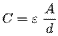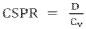Autonomous Lab Integration into Pharmaceutical and Biotech Industries
SEP 25, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Autonomous Lab Evolution and Objectives
The evolution of autonomous laboratories represents a significant paradigm shift in scientific research methodology, transitioning from manual experimentation to highly automated systems capable of self-directed discovery. Beginning in the early 2000s with basic robotic liquid handling systems, autonomous labs have evolved through several distinct phases, culminating in today's AI-driven platforms that can design, execute, and interpret experiments with minimal human intervention.
The pharmaceutical and biotech industries have historically relied on labor-intensive research processes, with scientists manually designing experiments, executing protocols, and analyzing results. This traditional approach faces inherent limitations in throughput, reproducibility, and the ability to explore vast experimental spaces. The emergence of autonomous lab technologies addresses these limitations by combining robotics, machine learning, and advanced analytical techniques into integrated systems.
Current autonomous lab platforms incorporate closed-loop experimentation capabilities, where AI algorithms can propose hypotheses, design experiments, interpret results, and refine subsequent experimental designs based on accumulated data. This represents a fundamental shift from automation as a tool for executing predefined protocols to autonomous systems capable of scientific reasoning and discovery.
The primary objectives of autonomous lab integration in pharmaceutical and biotech contexts include accelerating drug discovery timelines, enhancing research reproducibility, optimizing resource utilization, and enabling exploration of previously intractable research questions. By reducing the drug discovery cycle from years to months, these technologies promise to address critical industry challenges such as rising R&D costs and declining innovation productivity.
Technical evolution in this domain has been driven by advances in several interconnected fields, including robotics, sensor technology, microfluidics, and artificial intelligence. The convergence of these technologies has enabled increasingly sophisticated autonomous capabilities, from basic parameter optimization to complex experimental design and interpretation.
Looking forward, the trajectory of autonomous lab development points toward fully integrated discovery platforms capable of end-to-end research processes with minimal human oversight. This includes the potential for autonomous systems to formulate novel scientific hypotheses, design validation experiments, and contribute to theoretical model development based on experimental findings.
The ultimate goal of autonomous lab integration is not to replace human scientists but to augment their capabilities, allowing researchers to focus on creative and strategic aspects of scientific inquiry while delegating routine experimentation and data analysis to autonomous systems. This human-machine collaboration model represents the most promising path toward addressing complex biomedical challenges and accelerating scientific discovery.
The pharmaceutical and biotech industries have historically relied on labor-intensive research processes, with scientists manually designing experiments, executing protocols, and analyzing results. This traditional approach faces inherent limitations in throughput, reproducibility, and the ability to explore vast experimental spaces. The emergence of autonomous lab technologies addresses these limitations by combining robotics, machine learning, and advanced analytical techniques into integrated systems.
Current autonomous lab platforms incorporate closed-loop experimentation capabilities, where AI algorithms can propose hypotheses, design experiments, interpret results, and refine subsequent experimental designs based on accumulated data. This represents a fundamental shift from automation as a tool for executing predefined protocols to autonomous systems capable of scientific reasoning and discovery.
The primary objectives of autonomous lab integration in pharmaceutical and biotech contexts include accelerating drug discovery timelines, enhancing research reproducibility, optimizing resource utilization, and enabling exploration of previously intractable research questions. By reducing the drug discovery cycle from years to months, these technologies promise to address critical industry challenges such as rising R&D costs and declining innovation productivity.
Technical evolution in this domain has been driven by advances in several interconnected fields, including robotics, sensor technology, microfluidics, and artificial intelligence. The convergence of these technologies has enabled increasingly sophisticated autonomous capabilities, from basic parameter optimization to complex experimental design and interpretation.
Looking forward, the trajectory of autonomous lab development points toward fully integrated discovery platforms capable of end-to-end research processes with minimal human oversight. This includes the potential for autonomous systems to formulate novel scientific hypotheses, design validation experiments, and contribute to theoretical model development based on experimental findings.
