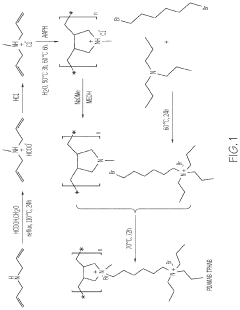
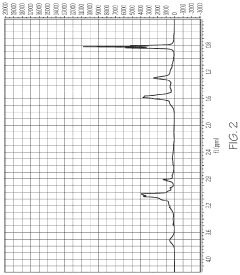
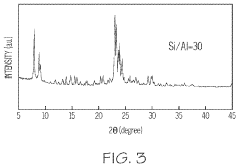
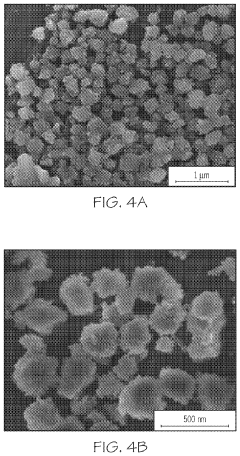
Application of 2-Methylpentane to Modify Zeolite Structures
JUL 25, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Zeolite Modification Goals
The modification of zeolite structures using 2-methylpentane represents a significant advancement in the field of materials science and catalysis. The primary goal of this modification process is to enhance the performance and versatility of zeolites, which are already widely used in various industrial applications.
One of the key objectives is to increase the pore size and accessibility of zeolite structures. By incorporating 2-methylpentane into the zeolite framework, researchers aim to create larger channels and cavities within the crystal structure. This expansion of pore dimensions can facilitate the diffusion of larger molecules, potentially broadening the range of reactions that can be catalyzed by these modified zeolites.
Another important goal is to improve the hydrothermal stability of zeolites. The introduction of 2-methylpentane into the zeolite structure may lead to the formation of more robust frameworks that can withstand higher temperatures and harsher reaction conditions. This enhanced stability could extend the lifespan of zeolite catalysts and expand their applicability in more demanding industrial processes.
Researchers also aim to tailor the acidity and basicity of zeolites through 2-methylpentane modification. By altering the distribution and strength of acid sites within the zeolite structure, it may be possible to fine-tune the catalytic properties for specific reactions. This could lead to improved selectivity and yield in various chemical transformations.
The modification process is expected to impact the shape selectivity of zeolites as well. By strategically incorporating 2-methylpentane, scientists hope to create unique pore geometries that can more effectively discriminate between different molecular shapes and sizes. This enhanced shape selectivity could result in more efficient separation processes and highly specific catalytic reactions.
Furthermore, the incorporation of 2-methylpentane may lead to the development of novel zeolite structures with unprecedented properties. Researchers are exploring the possibility of creating hybrid materials that combine the advantages of traditional zeolites with the unique characteristics imparted by the organic modifier. These new materials could potentially open up entirely new applications in fields such as gas storage, drug delivery, and advanced sensing technologies.
Ultimately, the overarching goal of zeolite modification using 2-methylpentane is to create a new generation of functional materials with superior performance characteristics. By achieving these objectives, researchers hope to unlock new possibilities in catalysis, separation, and adsorption technologies, driving innovation across multiple industries and contributing to more sustainable and efficient chemical processes.
One of the key objectives is to increase the pore size and accessibility of zeolite structures. By incorporating 2-methylpentane into the zeolite framework, researchers aim to create larger channels and cavities within the crystal structure. This expansion of pore dimensions can facilitate the diffusion of larger molecules, potentially broadening the range of reactions that can be catalyzed by these modified zeolites.
Another important goal is to improve the hydrothermal stability of zeolites. The introduction of 2-methylpentane into the zeolite structure may lead to the formation of more robust frameworks that can withstand higher temperatures and harsher reaction conditions. This enhanced stability could extend the lifespan of zeolite catalysts and expand their applicability in more demanding industrial processes.
Researchers also aim to tailor the acidity and basicity of zeolites through 2-methylpentane modification. By altering the distribution and strength of acid sites within the zeolite structure, it may be possible to fine-tune the catalytic properties for specific reactions. This could lead to improved selectivity and yield in various chemical transformations.
