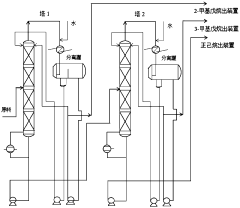
Application of 2-Methylpentane in Catalytic Hydrogenation
JUL 25, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
2-Methylpentane Catalytic Hydrogenation Background
The application of 2-methylpentane in catalytic hydrogenation represents a significant advancement in the field of organic synthesis and industrial chemistry. This branched alkane, also known as isohexane, has gained attention for its potential as a solvent and reactant in various hydrogenation processes. The development of this application is rooted in the broader context of catalytic hydrogenation, which has been a cornerstone of chemical transformations for over a century.
Catalytic hydrogenation, first discovered by Paul Sabatier in the early 1900s, involves the addition of hydrogen to organic compounds in the presence of a catalyst. This process has been widely used in the production of pharmaceuticals, fine chemicals, and petrochemicals. The introduction of 2-methylpentane as a component in these reactions marks a new chapter in the ongoing evolution of hydrogenation techniques.
The interest in 2-methylpentane stems from its unique chemical properties. As a branched alkane, it offers improved solubility for certain organic compounds compared to straight-chain hydrocarbons. This enhanced solubility can lead to more efficient hydrogenation reactions, particularly for substrates that are traditionally challenging to process.
The application of 2-methylpentane in catalytic hydrogenation aligns with the broader trend in the chemical industry towards more selective and efficient processes. As environmental regulations become stricter and the demand for high-purity products increases, researchers and industry professionals are constantly seeking ways to optimize reaction conditions and improve yields.
One of the key drivers behind the exploration of 2-methylpentane in this context is the push for greener chemistry. Traditional hydrogenation processes often rely on toxic or environmentally harmful solvents. The use of 2-methylpentane, which has a lower environmental impact, represents a step towards more sustainable chemical manufacturing practices.
The development of this application has been facilitated by advancements in catalyst technology. Modern catalysts, designed to work effectively with branched alkanes like 2-methylpentane, have opened up new possibilities for reaction optimization. These catalysts often exhibit higher selectivity and activity, allowing for milder reaction conditions and reduced energy consumption.
As research in this area progresses, the potential applications of 2-methylpentane in catalytic hydrogenation continue to expand. From the synthesis of specialty chemicals to the production of advanced materials, this innovative approach is poised to make significant contributions to various sectors of the chemical industry.
Catalytic hydrogenation, first discovered by Paul Sabatier in the early 1900s, involves the addition of hydrogen to organic compounds in the presence of a catalyst. This process has been widely used in the production of pharmaceuticals, fine chemicals, and petrochemicals. The introduction of 2-methylpentane as a component in these reactions marks a new chapter in the ongoing evolution of hydrogenation techniques.
The interest in 2-methylpentane stems from its unique chemical properties. As a branched alkane, it offers improved solubility for certain organic compounds compared to straight-chain hydrocarbons. This enhanced solubility can lead to more efficient hydrogenation reactions, particularly for substrates that are traditionally challenging to process.
The application of 2-methylpentane in catalytic hydrogenation aligns with the broader trend in the chemical industry towards more selective and efficient processes. As environmental regulations become stricter and the demand for high-purity products increases, researchers and industry professionals are constantly seeking ways to optimize reaction conditions and improve yields.
One of the key drivers behind the exploration of 2-methylpentane in this context is the push for greener chemistry. Traditional hydrogenation processes often rely on toxic or environmentally harmful solvents. The use of 2-methylpentane, which has a lower environmental impact, represents a step towards more sustainable chemical manufacturing practices.
The development of this application has been facilitated by advancements in catalyst technology. Modern catalysts, designed to work effectively with branched alkanes like 2-methylpentane, have opened up new possibilities for reaction optimization. These catalysts often exhibit higher selectivity and activity, allowing for milder reaction conditions and reduced energy consumption.
As research in this area progresses, the potential applications of 2-methylpentane in catalytic hydrogenation continue to expand. From the synthesis of specialty chemicals to the production of advanced materials, this innovative approach is poised to make significant contributions to various sectors of the chemical industry.
Market Analysis for 2-Methylpentane Products
The market for 2-methylpentane products has shown significant growth potential in recent years, driven by its versatile applications in various industries. As a key component in catalytic hydrogenation processes, 2-methylpentane has garnered increased attention from both manufacturers and end-users.