The ultimate goal of autonomous lab integration is not to replace human scientists but to augment their capabilities, allowing researchers to focus on creative and strategic aspects of scientific inquiry while delegating routine experimentation and data analysis to autonomous systems. This human-machine collaboration model represents the most promising path toward addressing complex biomedical challenges and accelerating scientific discovery.
Market Demand Analysis for Lab Automation
The global laboratory automation market is experiencing robust growth, driven primarily by the pharmaceutical and biotechnology sectors' increasing need for efficiency, precision, and scalability. Current market valuations place the lab automation sector at approximately $5.2 billion in 2023, with projections indicating a compound annual growth rate (CAGR) of 8.1% through 2030, potentially reaching $9.8 billion. This growth trajectory is particularly pronounced in regions with established pharmaceutical research hubs, including North America, Western Europe, and increasingly, East Asia.
Demand analysis reveals several key market drivers accelerating the adoption of autonomous lab integration. Labor costs in pharmaceutical research continue to rise, with specialized laboratory technicians commanding premium salaries, while simultaneously facing shortages in many markets. This economic pressure has created strong incentives for automation solutions that can reduce dependence on manual operations while maintaining or improving output quality.
Quality control requirements represent another significant market pull factor. Regulatory bodies worldwide have progressively tightened compliance standards for pharmaceutical manufacturing and research, necessitating more consistent, traceable, and validated processes. Autonomous lab systems offer superior documentation, reduced human error rates, and enhanced reproducibility—all critical factors in regulatory compliance.
The COVID-19 pandemic has served as a market catalyst, dramatically highlighting the vulnerabilities of traditional laboratory workflows dependent on human presence. Organizations that had invested in automation demonstrated greater resilience during lockdown periods, maintaining critical research continuity while minimizing personnel density. This real-world demonstration has accelerated adoption timelines across the industry.
Data-driven drug discovery represents a particularly high-growth segment within the market. The integration of artificial intelligence with laboratory automation has created demand for systems capable of designing, executing, and analyzing experiments with minimal human intervention. Companies pursuing AI-driven discovery approaches report 30-40% reductions in early-stage research timelines when utilizing fully automated laboratory workflows.
Market segmentation analysis indicates that mid-sized biotechnology companies (annual revenue $100M-$1B) represent the fastest-growing customer segment, with adoption rates increasing 12% annually. These organizations typically lack the extensive legacy infrastructure of larger pharmaceutical companies while possessing sufficient capital to invest in transformative automation technologies.
Customer surveys indicate that return on investment expectations have evolved, with organizations now prioritizing flexibility and scalability over immediate cost savings. This shift reflects a maturing market understanding that the primary value of autonomous lab integration lies in accelerating research timelines and improving experimental reproducibility rather than simple labor cost reduction.
Demand analysis reveals several key market drivers accelerating the adoption of autonomous lab integration. Labor costs in pharmaceutical research continue to rise, with specialized laboratory technicians commanding premium salaries, while simultaneously facing shortages in many markets. This economic pressure has created strong incentives for automation solutions that can reduce dependence on manual operations while maintaining or improving output quality.
Quality control requirements represent another significant market pull factor. Regulatory bodies worldwide have progressively tightened compliance standards for pharmaceutical manufacturing and research, necessitating more consistent, traceable, and validated processes. Autonomous lab systems offer superior documentation, reduced human error rates, and enhanced reproducibility—all critical factors in regulatory compliance.
The COVID-19 pandemic has served as a market catalyst, dramatically highlighting the vulnerabilities of traditional laboratory workflows dependent on human presence. Organizations that had invested in automation demonstrated greater resilience during lockdown periods, maintaining critical research continuity while minimizing personnel density. This real-world demonstration has accelerated adoption timelines across the industry.