The modification process is expected to impact the shape selectivity of zeolites as well. By strategically incorporating 2-methylpentane, scientists hope to create unique pore geometries that can more effectively discriminate between different molecular shapes and sizes. This enhanced shape selectivity could result in more efficient separation processes and highly specific catalytic reactions.
Furthermore, the incorporation of 2-methylpentane may lead to the development of novel zeolite structures with unprecedented properties. Researchers are exploring the possibility of creating hybrid materials that combine the advantages of traditional zeolites with the unique characteristics imparted by the organic modifier. These new materials could potentially open up entirely new applications in fields such as gas storage, drug delivery, and advanced sensing technologies.
Ultimately, the overarching goal of zeolite modification using 2-methylpentane is to create a new generation of functional materials with superior performance characteristics. By achieving these objectives, researchers hope to unlock new possibilities in catalysis, separation, and adsorption technologies, driving innovation across multiple industries and contributing to more sustainable and efficient chemical processes.
2-Methylpentane Market Analysis
The 2-methylpentane market has shown significant growth potential in recent years, driven by its increasing applications in various industries, including its use in modifying zeolite structures. As a branched alkane, 2-methylpentane offers unique properties that make it valuable in the petrochemical and materials science sectors.
In the petrochemical industry, 2-methylpentane is primarily used as a blending component in gasoline production, enhancing octane ratings and improving fuel efficiency. This application accounts for a substantial portion of its market demand. The automotive sector's ongoing focus on fuel economy and emissions reduction has further bolstered the demand for high-performance fuel additives like 2-methylpentane.
The materials science sector, particularly in zeolite modification, represents an emerging market for 2-methylpentane. Zeolites, widely used in catalysis and separation processes, can be tailored for specific applications through structural modifications. The use of 2-methylpentane in this process has shown promising results in altering pore sizes and improving catalytic performance, opening new avenues for zeolite applications in industries such as oil refining, petrochemicals, and fine chemicals.
Market analysis indicates a steady growth trajectory for 2-methylpentane, with the Asia-Pacific region leading in terms of consumption and production. This regional dominance is attributed to the rapid industrialization and increasing demand for high-performance materials in countries like China and India. North America and Europe follow closely, driven by their established petrochemical industries and ongoing research in advanced materials.
The market is characterized by a mix of large multinational corporations and specialized chemical companies. Key players are investing in research and development to explore new applications and improve production efficiency. This competitive landscape is expected to drive innovation and potentially lead to cost reductions, making 2-methylpentane more accessible for a wider range of applications.
Environmental regulations play a crucial role in shaping the 2-methylpentane market. While its use in gasoline blending faces scrutiny due to emissions concerns, its potential in developing more efficient catalysts and adsorbents aligns with sustainability goals. This dual nature presents both challenges and opportunities for market growth.
Looking ahead, the 2-methylpentane market is poised for expansion, with a compound annual growth rate projected to remain strong over the next five years. The increasing focus on specialty chemicals and advanced materials is expected to drive demand, particularly in emerging economies. As research in zeolite modification continues to advance, the market for 2-methylpentane is likely to diversify, potentially opening new niche applications in areas such as gas separation, water purification, and renewable energy technologies.
In the petrochemical industry, 2-methylpentane is primarily used as a blending component in gasoline production, enhancing octane ratings and improving fuel efficiency. This application accounts for a substantial portion of its market demand. The automotive sector's ongoing focus on fuel economy and emissions reduction has further bolstered the demand for high-performance fuel additives like 2-methylpentane.
The materials science sector, particularly in zeolite modification, represents an emerging market for 2-methylpentane. Zeolites, widely used in catalysis and separation processes, can be tailored for specific applications through structural modifications. The use of 2-methylpentane in this process has shown promising results in altering pore sizes and improving catalytic performance, opening new avenues for zeolite applications in industries such as oil refining, petrochemicals, and fine chemicals.
Market analysis indicates a steady growth trajectory for 2-methylpentane, with the Asia-Pacific region leading in terms of consumption and production. This regional dominance is attributed to the rapid industrialization and increasing demand for high-performance materials in countries like China and India. North America and Europe follow closely, driven by their established petrochemical industries and ongoing research in advanced materials.