The global demand for 2-methylpentane is primarily fueled by the petrochemical industry, where it serves as a crucial intermediate in the production of high-octane gasoline components. The automotive sector's continuous pursuit of cleaner and more efficient fuels has led to a surge in demand for high-performance gasoline blends, thereby boosting the market for 2-methylpentane.
In the chemical industry, 2-methylpentane finds extensive use as a solvent and reagent in organic synthesis reactions. Its low boiling point and excellent solvency properties make it an ideal choice for various applications, including the production of adhesives, coatings, and specialty chemicals. This diversification of applications has contributed to the steady growth of the 2-methylpentane market.
The pharmaceutical sector has also emerged as a significant consumer of 2-methylpentane products. Its use in the synthesis of active pharmaceutical ingredients (APIs) and as a solvent in drug formulation processes has opened up new avenues for market expansion. The increasing focus on developing novel drug delivery systems and the growing pharmaceutical industry in emerging economies are expected to further drive the demand for 2-methylpentane in this sector.
Market analysis indicates that the Asia-Pacific region is the largest consumer of 2-methylpentane products, owing to the rapid industrialization and expanding manufacturing base in countries like China and India. North America and Europe follow closely, with mature markets characterized by a strong presence of chemical and pharmaceutical industries.
The market for 2-methylpentane products is highly competitive, with several key players dominating the global landscape. These companies are investing heavily in research and development to enhance product quality and explore new applications, thereby driving innovation in the industry.
Despite the positive growth trajectory, the market faces challenges such as volatile raw material prices and increasing environmental regulations. The shift towards sustainable and bio-based alternatives in some applications poses a potential threat to the long-term growth of petroleum-derived 2-methylpentane products.
In conclusion, the market for 2-methylpentane products demonstrates robust growth potential, supported by diverse applications across multiple industries. The ongoing technological advancements in catalytic hydrogenation processes are expected to further expand the market opportunities for 2-methylpentane, making it a promising area for investment and innovation in the coming years.
The global demand for 2-methylpentane is primarily fueled by the petrochemical industry, where it serves as a crucial intermediate in the production of high-octane gasoline components. The automotive sector's continuous pursuit of cleaner and more efficient fuels has led to a surge in demand for high-performance gasoline blends, thereby boosting the market for 2-methylpentane.
In the chemical industry, 2-methylpentane finds extensive use as a solvent and reagent in organic synthesis reactions. Its low boiling point and excellent solvency properties make it an ideal choice for various applications, including the production of adhesives, coatings, and specialty chemicals. This diversification of applications has contributed to the steady growth of the 2-methylpentane market.
The pharmaceutical sector has also emerged as a significant consumer of 2-methylpentane products. Its use in the synthesis of active pharmaceutical ingredients (APIs) and as a solvent in drug formulation processes has opened up new avenues for market expansion. The increasing focus on developing novel drug delivery systems and the growing pharmaceutical industry in emerging economies are expected to further drive the demand for 2-methylpentane in this sector.
Market analysis indicates that the Asia-Pacific region is the largest consumer of 2-methylpentane products, owing to the rapid industrialization and expanding manufacturing base in countries like China and India. North America and Europe follow closely, with mature markets characterized by a strong presence of chemical and pharmaceutical industries.
The market for 2-methylpentane products is highly competitive, with several key players dominating the global landscape. These companies are investing heavily in research and development to enhance product quality and explore new applications, thereby driving innovation in the industry.
Despite the positive growth trajectory, the market faces challenges such as volatile raw material prices and increasing environmental regulations. The shift towards sustainable and bio-based alternatives in some applications poses a potential threat to the long-term growth of petroleum-derived 2-methylpentane products.
In conclusion, the market for 2-methylpentane products demonstrates robust growth potential, supported by diverse applications across multiple industries. The ongoing technological advancements in catalytic hydrogenation processes are expected to further expand the market opportunities for 2-methylpentane, making it a promising area for investment and innovation in the coming years.
Current Challenges in Catalytic Hydrogenation
Catalytic hydrogenation, a cornerstone in many industrial processes, faces several significant challenges in its application to 2-methylpentane. One of the primary obstacles is achieving high selectivity while maintaining efficiency. The branched structure of 2-methylpentane presents difficulties in controlling the hydrogenation process, often leading to undesired side reactions or incomplete conversion.