Data-driven drug discovery represents a particularly high-growth segment within the market. The integration of artificial intelligence with laboratory automation has created demand for systems capable of designing, executing, and analyzing experiments with minimal human intervention. Companies pursuing AI-driven discovery approaches report 30-40% reductions in early-stage research timelines when utilizing fully automated laboratory workflows.
Market segmentation analysis indicates that mid-sized biotechnology companies (annual revenue $100M-$1B) represent the fastest-growing customer segment, with adoption rates increasing 12% annually. These organizations typically lack the extensive legacy infrastructure of larger pharmaceutical companies while possessing sufficient capital to invest in transformative automation technologies.
Customer surveys indicate that return on investment expectations have evolved, with organizations now prioritizing flexibility and scalability over immediate cost savings. This shift reflects a maturing market understanding that the primary value of autonomous lab integration lies in accelerating research timelines and improving experimental reproducibility rather than simple labor cost reduction.
Current Autonomous Lab Technologies and Barriers
Autonomous laboratories have emerged as a transformative technology in pharmaceutical and biotech industries, with current implementations ranging from basic automated systems to fully integrated AI-driven platforms. Leading technologies include robotic liquid handling systems that can perform precise sample preparation and dispensing with minimal human intervention. These systems typically feature multi-axis robotic arms capable of manipulating labware across various instruments, achieving throughput rates up to 1,000 samples per day with precision levels of ±0.1mm positioning accuracy.
Cloud-based laboratory information management systems (LIMS) represent another critical component, enabling remote experiment design, monitoring, and data analysis. Advanced LIMS platforms now incorporate machine learning algorithms that can analyze experimental results in real-time, suggesting modifications to protocols based on emerging data patterns, with some systems demonstrating up to 30% improvement in experimental success rates compared to traditional approaches.
Computer vision systems integrated with high-throughput screening platforms can process and analyze thousands of microscopy images per hour, identifying cellular phenotypes and molecular interactions that might be missed by human observers. These systems have achieved classification accuracies exceeding 95% for certain standardized assays, significantly outperforming manual analysis methods.
Despite these advancements, significant barriers impede widespread adoption of autonomous lab technologies. Technical challenges include integration difficulties between equipment from different manufacturers due to proprietary interfaces and communication protocols. The lack of standardization across the industry creates "islands of automation" rather than truly integrated systems, with an estimated 40-60% of development time spent on integration rather than core functionality.
Data management presents another substantial hurdle, as autonomous labs generate massive datasets requiring sophisticated storage, processing, and analysis infrastructure. Current systems struggle with data heterogeneity across different experimental modalities, with interoperability issues between analytical platforms limiting the potential for comprehensive data mining and knowledge discovery.
Regulatory compliance represents a significant barrier, particularly for pharmaceutical applications where validation requirements for automated systems remain complex and time-consuming. Documentation requirements for GMP-compliant autonomous systems can extend development timelines by 12-18 months compared to non-regulated implementations.
Cost barriers also remain substantial, with fully integrated autonomous lab systems requiring capital investments of $2-10 million depending on scale and capabilities. This creates significant adoption challenges for smaller organizations and academic institutions, limiting innovation potential across the broader research ecosystem.
Cloud-based laboratory information management systems (LIMS) represent another critical component, enabling remote experiment design, monitoring, and data analysis. Advanced LIMS platforms now incorporate machine learning algorithms that can analyze experimental results in real-time, suggesting modifications to protocols based on emerging data patterns, with some systems demonstrating up to 30% improvement in experimental success rates compared to traditional approaches.
Computer vision systems integrated with high-throughput screening platforms can process and analyze thousands of microscopy images per hour, identifying cellular phenotypes and molecular interactions that might be missed by human observers. These systems have achieved classification accuracies exceeding 95% for certain standardized assays, significantly outperforming manual analysis methods.
Despite these advancements, significant barriers impede widespread adoption of autonomous lab technologies. Technical challenges include integration difficulties between equipment from different manufacturers due to proprietary interfaces and communication protocols. The lack of standardization across the industry creates "islands of automation" rather than truly integrated systems, with an estimated 40-60% of development time spent on integration rather than core functionality.