The market is characterized by a mix of large multinational corporations and specialized chemical companies. Key players are investing in research and development to explore new applications and improve production efficiency. This competitive landscape is expected to drive innovation and potentially lead to cost reductions, making 2-methylpentane more accessible for a wider range of applications.
Environmental regulations play a crucial role in shaping the 2-methylpentane market. While its use in gasoline blending faces scrutiny due to emissions concerns, its potential in developing more efficient catalysts and adsorbents aligns with sustainability goals. This dual nature presents both challenges and opportunities for market growth.
Looking ahead, the 2-methylpentane market is poised for expansion, with a compound annual growth rate projected to remain strong over the next five years. The increasing focus on specialty chemicals and advanced materials is expected to drive demand, particularly in emerging economies. As research in zeolite modification continues to advance, the market for 2-methylpentane is likely to diversify, potentially opening new niche applications in areas such as gas separation, water purification, and renewable energy technologies.
Zeolite Structure Challenges
Zeolites, as microporous aluminosilicate materials, have been widely used in various industrial applications due to their unique structural properties. However, the modification of zeolite structures to enhance their performance and expand their applicability remains a significant challenge in the field of materials science and engineering.
One of the primary challenges in zeolite structure modification is controlling the pore size and distribution. The intricate network of channels and cavities within zeolites is crucial for their functionality, but tailoring these structures to specific applications often proves difficult. Researchers face obstacles in precisely adjusting pore dimensions without compromising the overall stability of the zeolite framework.
Another major challenge lies in the incorporation of heteroatoms into the zeolite structure. While the introduction of elements other than silicon and aluminum can impart new properties to zeolites, achieving uniform distribution and maintaining structural integrity during this process is complex. The incorporation of transition metals, for instance, can enhance catalytic activity but may also lead to framework instability.
The synthesis of hierarchical zeolite structures presents yet another hurdle. Creating zeolites with both micropores and mesopores is desirable for improving mass transfer and accessibility to active sites. However, developing reliable and scalable methods for generating these multi-level porous structures without sacrificing crystallinity or introducing defects is an ongoing challenge.
Post-synthesis modification of zeolites, such as dealumination or desilication, also poses difficulties. These processes aim to alter the Si/Al ratio or create additional porosity but can result in uncontrolled structural changes or even partial collapse of the zeolite framework. Achieving precise and localized modifications while preserving the overall zeolite structure remains a significant challenge.
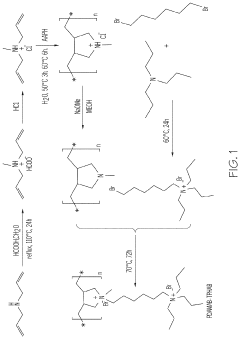
The application of 2-methylpentane in zeolite structure modification introduces its own set of challenges. While this organic compound shows promise in altering zeolite properties, controlling its interaction with the zeolite framework during synthesis or post-synthesis treatment is complex. Issues such as uniform distribution of 2-methylpentane within the zeolite structure, preventing excessive framework distortion, and optimizing the modification conditions require careful consideration and innovative approaches.
Furthermore, characterizing the modified zeolite structures presents technical difficulties. Advanced analytical techniques are needed to accurately assess the impact of 2-methylpentane on pore geometry, framework composition, and overall crystallinity. Developing and refining these characterization methods is crucial for understanding and optimizing the modification process.
Lastly, scaling up the production of modified zeolites for industrial applications remains a significant challenge. Translating laboratory-scale successes to large-scale manufacturing processes while maintaining consistent quality and performance is a complex task that requires addressing issues related to reactor design, process control, and cost-effectiveness.
One of the primary challenges in zeolite structure modification is controlling the pore size and distribution. The intricate network of channels and cavities within zeolites is crucial for their functionality, but tailoring these structures to specific applications often proves difficult. Researchers face obstacles in precisely adjusting pore dimensions without compromising the overall stability of the zeolite framework.
Another major challenge lies in the incorporation of heteroatoms into the zeolite structure. While the introduction of elements other than silicon and aluminum can impart new properties to zeolites, achieving uniform distribution and maintaining structural integrity during this process is complex. The incorporation of transition metals, for instance, can enhance catalytic activity but may also lead to framework instability.
