-organic frameworks (MOFs) and conductive polymers generally demonstrate more favorable environmental profiles compared to carbon nanostructures. Their synthesis typically requires lower processing temperatures and fewer toxic reagents. However, the scalability of these materials remains a challenge that could affect their overall sustainability when considering mass production scenarios.
Metal sulfides and oxides present an interesting environmental trade-off. While some require energy-intensive mining operations, others can be synthesized from abundant precursors or even recycled materials. Their durability and potential for recycling may offset initial environmental costs over multiple battery lifecycles. The leaching of heavy metals remains a concern that necessitates careful end-of-life management strategies.
The manufacturing processes for different host materials vary significantly in their energy consumption and waste generation. Carbon-based materials typically require high temperatures for activation and functionalization, contributing to their carbon footprint. In contrast, solution-based synthesis methods for polymeric hosts generally consume less energy but may involve organic solvents that require proper handling and disposal.
Water usage represents another important environmental consideration. Hydrothermal synthesis methods common for some metal-based hosts can be water-intensive, while dry synthesis routes for carbon materials may conserve water but consume more energy. The availability of recycling technologies for different host materials also influences their long-term environmental sustainability.
Resource depletion concerns differ among host materials. While carbon is abundant, specialized forms like graphene rely on high-purity precursors. Metal-based hosts may incorporate critical or rare elements, potentially creating supply chain vulnerabilities and environmental justice concerns in mining regions. Developing hosts from abundant, non-critical materials represents a promising direction for sustainable Li-S battery technology.
End-of-life management capabilities vary significantly across host materials. Some carbon-based materials can be recovered and repurposed, while complex composites may present recycling challenges. The development of design-for-recycling approaches specific to each host material type will be crucial for minimizing the environmental footprint of Li-S batteries throughout their complete lifecycle.
Metal-organic frameworks (MOFs) and conductive polymers generally demonstrate more favorable environmental profiles compared to carbon nanostructures. Their synthesis typically requires lower processing temperatures and fewer toxic reagents. However, the scalability of these materials remains a challenge that could affect their overall sustainability when considering mass production scenarios.
Metal sulfides and oxides present an interesting environmental trade-off. While some require energy-intensive mining operations, others can be synthesized from abundant precursors or even recycled materials. Their durability and potential for recycling may offset initial environmental costs over multiple battery lifecycles. The leaching of heavy metals remains a concern that necessitates careful end-of-life management strategies.
The manufacturing processes for different host materials vary significantly in their energy consumption and waste generation. Carbon-based materials typically require high temperatures for activation and functionalization, contributing to their carbon footprint. In contrast, solution-based synthesis methods for polymeric hosts generally consume less energy but may involve organic solvents that require proper handling and disposal.
Water usage represents another important environmental consideration. Hydrothermal synthesis methods common for some metal-based hosts can be water-intensive, while dry synthesis routes for carbon materials may conserve water but consume more energy. The availability of recycling technologies for different host materials also influences their long-term environmental sustainability.
Resource depletion concerns differ among host materials. While carbon is abundant, specialized forms like graphene rely on high-purity precursors. Metal-based hosts may incorporate critical or rare elements, potentially creating supply chain vulnerabilities and environmental justice concerns in mining regions. Developing hosts from abundant, non-critical materials represents a promising direction for sustainable Li-S battery technology.
End-of-life management capabilities vary significantly across host materials. Some carbon-based materials can be recovered and repurposed, while complex composites may present recycling challenges. The development of design-for-recycling approaches specific to each host material type will be crucial for minimizing the environmental footprint of Li-S batteries throughout their complete lifecycle.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!