Data management presents another substantial hurdle, as autonomous labs generate massive datasets requiring sophisticated storage, processing, and analysis infrastructure. Current systems struggle with data heterogeneity across different experimental modalities, with interoperability issues between analytical platforms limiting the potential for comprehensive data mining and knowledge discovery.
Regulatory compliance represents a significant barrier, particularly for pharmaceutical applications where validation requirements for automated systems remain complex and time-consuming. Documentation requirements for GMP-compliant autonomous systems can extend development timelines by 12-18 months compared to non-regulated implementations.
Cost barriers also remain substantial, with fully integrated autonomous lab systems requiring capital investments of $2-10 million depending on scale and capabilities. This creates significant adoption challenges for smaller organizations and academic institutions, limiting innovation potential across the broader research ecosystem.
Current Implementation Solutions for Autonomous Labs
01 Automated laboratory systems for scientific research
Autonomous laboratory systems that integrate robotics, AI, and automation technologies to conduct scientific experiments with minimal human intervention. These systems can perform complex research tasks, data collection, and analysis in various scientific fields, improving efficiency and reproducibility of experiments while reducing human error.- Automated laboratory systems for scientific research: Autonomous laboratory systems that automate scientific research processes, including experiment design, execution, and analysis. These systems integrate robotics, AI, and machine learning to perform experiments with minimal human intervention, increasing efficiency and reproducibility in scientific discovery. They can handle complex workflows, sample preparation, and data collection while adapting to experimental outcomes.
- Robotic systems for laboratory automation: Robotic systems designed specifically for laboratory environments that can manipulate laboratory equipment, handle samples, and perform precise movements required for scientific experiments. These systems include robotic arms, automated liquid handling systems, and mobile robots that can navigate laboratory spaces to transport materials between workstations, enabling continuous operation with minimal human supervision.
- AI and machine learning for experiment optimization: Integration of artificial intelligence and machine learning algorithms in laboratory systems to optimize experimental parameters, predict outcomes, and make autonomous decisions about subsequent experimental steps. These technologies enable systems to learn from previous experiments, adapt protocols based on results, and discover new scientific insights more efficiently than traditional methods.
- Autonomous quality control and monitoring systems: Systems that continuously monitor laboratory conditions, equipment performance, and experimental progress to ensure quality and reliability of results. These systems can detect anomalies, calibrate instruments automatically, and maintain optimal environmental conditions for experiments. They provide real-time feedback and can adjust parameters to maintain experimental integrity without human intervention.
- Cloud-connected laboratory infrastructure: Laboratory systems that leverage cloud computing and IoT technologies to enable remote operation, data sharing, and collaborative research across distributed teams. These systems allow researchers to design experiments, monitor progress, and analyze results from anywhere, while also facilitating integration with external databases and computational resources for enhanced data analysis and knowledge discovery.