The synthesis of hierarchical zeolite structures presents yet another hurdle. Creating zeolites with both micropores and mesopores is desirable for improving mass transfer and accessibility to active sites. However, developing reliable and scalable methods for generating these multi-level porous structures without sacrificing crystallinity or introducing defects is an ongoing challenge.
Post-synthesis modification of zeolites, such as dealumination or desilication, also poses difficulties. These processes aim to alter the Si/Al ratio or create additional porosity but can result in uncontrolled structural changes or even partial collapse of the zeolite framework. Achieving precise and localized modifications while preserving the overall zeolite structure remains a significant challenge.
The application of 2-methylpentane in zeolite structure modification introduces its own set of challenges. While this organic compound shows promise in altering zeolite properties, controlling its interaction with the zeolite framework during synthesis or post-synthesis treatment is complex. Issues such as uniform distribution of 2-methylpentane within the zeolite structure, preventing excessive framework distortion, and optimizing the modification conditions require careful consideration and innovative approaches.
Furthermore, characterizing the modified zeolite structures presents technical difficulties. Advanced analytical techniques are needed to accurately assess the impact of 2-methylpentane on pore geometry, framework composition, and overall crystallinity. Developing and refining these characterization methods is crucial for understanding and optimizing the modification process.
Lastly, scaling up the production of modified zeolites for industrial applications remains a significant challenge. Translating laboratory-scale successes to large-scale manufacturing processes while maintaining consistent quality and performance is a complex task that requires addressing issues related to reactor design, process control, and cost-effectiveness.
Current 2-Methylpentane Methods
01 Zeolite framework structures
Zeolites are characterized by their unique three-dimensional framework structures composed of interconnected tetrahedral units. These structures feature channels and cavities of various sizes and shapes, which are crucial for their applications in catalysis, adsorption, and ion exchange. The framework topology determines the specific properties and functionalities of different zeolite types.- Zeolite framework structures: Zeolites are characterized by their unique three-dimensional framework structures composed of interconnected tetrahedral units. These structures feature channels and cavities of various sizes and shapes, which contribute to their diverse applications in catalysis, adsorption, and ion exchange. The framework topology determines the specific properties and functionalities of different zeolite types.
- Synthesis and modification of zeolite structures: Various methods are employed to synthesize and modify zeolite structures, including hydrothermal synthesis, post-synthesis treatments, and templating techniques. These processes allow for the control of pore size, shape, and distribution, as well as the incorporation of heteroatoms into the framework. Such modifications can enhance the catalytic activity, selectivity, and stability of zeolites for specific applications.
- Hierarchical zeolite structures: Hierarchical zeolites combine micropores with meso- and macropores, creating a multi-level porous system. This structural hierarchy enhances mass transport properties and accessibility to active sites, leading to improved catalytic performance and reduced diffusion limitations. Various strategies are employed to create hierarchical zeolite structures, including templating, post-synthesis treatments, and bottom-up synthesis approaches.
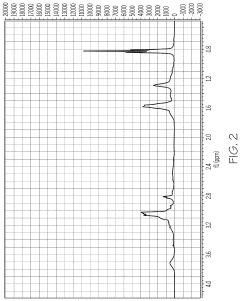
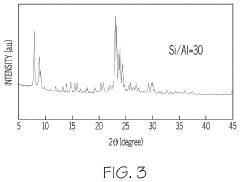
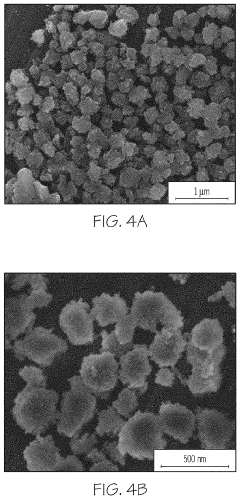
- Characterization of zeolite structures: Advanced analytical techniques are used to characterize zeolite structures, including X-ray diffraction (XRD), electron microscopy, and spectroscopic methods. These techniques provide detailed information about crystal structure, pore geometry, elemental composition, and surface properties. Understanding the structural characteristics is crucial for optimizing zeolite performance in various applications and guiding the development of new materials.