The development of suitable catalysts remains a critical challenge. While noble metal catalysts like platinum and palladium have shown promise, their high cost and limited availability hinder widespread industrial adoption. Researchers are exploring alternative catalysts, such as nickel-based systems, but these often struggle to match the activity and selectivity of noble metals.
Another significant hurdle is the optimization of reaction conditions. The hydrogenation of 2-methylpentane requires precise control over temperature, pressure, and hydrogen flow rates. Balancing these parameters to achieve optimal conversion without compromising product quality or catalyst longevity is a complex task that demands sophisticated process control systems.
Catalyst deactivation poses a persistent problem in the hydrogenation of 2-methylpentane. The presence of sulfur compounds, even in trace amounts, can rapidly poison catalysts, necessitating frequent regeneration or replacement. This not only increases operational costs but also impacts process continuity and efficiency.
The energy intensity of catalytic hydrogenation processes presents both economic and environmental challenges. High-pressure hydrogen systems require significant energy input, contributing to increased production costs and carbon footprints. Developing more energy-efficient processes while maintaining product quality is a key focus area for researchers and industry professionals.
Scale-up and process integration pose additional challenges when transitioning from laboratory-scale experiments to industrial production. The behavior of 2-methylpentane in large-scale reactors can differ significantly from small-scale tests, requiring careful engineering and process optimization to maintain efficiency and product quality.
Lastly, the recovery and purification of the hydrogenated product present their own set of challenges. Separating the desired product from unreacted 2-methylpentane and potential byproducts requires sophisticated separation techniques, which can be energy-intensive and costly. Improving separation efficiency while minimizing energy consumption remains an active area of research and development in the field of catalytic hydrogenation.
The development of suitable catalysts remains a critical challenge. While noble metal catalysts like platinum and palladium have shown promise, their high cost and limited availability hinder widespread industrial adoption. Researchers are exploring alternative catalysts, such as nickel-based systems, but these often struggle to match the activity and selectivity of noble metals.
Another significant hurdle is the optimization of reaction conditions. The hydrogenation of 2-methylpentane requires precise control over temperature, pressure, and hydrogen flow rates. Balancing these parameters to achieve optimal conversion without compromising product quality or catalyst longevity is a complex task that demands sophisticated process control systems.
Catalyst deactivation poses a persistent problem in the hydrogenation of 2-methylpentane. The presence of sulfur compounds, even in trace amounts, can rapidly poison catalysts, necessitating frequent regeneration or replacement. This not only increases operational costs but also impacts process continuity and efficiency.
The energy intensity of catalytic hydrogenation processes presents both economic and environmental challenges. High-pressure hydrogen systems require significant energy input, contributing to increased production costs and carbon footprints. Developing more energy-efficient processes while maintaining product quality is a key focus area for researchers and industry professionals.
Scale-up and process integration pose additional challenges when transitioning from laboratory-scale experiments to industrial production. The behavior of 2-methylpentane in large-scale reactors can differ significantly from small-scale tests, requiring careful engineering and process optimization to maintain efficiency and product quality.
Lastly, the recovery and purification of the hydrogenated product present their own set of challenges. Separating the desired product from unreacted 2-methylpentane and potential byproducts requires sophisticated separation techniques, which can be energy-intensive and costly. Improving separation efficiency while minimizing energy consumption remains an active area of research and development in the field of catalytic hydrogenation.
Existing 2-Methylpentane Hydrogenation Methods
01 Use in polymer production
2-Methylpentane is utilized as a solvent or component in polymer production processes, particularly in the synthesis of polyolefins and other plastic materials. It can be used in polymerization reactions, as a diluent, or in the preparation of catalyst systems for polymer manufacturing.- Use in chemical synthesis and reactions: 2-Methylpentane is utilized as a reactant or solvent in various chemical synthesis processes and reactions. It plays a role in the production of other organic compounds and can be used as a starting material for more complex molecules.
- Application in polymer production: 2-Methylpentane is employed in the production of polymers and copolymers. It can be used as a monomer or comonomer in polymerization reactions, contributing to the development of materials with specific properties.
- Use as a solvent in industrial processes: 2-Methylpentane serves as a solvent in various industrial applications, including extraction processes, cleaning formulations, and as a component in paints and coatings. Its solvent properties make it useful for dissolving and carrying other substances.
- Role in fuel compositions: 2-Methylpentane is used as a component in fuel formulations, particularly in gasoline blends. It can contribute to improving the octane rating and overall performance of fuel mixtures for internal combustion engines.