02 Self-driving and autonomous vehicle testing laboratories
Specialized laboratory environments designed for testing and validating autonomous vehicle technologies. These facilities include simulation capabilities, sensor testing equipment, and controlled testing environments that allow for the development and verification of self-driving systems under various conditions before deployment on public roads.Expand Specific Solutions03 AI-powered laboratory automation and management systems
Intelligent systems that manage laboratory operations through artificial intelligence, machine learning, and data analytics. These solutions optimize workflow, resource allocation, and experimental design while providing predictive maintenance for lab equipment and real-time monitoring of experiments.Expand Specific Solutions04 Remote and cloud-connected laboratory infrastructure
Laboratory systems that enable remote operation and monitoring through cloud connectivity and IoT technologies. These solutions allow researchers to conduct experiments, collect data, and collaborate from different locations, providing flexibility in research operations and facilitating global scientific collaboration.Expand Specific Solutions05 Modular and reconfigurable autonomous lab equipment
Flexible laboratory equipment and instruments designed with modular components that can be reconfigured for different experimental protocols. These systems feature interchangeable parts, standardized interfaces, and programmable functions that allow laboratories to adapt to changing research needs without requiring complete equipment replacement.Expand Specific Solutions
Key Industry Players and Competitive Landscape
The autonomous lab integration market in pharmaceutical and biotech industries is currently in a growth phase, with increasing adoption driven by efficiency demands and technological advancements. The market size is expanding rapidly, projected to reach significant value as major players invest in automation solutions. Technologically, the field shows varying maturity levels, with established companies like Roche, Amgen, and Regeneron leading commercial implementation, while Beckman Coulter and Tecan provide specialized instrumentation platforms. Academic institutions including Tsinghua University and USC are advancing research foundations, while emerging players like Andrew Alliance and KyooBe Tech focus on innovative integration solutions. The ecosystem demonstrates a collaborative dynamic between pharmaceutical giants, equipment manufacturers, and research institutions, collectively driving the evolution of autonomous laboratory systems.
Amgen, Inc.
Technical Solution: Amgen has developed an in-house autonomous laboratory platform called "Digital Biologics Factory" that integrates robotics, AI, and advanced analytics to accelerate drug discovery and development. Their system employs a distributed architecture where multiple autonomous workstations communicate through a centralized orchestration layer, enabling parallel processing of complex biological experiments. Amgen's platform incorporates advanced predictive modeling that suggests optimal experimental conditions based on historical data and molecular characteristics. Their technology features adaptive learning algorithms that continuously refine experimental protocols based on outcomes, creating a self-improving research ecosystem. Amgen has implemented digital twin technology that creates virtual representations of physical experiments, allowing researchers to simulate outcomes before committing resources. Their autonomous lab infrastructure includes sophisticated environmental monitoring systems that maintain precise conditions required for biological research while automatically documenting compliance with regulatory requirements.
Strengths: Deep integration with biological research workflows; advanced predictive modeling capabilities; proven success in accelerating antibody discovery timelines. Weaknesses: Primarily developed for internal use with limited commercial availability; requires significant biological domain expertise to fully leverage capabilities.
Tecan Trading AG
Technical Solution: Tecan has pioneered the Fluent Automation Workstation, a highly flexible autonomous lab platform specifically designed for pharmaceutical applications. Their system utilizes a combination of robotic arms, precision liquid handling modules, and integrated analytical instruments to create a seamless workflow. Tecan's technology incorporates adaptive scheduling algorithms that dynamically adjust experimental priorities based on real-time results and resource availability. Their platform features a modular design that allows pharmaceutical companies to customize configurations based on specific research needs while maintaining standardization across sites. Tecan has implemented advanced computer vision systems for real-time monitoring of experimental progress and quality control. Their autonomous lab solutions include sophisticated data management capabilities that integrate with cloud-based analytics platforms, enabling remote monitoring and collaborative research across distributed teams.
Strengths: Exceptional precision in liquid handling operations; highly modular and adaptable to different research applications; strong track record in pharmaceutical industry implementations. Weaknesses: Integration with third-party instruments can sometimes require custom development; higher maintenance requirements compared to some competitors.
Core Technologies and Patents in Lab Automation
Automated biomass-based perfusion control in the manufacturing of biologics
PatentWO2020252442A9
Innovation
- An automated biomass-based perfusion control system for continuous manufacturing processes that adjusts cell concentration and perfusion rates in real-time using permittivity probes and Raman spectroscopy to maintain optimal biomass levels, reducing lactate production and improving product quality and quantity.
An autonomous sampling system
PatentInactiveEP3516364A2
Innovation
- An autonomous sampling system with a base for autonomous locomotion, equipped with a robotic arm, disinfecting assemblies, video monitoring, and secure data transmission, allowing for autonomous sampling and monitoring without human intervention, including wheel disinfection to maintain sterility and a carousel for multiple sample reservoirs for efficient sampling.