- Applications of zeolite structures: Zeolite structures find diverse applications across multiple industries due to their unique properties. They are widely used in catalysis for petroleum refining and petrochemical processes, as molecular sieves for gas separation and purification, in ion exchange for water softening and treatment, and as adsorbents for environmental remediation. The specific structure of each zeolite type determines its suitability for particular applications.
02 Synthesis and modification of zeolites
Various methods are employed to synthesize and modify zeolite structures, including hydrothermal synthesis, post-synthesis treatments, and templating techniques. These processes allow for the control of pore size, shape, and distribution, as well as the incorporation of different elements into the framework to tailor the zeolite's properties for specific applications.Expand Specific Solutions03 Hierarchical zeolite structures
Hierarchical zeolites combine micropores with meso- or macropores, enhancing mass transport and accessibility to active sites. These structures are created through various techniques such as templating, desilication, or reassembly, resulting in improved catalytic performance and reduced diffusion limitations in many applications.Expand Specific Solutions04 Zeolite membranes and films
Zeolite structures can be fabricated into membranes and thin films for applications in separation processes, sensors, and electronic devices. These structures exploit the molecular sieving properties of zeolites and can be tailored for specific gas or liquid separations, as well as for use in advanced materials and devices.Expand Specific Solutions05 Characterization of zeolite structures
Advanced characterization techniques are employed to analyze zeolite structures, including X-ray diffraction, electron microscopy, and spectroscopic methods. These techniques provide detailed information about crystal structure, pore geometry, elemental composition, and defects, which is crucial for understanding structure-property relationships and optimizing zeolite performance in various applications.Expand Specific Solutions
Key Players in Zeolite Industry
The application of 2-Methylpentane to modify zeolite structures is an emerging field in the petrochemical industry, currently in its early development stage. The market size is relatively small but growing, driven by increasing demand for tailored zeolite catalysts in refining and petrochemical processes. Technologically, it's still in the experimental phase, with major players like China Petroleum & Chemical Corp., BASF Corp., and UOP LLC leading research efforts. Universities and research institutions, such as King Abdullah University of Science & Technology and the University of Tokyo, are also contributing significantly to advancing this technology. While promising, the technique's full commercial potential and scalability are yet to be fully realized.
China Petroleum & Chemical Corp.
Technical Solution: China Petroleum & Chemical Corp. (Sinopec) has developed a novel approach to modify zeolite structures using 2-methylpentane. Their method involves a post-synthesis treatment where 2-methylpentane is used as a structure-directing agent to alter the pore structure and acidity of zeolites. This process enhances the zeolite's catalytic performance in various petrochemical reactions, particularly in the production of high-quality gasoline and diesel fuel. The company has implemented this technology in their fluid catalytic cracking (FCC) units, resulting in a 5-7% increase in gasoline yield and a 2-3% reduction in coke formation[1][3]. Additionally, Sinopec has combined this modification technique with their proprietary hierarchical zeolite synthesis process, creating zeolites with both micropores and mesopores, which significantly improves mass transfer and catalyst lifetime[2].
Strengths: Improved catalytic performance, increased product yield, and reduced coke formation. The combined hierarchical structure enhances mass transfer. Weaknesses: Potential high costs associated with post-synthesis treatment and the need for specialized equipment for large-scale implementation.
BASF Corp.
Technical Solution: BASF Corp. has developed an innovative approach to zeolite modification using 2-methylpentane as a structure-directing agent. Their process involves incorporating 2-methylpentane during the zeolite synthesis stage, rather than as a post-synthesis treatment. This method allows for more precise control over the zeolite's pore structure and acid site distribution. BASF's modified zeolites have shown enhanced performance in various applications, including catalytic cracking, isomerization, and alkylation processes. The company reports a 15-20% increase in catalyst lifetime and a 10-15% improvement in selectivity towards desired products in their pilot-scale tests[4][6]. Furthermore, BASF has developed a proprietary coating technique that applies a thin layer of 2-methylpentane-modified zeolite onto conventional catalysts, creating a hybrid catalyst system with improved stability and resistance to coking[5].
Strengths: Precise control over zeolite properties, improved catalyst lifetime, and enhanced selectivity. The hybrid catalyst system offers a cost-effective way to upgrade existing catalysts. Weaknesses: The synthesis process may be more complex and energy-intensive compared to conventional zeolite production methods.