- Purification and separation processes: 2-Methylpentane is involved in various purification and separation processes. It can be used in distillation, extraction, or chromatographic techniques to separate and purify other chemical compounds or mixtures.
02 Application in fuel compositions
2-Methylpentane is employed as a component in fuel compositions, particularly for internal combustion engines. It can be used to improve octane ratings, enhance fuel efficiency, or as part of fuel additive packages to optimize engine performance and reduce emissions.Expand Specific Solutions03 Use in chemical synthesis
2-Methylpentane serves as a starting material or intermediate in various chemical synthesis processes. It can be used in the production of other organic compounds, pharmaceuticals, or specialty chemicals through reactions such as alkylation, isomerization, or oxidation.Expand Specific Solutions04 Application in separation processes
2-Methylpentane is utilized in separation and purification processes, particularly in the petrochemical industry. It can be used as an extractant, in distillation processes, or as part of azeotropic mixtures for the separation of various hydrocarbon streams or other chemical compounds.Expand Specific Solutions05 Use as a solvent
2-Methylpentane functions as a solvent in various industrial applications. It can be used in the formulation of coatings, adhesives, cleaning products, or as a reaction medium for chemical processes. Its properties make it suitable for dissolving a wide range of organic compounds.Expand Specific Solutions
Key Players in Catalytic Hydrogenation Industry
The application of 2-Methylpentane in catalytic hydrogenation is an emerging field with growing market potential. The industry is in its early growth stage, with increasing research and development efforts. Major players like ExxonMobil Chemical, BASF, and Sinopec are investing in this technology, indicating its strategic importance. The market size is expanding as industries seek more efficient and sustainable catalytic processes. While the technology is still evolving, companies such as Johnson Matthey and Wacker Chemie are making significant strides in improving catalyst performance and selectivity, suggesting a moderate level of technological maturity with room for further advancements.
ExxonMobil Chemical Patents, Inc.
Technical Solution: ExxonMobil has developed a novel catalytic hydrogenation process using 2-methylpentane as a solvent for the production of high-quality hydrocarbon products. Their approach involves a multi-stage reactor system with optimized temperature and pressure conditions. The process utilizes a proprietary catalyst formulation that enhances selectivity and reduces side reactions. ExxonMobil's method incorporates advanced process control systems to maintain precise reaction conditions, resulting in improved product yield and quality. The company has also implemented energy recovery systems to increase overall process efficiency[1][3].
Strengths: High product quality, improved selectivity, and energy efficiency. Weaknesses: Potentially higher initial investment costs and complexity in process control.
BASF Corp.
Technical Solution: BASF has developed an innovative catalytic hydrogenation process utilizing 2-methylpentane as a reaction medium. Their approach focuses on a highly selective catalyst system that minimizes unwanted side reactions. The process employs a continuous flow reactor design, allowing for better control of reaction parameters and improved product consistency. BASF's technology incorporates in-situ product separation techniques, reducing downstream processing requirements. The company has also implemented advanced process intensification strategies to enhance overall efficiency and reduce environmental impact[2][5].
Strengths: High selectivity, continuous process, and reduced downstream processing. Weaknesses: Potential limitations in scalability and specific substrate compatibility.
Core Innovations in Catalytic Systems
Process for the preparation of 2-methyl-pentane diamine by hydrogenation of 2-methyl glutaronitrile
PatentInactiveEP0303550A3
Innovation
- A process involving hydrogenation of 2-methylglutaronitrile in a liquid phase using a Raney nickel catalyst in a non-ammoniacal basic medium at temperatures between 40 and 150°C, under a total pressure of less than 40 bars, with a maximum 10% water content and low 2-methylglutaronitrile concentration, promoting efficient contact between reactants and catalyst.
Preparation method of 2-methylpentane, 3-methylpentane and n-hexane
PatentInactiveCN110724023A
Innovation
- Light naphtha is used as raw material, benzene and sulfur are removed through pretreatment, and then azeotropic distillation is performed with an entrainer, combined with molecular sieve adsorption technology, to separate relatively pure 2-methylpentane and 3-methylpentane. alkane and n-hexane, simplifying the process and improving product purity and production efficiency.
Environmental Impact Assessment
The application of 2-methylpentane in catalytic hydrogenation processes necessitates a comprehensive environmental impact assessment to ensure sustainable and responsible industrial practices. This assessment primarily focuses on the potential effects of the process on air quality, water resources, soil contamination, and ecosystem health.