Regulatory Compliance and Validation Requirements
The integration of autonomous laboratories into pharmaceutical and biotech industries faces a complex regulatory landscape that varies significantly across global markets. In the United States, the FDA's regulatory framework requires autonomous lab systems to comply with 21 CFR Part 11 for electronic records and signatures, ensuring data integrity and system validation. Additionally, Good Laboratory Practices (GLP) and Good Manufacturing Practices (GMP) standards must be rigorously applied to autonomous systems, with particular emphasis on traceability and reproducibility of experimental results.
European regulatory bodies, including the European Medicines Agency (EMA), impose their own set of compliance requirements through EU GMP Annex 11 for computerized systems. These regulations demand comprehensive validation documentation and risk assessment protocols specifically tailored for autonomous laboratory environments. The challenge for implementation teams lies in developing validation strategies that address both hardware components and sophisticated AI/ML algorithms that may evolve over time.
System validation for autonomous labs requires a multi-layered approach encompassing Installation Qualification (IQ), Operational Qualification (OQ), and Performance Qualification (PQ). The validation process must verify that autonomous systems consistently perform as intended across various experimental conditions while maintaining data integrity. This presents unique challenges when validating self-learning systems whose decision-making processes may change through machine learning iterations.
Data integrity requirements present particular challenges for autonomous labs, as regulatory bodies increasingly scrutinize automated data collection and analysis processes. Companies must implement robust audit trail mechanisms that document all system activities, including automated decision points and algorithm-driven experimental adjustments. The concept of "data provenance" has gained prominence, requiring systems to maintain complete records of how each data point was generated, processed, and utilized.
Change management protocols represent another critical regulatory consideration. As autonomous lab systems evolve through software updates and algorithm refinements, organizations must establish formal change control procedures that assess the impact of modifications on system validation status. This includes regression testing protocols to ensure that system enhancements do not compromise previously validated functionalities.
Regulatory agencies are gradually developing more specific guidance for autonomous laboratory systems, recognizing their transformative potential in drug discovery and development. Industry consortia and standards organizations are simultaneously working to establish consensus-based validation approaches that balance innovation with compliance requirements. Companies at the forefront of autonomous lab implementation are advised to maintain proactive engagement with regulatory authorities through early consultation and participation in emerging guidance development.
European regulatory bodies, including the European Medicines Agency (EMA), impose their own set of compliance requirements through EU GMP Annex 11 for computerized systems. These regulations demand comprehensive validation documentation and risk assessment protocols specifically tailored for autonomous laboratory environments. The challenge for implementation teams lies in developing validation strategies that address both hardware components and sophisticated AI/ML algorithms that may evolve over time.
System validation for autonomous labs requires a multi-layered approach encompassing Installation Qualification (IQ), Operational Qualification (OQ), and Performance Qualification (PQ). The validation process must verify that autonomous systems consistently perform as intended across various experimental conditions while maintaining data integrity. This presents unique challenges when validating self-learning systems whose decision-making processes may change through machine learning iterations.
Data integrity requirements present particular challenges for autonomous labs, as regulatory bodies increasingly scrutinize automated data collection and analysis processes. Companies must implement robust audit trail mechanisms that document all system activities, including automated decision points and algorithm-driven experimental adjustments. The concept of "data provenance" has gained prominence, requiring systems to maintain complete records of how each data point was generated, processed, and utilized.
Change management protocols represent another critical regulatory consideration. As autonomous lab systems evolve through software updates and algorithm refinements, organizations must establish formal change control procedures that assess the impact of modifications on system validation status. This includes regression testing protocols to ensure that system enhancements do not compromise previously validated functionalities.
Regulatory agencies are gradually developing more specific guidance for autonomous laboratory systems, recognizing their transformative potential in drug discovery and development. Industry consortia and standards organizations are simultaneously working to establish consensus-based validation approaches that balance innovation with compliance requirements. Companies at the forefront of autonomous lab implementation are advised to maintain proactive engagement with regulatory authorities through early consultation and participation in emerging guidance development.