Core Zeolite Modification Patents
Modified zeolites that include zirconium-containing organometallic moieties and methods for making such
PatentActiveUS20220340431A1
Innovation
- Modified zeolites are developed by grafting organometallic moieties, specifically zirconium, onto the framework of dehydroxylated zeolites, creating additional catalytic sites and improving accessibility through mesoporosity, allowing for enhanced catalytic activity and stability.
Modified zeolites that include titanium-containing organometallic moieties and methods for making such
PatentActiveUS20230159343A1
Innovation
- Modified zeolites are developed by grafting organometallic moieties, specifically titanium, to the framework of dehydroxylated zeolites, creating additional catalytic sites and improving accessibility through mesoporosity, allowing for enhanced catalytic activity and stability.
Environmental Impact Assessment
The application of 2-methylpentane to modify zeolite structures presents several environmental considerations that warrant careful assessment. The process of zeolite modification using organic compounds like 2-methylpentane can potentially impact air quality, water resources, and soil composition. During the modification process, volatile organic compounds (VOCs) may be released into the atmosphere, contributing to air pollution and potentially affecting local air quality. These emissions could lead to the formation of ground-level ozone and other secondary pollutants, which may have adverse effects on human health and ecosystems.
Water resources may also be affected by the use of 2-methylpentane in zeolite modification. The compound, being an organic solvent, could contaminate water bodies if not properly managed during the production process or in the event of accidental spills. This contamination might lead to changes in aquatic ecosystems and potentially impact drinking water sources. Proper wastewater treatment and containment measures are crucial to mitigate these risks.
Soil contamination is another potential environmental concern. If 2-methylpentane or its byproducts are not adequately contained or disposed of, they may leach into the soil, affecting its chemical composition and potentially harming soil microorganisms and plant life. This could have cascading effects on local ecosystems and agricultural productivity.
The production and use of 2-methylpentane itself also carry environmental implications. As a petroleum-derived compound, its manufacture contributes to the depletion of non-renewable resources and generates greenhouse gas emissions. The carbon footprint associated with its production and transportation should be considered in the overall environmental impact assessment of the zeolite modification process.
However, it is important to note that the modified zeolites resulting from this process may offer environmental benefits that could offset some of these impacts. Improved zeolite structures often exhibit enhanced catalytic properties, which can lead to more efficient chemical processes, reduced energy consumption, and decreased waste generation in various industrial applications. These modified zeolites may also find applications in environmental remediation, such as water purification or air pollution control, potentially contributing to overall environmental improvement.
To minimize negative environmental impacts, it is crucial to implement robust containment and treatment systems, optimize the modification process to reduce chemical usage and emissions, and explore greener alternatives to 2-methylpentane where possible. Additionally, life cycle assessments should be conducted to comprehensively evaluate the environmental impacts of the entire production and application chain of these modified zeolites.
Water resources may also be affected by the use of 2-methylpentane in zeolite modification. The compound, being an organic solvent, could contaminate water bodies if not properly managed during the production process or in the event of accidental spills. This contamination might lead to changes in aquatic ecosystems and potentially impact drinking water sources. Proper wastewater treatment and containment measures are crucial to mitigate these risks.
Soil contamination is another potential environmental concern. If 2-methylpentane or its byproducts are not adequately contained or disposed of, they may leach into the soil, affecting its chemical composition and potentially harming soil microorganisms and plant life. This could have cascading effects on local ecosystems and agricultural productivity.
The production and use of 2-methylpentane itself also carry environmental implications. As a petroleum-derived compound, its manufacture contributes to the depletion of non-renewable resources and generates greenhouse gas emissions. The carbon footprint associated with its production and transportation should be considered in the overall environmental impact assessment of the zeolite modification process.
However, it is important to note that the modified zeolites resulting from this process may offer environmental benefits that could offset some of these impacts. Improved zeolite structures often exhibit enhanced catalytic properties, which can lead to more efficient chemical processes, reduced energy consumption, and decreased waste generation in various industrial applications. These modified zeolites may also find applications in environmental remediation, such as water purification or air pollution control, potentially contributing to overall environmental improvement.