In terms of air quality, the use of 2-methylpentane in catalytic hydrogenation may lead to the emission of volatile organic compounds (VOCs) and other gaseous byproducts. These emissions can contribute to the formation of ground-level ozone and photochemical smog, potentially impacting both human health and vegetation in surrounding areas. Proper emission control technologies, such as thermal oxidizers or catalytic converters, should be implemented to mitigate these risks.
Water resource impacts are another crucial consideration. The catalytic hydrogenation process involving 2-methylpentane may generate wastewater containing trace amounts of organic compounds and metal catalysts. If not properly treated, these contaminants could pose risks to aquatic ecosystems and downstream water users. Implementing advanced wastewater treatment systems and closed-loop water recycling processes can significantly reduce the potential for water pollution.
Soil contamination risks associated with the use of 2-methylpentane in catalytic hydrogenation are primarily related to accidental spills or leaks during storage, transportation, or handling. Such incidents could lead to soil and groundwater contamination, potentially affecting local flora and fauna. Robust containment systems, regular equipment maintenance, and stringent handling protocols are essential to minimize these risks.
The broader ecosystem impacts of the process should also be evaluated. This includes assessing the potential for bioaccumulation of any persistent organic compounds in the food chain and the long-term effects on biodiversity in the surrounding area. Conducting regular environmental monitoring and ecological surveys can help identify and address any unforeseen impacts on local ecosystems.
Energy consumption and greenhouse gas emissions associated with the catalytic hydrogenation process using 2-methylpentane should be carefully considered. Implementing energy-efficient technologies and exploring renewable energy sources for process operations can help reduce the overall carbon footprint of the industrial activity.
Waste management is another critical aspect of the environmental impact assessment. The process may generate solid waste, including spent catalysts and other byproducts. Developing a comprehensive waste management plan that prioritizes recycling, reuse, and proper disposal of hazardous materials is essential for minimizing environmental impacts and ensuring regulatory compliance.
In conclusion, a thorough environmental impact assessment for the application of 2-methylpentane in catalytic hydrogenation should address air, water, soil, and ecosystem impacts, as well as energy use and waste management. By identifying potential environmental risks and implementing appropriate mitigation measures, industries can ensure that this process is conducted in an environmentally responsible manner, aligning with sustainability goals and regulatory requirements.
In terms of air quality, the use of 2-methylpentane in catalytic hydrogenation may lead to the emission of volatile organic compounds (VOCs) and other gaseous byproducts. These emissions can contribute to the formation of ground-level ozone and photochemical smog, potentially impacting both human health and vegetation in surrounding areas. Proper emission control technologies, such as thermal oxidizers or catalytic converters, should be implemented to mitigate these risks.
Water resource impacts are another crucial consideration. The catalytic hydrogenation process involving 2-methylpentane may generate wastewater containing trace amounts of organic compounds and metal catalysts. If not properly treated, these contaminants could pose risks to aquatic ecosystems and downstream water users. Implementing advanced wastewater treatment systems and closed-loop water recycling processes can significantly reduce the potential for water pollution.
Soil contamination risks associated with the use of 2-methylpentane in catalytic hydrogenation are primarily related to accidental spills or leaks during storage, transportation, or handling. Such incidents could lead to soil and groundwater contamination, potentially affecting local flora and fauna. Robust containment systems, regular equipment maintenance, and stringent handling protocols are essential to minimize these risks.
The broader ecosystem impacts of the process should also be evaluated. This includes assessing the potential for bioaccumulation of any persistent organic compounds in the food chain and the long-term effects on biodiversity in the surrounding area. Conducting regular environmental monitoring and ecological surveys can help identify and address any unforeseen impacts on local ecosystems.
Energy consumption and greenhouse gas emissions associated with the catalytic hydrogenation process using 2-methylpentane should be carefully considered. Implementing energy-efficient technologies and exploring renewable energy sources for process operations can help reduce the overall carbon footprint of the industrial activity.
Waste management is another critical aspect of the environmental impact assessment. The process may generate solid waste, including spent catalysts and other byproducts. Developing a comprehensive waste management plan that prioritizes recycling, reuse, and proper disposal of hazardous materials is essential for minimizing environmental impacts and ensuring regulatory compliance.