ROI Analysis and Implementation Strategies
The implementation of autonomous laboratory systems in pharmaceutical and biotech industries requires substantial initial investment, yet offers compelling long-term returns. Financial analysis indicates that most organizations achieve full ROI within 24-36 months, with cost recovery accelerating after the initial integration period. Key financial benefits include a 30-45% reduction in operational costs through decreased reagent usage, minimized human error, and optimized resource allocation.
Labor cost savings represent a significant component of ROI calculations, with autonomous systems reducing routine laboratory tasks by up to 70%. This allows redeployment of skilled scientists to higher-value activities such as experimental design and data interpretation, effectively increasing the intellectual output per employee. Additionally, throughput improvements of 200-300% compared to traditional methods contribute substantially to accelerated product development timelines.
Implementation strategies must follow a phased approach to maximize ROI while minimizing operational disruption. The recommended pathway begins with a focused pilot program targeting high-volume, standardized processes where automation can demonstrate immediate value. This initial phase should include comprehensive data collection to establish baseline metrics for subsequent ROI validation.
Following successful pilot implementation, organizations should adopt a modular expansion strategy, gradually integrating autonomous systems across laboratory functions. This approach allows for calibration of investment timing based on demonstrated returns from earlier phases. Critical to success is the establishment of cross-functional implementation teams comprising laboratory scientists, IT specialists, and financial analysts to ensure technical compatibility and accurate ROI tracking.
Training programs represent a crucial yet often underestimated component of implementation strategies. Organizations achieving the highest ROI typically allocate 8-12% of total project budget to comprehensive staff training, focusing on both technical operation and data interpretation skills. This investment significantly reduces the productivity dip commonly observed during technology transitions.
Vendor selection criteria should prioritize systems offering open architecture and integration capabilities with existing laboratory infrastructure. While closed proprietary systems may offer superior performance in isolated applications, they typically deliver lower overall ROI due to limited scalability and higher long-term maintenance costs. Implementation contracts should include performance guarantees tied to specific ROI metrics, with vendor compensation partially contingent on achieving agreed financial outcomes.
Labor cost savings represent a significant component of ROI calculations, with autonomous systems reducing routine laboratory tasks by up to 70%. This allows redeployment of skilled scientists to higher-value activities such as experimental design and data interpretation, effectively increasing the intellectual output per employee. Additionally, throughput improvements of 200-300% compared to traditional methods contribute substantially to accelerated product development timelines.
Implementation strategies must follow a phased approach to maximize ROI while minimizing operational disruption. The recommended pathway begins with a focused pilot program targeting high-volume, standardized processes where automation can demonstrate immediate value. This initial phase should include comprehensive data collection to establish baseline metrics for subsequent ROI validation.
Following successful pilot implementation, organizations should adopt a modular expansion strategy, gradually integrating autonomous systems across laboratory functions. This approach allows for calibration of investment timing based on demonstrated returns from earlier phases. Critical to success is the establishment of cross-functional implementation teams comprising laboratory scientists, IT specialists, and financial analysts to ensure technical compatibility and accurate ROI tracking.
Training programs represent a crucial yet often underestimated component of implementation strategies. Organizations achieving the highest ROI typically allocate 8-12% of total project budget to comprehensive staff training, focusing on both technical operation and data interpretation skills. This investment significantly reduces the productivity dip commonly observed during technology transitions.
Vendor selection criteria should prioritize systems offering open architecture and integration capabilities with existing laboratory infrastructure. While closed proprietary systems may offer superior performance in isolated applications, they typically deliver lower overall ROI due to limited scalability and higher long-term maintenance costs. Implementation contracts should include performance guarantees tied to specific ROI metrics, with vendor compensation partially contingent on achieving agreed financial outcomes.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!