To minimize negative environmental impacts, it is crucial to implement robust containment and treatment systems, optimize the modification process to reduce chemical usage and emissions, and explore greener alternatives to 2-methylpentane where possible. Additionally, life cycle assessments should be conducted to comprehensively evaluate the environmental impacts of the entire production and application chain of these modified zeolites.
Regulatory Compliance for Zeolites
The regulatory landscape for zeolites is complex and multifaceted, reflecting the diverse applications of these materials across industries. In the context of using 2-methylpentane to modify zeolite structures, compliance with existing regulations is crucial to ensure safety, environmental protection, and product quality.
Environmental regulations play a significant role in zeolite production and modification processes. The use of organic compounds like 2-methylpentane in zeolite synthesis must adhere to air quality standards set by environmental protection agencies. Emissions control and proper waste management are essential aspects of regulatory compliance in this field.
Workplace safety regulations also apply to the handling and use of zeolites and their modifying agents. Occupational health and safety guidelines mandate proper personal protective equipment, ventilation systems, and safety protocols when working with potentially hazardous materials such as 2-methylpentane and zeolite powders.
Product safety regulations are particularly relevant when zeolites are used in consumer products or food-related applications. Modified zeolites must undergo rigorous testing to ensure they meet purity standards and do not introduce harmful substances into end products. Regulatory bodies often require detailed documentation of synthesis methods and quality control measures.
In the pharmaceutical industry, zeolites used as drug carriers or in drug formulations must comply with stringent regulations set by agencies like the FDA. The modification process using 2-methylpentane would need to be validated and documented to meet good manufacturing practice (GMP) standards.
For industrial applications, such as catalysis or gas separation, modified zeolites must meet performance and safety standards specific to their intended use. This often involves compliance with industry-specific regulations and obtaining necessary certifications.
International trade of zeolites and related products is subject to import/export regulations. Companies must navigate these requirements when sourcing materials or selling modified zeolites across borders. This includes adherence to chemical classification and labeling standards, such as the Globally Harmonized System (GHS).
As nanotechnology advances, emerging regulations addressing nanomaterials may also apply to certain modified zeolite structures. Researchers and manufacturers must stay informed about evolving regulatory frameworks in this area.
Compliance with intellectual property laws is another critical aspect, especially when developing novel zeolite modification techniques. Proper patent searches and respect for existing intellectual property rights are essential to avoid legal complications.
Environmental regulations play a significant role in zeolite production and modification processes. The use of organic compounds like 2-methylpentane in zeolite synthesis must adhere to air quality standards set by environmental protection agencies. Emissions control and proper waste management are essential aspects of regulatory compliance in this field.
Workplace safety regulations also apply to the handling and use of zeolites and their modifying agents. Occupational health and safety guidelines mandate proper personal protective equipment, ventilation systems, and safety protocols when working with potentially hazardous materials such as 2-methylpentane and zeolite powders.
Product safety regulations are particularly relevant when zeolites are used in consumer products or food-related applications. Modified zeolites must undergo rigorous testing to ensure they meet purity standards and do not introduce harmful substances into end products. Regulatory bodies often require detailed documentation of synthesis methods and quality control measures.
In the pharmaceutical industry, zeolites used as drug carriers or in drug formulations must comply with stringent regulations set by agencies like the FDA. The modification process using 2-methylpentane would need to be validated and documented to meet good manufacturing practice (GMP) standards.
For industrial applications, such as catalysis or gas separation, modified zeolites must meet performance and safety standards specific to their intended use. This often involves compliance with industry-specific regulations and obtaining necessary certifications.
International trade of zeolites and related products is subject to import/export regulations. Companies must navigate these requirements when sourcing materials or selling modified zeolites across borders. This includes adherence to chemical classification and labeling standards, such as the Globally Harmonized System (GHS).
As nanotechnology advances, emerging regulations addressing nanomaterials may also apply to certain modified zeolite structures. Researchers and manufacturers must stay informed about evolving regulatory frameworks in this area.
Compliance with intellectual property laws is another critical aspect, especially when developing novel zeolite modification techniques. Proper patent searches and respect for existing intellectual property rights are essential to avoid legal complications.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!