In conclusion, a thorough environmental impact assessment for the application of 2-methylpentane in catalytic hydrogenation should address air, water, soil, and ecosystem impacts, as well as energy use and waste management. By identifying potential environmental risks and implementing appropriate mitigation measures, industries can ensure that this process is conducted in an environmentally responsible manner, aligning with sustainability goals and regulatory requirements.
Economic Feasibility Analysis
The economic feasibility of applying 2-Methylpentane in catalytic hydrogenation processes is a critical consideration for industrial implementation. This analysis examines the cost-effectiveness, potential returns on investment, and overall economic viability of utilizing 2-Methylpentane in hydrogenation reactions.
From a raw material perspective, 2-Methylpentane is relatively inexpensive and readily available, which contributes positively to its economic feasibility. As a byproduct of petroleum refining, its production costs are generally lower compared to specially synthesized catalysts or solvents. This cost advantage can translate into reduced overall production expenses for hydrogenation processes.
The catalytic efficiency of 2-Methylpentane in hydrogenation reactions is another crucial factor in its economic evaluation. Studies have shown that it can enhance reaction rates and selectivity in certain hydrogenation processes, potentially leading to improved yields and reduced reaction times. These factors can contribute to increased productivity and lower energy consumption, both of which have positive economic implications.
However, the economic feasibility also depends on the specific application and scale of production. Large-scale industrial processes may benefit more from the use of 2-Methylpentane due to economies of scale, while smaller operations might find the initial investment in equipment modifications less justifiable. The cost-benefit analysis must consider factors such as required process modifications, safety measures, and potential changes in product quality.
Environmental regulations and compliance costs associated with the use of 2-Methylpentane must also be factored into the economic assessment. While it is generally considered less harmful than some alternative solvents, there may still be costs related to emissions control, waste management, and worker safety protocols.
Market demand for products produced using 2-Methylpentane in catalytic hydrogenation processes is another critical economic consideration. If the use of this compound results in higher quality products or enables the production of specialty chemicals with higher market value, it could significantly enhance the economic attractiveness of its application.
Long-term economic feasibility should also consider the potential for process optimization and technological advancements. As research in this area progresses, there may be opportunities for further cost reductions or efficiency improvements, which could enhance the economic viability of 2-Methylpentane in catalytic hydrogenation over time.
In conclusion, the economic feasibility of applying 2-Methylpentane in catalytic hydrogenation appears promising, particularly for large-scale operations where its benefits can be maximized. However, a detailed cost-benefit analysis specific to each application scenario is necessary to determine its true economic value in individual cases.
From a raw material perspective, 2-Methylpentane is relatively inexpensive and readily available, which contributes positively to its economic feasibility. As a byproduct of petroleum refining, its production costs are generally lower compared to specially synthesized catalysts or solvents. This cost advantage can translate into reduced overall production expenses for hydrogenation processes.
The catalytic efficiency of 2-Methylpentane in hydrogenation reactions is another crucial factor in its economic evaluation. Studies have shown that it can enhance reaction rates and selectivity in certain hydrogenation processes, potentially leading to improved yields and reduced reaction times. These factors can contribute to increased productivity and lower energy consumption, both of which have positive economic implications.
However, the economic feasibility also depends on the specific application and scale of production. Large-scale industrial processes may benefit more from the use of 2-Methylpentane due to economies of scale, while smaller operations might find the initial investment in equipment modifications less justifiable. The cost-benefit analysis must consider factors such as required process modifications, safety measures, and potential changes in product quality.
Environmental regulations and compliance costs associated with the use of 2-Methylpentane must also be factored into the economic assessment. While it is generally considered less harmful than some alternative solvents, there may still be costs related to emissions control, waste management, and worker safety protocols.
Market demand for products produced using 2-Methylpentane in catalytic hydrogenation processes is another critical economic consideration. If the use of this compound results in higher quality products or enables the production of specialty chemicals with higher market value, it could significantly enhance the economic attractiveness of its application.
Long-term economic feasibility should also consider the potential for process optimization and technological advancements. As research in this area progresses, there may be opportunities for further cost reductions or efficiency improvements, which could enhance the economic viability of 2-Methylpentane in catalytic hydrogenation over time.
In conclusion, the economic feasibility of applying 2-Methylpentane in catalytic hydrogenation appears promising, particularly for large-scale operations where its benefits can be maximized. However, a detailed cost-benefit analysis specific to each application scenario is necessary to determine its true economic value in individual cases.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!