What Factors Affect the Adoption of Autonomous Lab in Clinical Trials
SEP 25, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Autonomous Lab Evolution and Objectives
The evolution of autonomous laboratories represents a significant paradigm shift in clinical research methodologies. Originating from basic laboratory automation in the 1980s, autonomous labs have evolved through several distinct phases, from simple robotic sample handling to today's AI-driven fully integrated systems. This technological progression has been accelerated by advances in machine learning, robotics, and cloud computing, creating platforms capable of designing, executing, and analyzing experiments with minimal human intervention.
The autonomous lab concept emerged from the convergence of high-throughput screening technologies and artificial intelligence, initially gaining traction in pharmaceutical discovery before expanding into clinical trial applications. Key evolutionary milestones include the development of standardized laboratory automation protocols in the early 2000s, the integration of machine learning for experimental design around 2010, and the recent emergence of cloud-connected autonomous systems capable of multi-site coordination.
Current technological objectives for autonomous labs in clinical trials focus on several critical areas. Primary among these is achieving end-to-end automation of complex clinical sample processing workflows while maintaining regulatory compliance. There is also significant emphasis on developing adaptive experimental systems that can modify protocols in real-time based on incoming data, potentially revolutionizing the traditional fixed-protocol approach to clinical trials.
Another crucial objective is the seamless integration of autonomous lab systems with electronic health records and clinical data management systems, creating unified data ecosystems that enhance trial efficiency and data integrity. Reducing the time from sample collection to actionable results represents a key performance metric, with current targets aiming for 60-80% reductions compared to traditional laboratory processes.
The industry is also pursuing standardization objectives, working toward common interfaces and protocols that would allow interoperability between different autonomous lab platforms and components. This standardization is essential for widespread adoption across diverse clinical trial environments and therapeutic areas.
Looking forward, the technological roadmap for autonomous labs in clinical trials includes developing more sophisticated AI capabilities for protocol optimization, enhancing remote monitoring and operation capabilities, and creating systems capable of handling increasingly complex biological assays with minimal human oversight. The ultimate objective is to establish autonomous labs as the standard methodology for clinical trial sample processing, enabling more adaptive, efficient, and data-rich clinical research paradigms.
The autonomous lab concept emerged from the convergence of high-throughput screening technologies and artificial intelligence, initially gaining traction in pharmaceutical discovery before expanding into clinical trial applications. Key evolutionary milestones include the development of standardized laboratory automation protocols in the early 2000s, the integration of machine learning for experimental design around 2010, and the recent emergence of cloud-connected autonomous systems capable of multi-site coordination.
Current technological objectives for autonomous labs in clinical trials focus on several critical areas. Primary among these is achieving end-to-end automation of complex clinical sample processing workflows while maintaining regulatory compliance. There is also significant emphasis on developing adaptive experimental systems that can modify protocols in real-time based on incoming data, potentially revolutionizing the traditional fixed-protocol approach to clinical trials.
Another crucial objective is the seamless integration of autonomous lab systems with electronic health records and clinical data management systems, creating unified data ecosystems that enhance trial efficiency and data integrity. Reducing the time from sample collection to actionable results represents a key performance metric, with current targets aiming for 60-80% reductions compared to traditional laboratory processes.
The industry is also pursuing standardization objectives, working toward common interfaces and protocols that would allow interoperability between different autonomous lab platforms and components. This standardization is essential for widespread adoption across diverse clinical trial environments and therapeutic areas.
Looking forward, the technological roadmap for autonomous labs in clinical trials includes developing more sophisticated AI capabilities for protocol optimization, enhancing remote monitoring and operation capabilities, and creating systems capable of handling increasingly complex biological assays with minimal human oversight. The ultimate objective is to establish autonomous labs as the standard methodology for clinical trial sample processing, enabling more adaptive, efficient, and data-rich clinical research paradigms.
Clinical Trial Market Demand Analysis
The clinical trial market is experiencing significant growth, with the global market valued at approximately $44.3 billion in 2020 and projected to reach $69.8 billion by 2028, representing a compound annual growth rate (CAGR) of 5.7%. This expansion is driven by increasing R&D investments from pharmaceutical and biotechnology companies seeking to develop novel therapeutics and medical devices. Within this growing market, there is a substantial demand for technologies that can enhance efficiency, reduce costs, and improve data quality.
Autonomous laboratories represent a transformative approach to clinical trials, offering potential solutions to longstanding challenges in the industry. Market research indicates that approximately 80% of clinical trials fail to meet enrollment timelines, and nearly 30% of phase III clinical trials fail due to issues with data collection and management. These inefficiencies create a compelling market need for autonomous lab technologies that can streamline processes and minimize human error.
The COVID-19 pandemic has accelerated the adoption of decentralized and virtual clinical trial models, with 76% of research organizations implementing some form of remote monitoring during the pandemic. This shift has created an environment more receptive to autonomous lab technologies that can support remote sample processing, analysis, and data management. Industry surveys reveal that 64% of clinical trial sponsors are actively exploring automation technologies to enhance trial operations.
Regulatory bodies are increasingly supportive of innovative approaches in clinical trials. The FDA's Clinical Trial Transformation Initiative and the EMA's Regulatory Science Strategy both emphasize the importance of modernizing clinical trials through technology adoption. This regulatory environment creates favorable conditions for autonomous lab implementation, particularly as these technologies demonstrate their ability to maintain or improve data integrity and patient safety.
Cost considerations remain a significant market driver, with the average cost of bringing a drug to market exceeding $2.6 billion. Autonomous labs offer potential cost reductions through decreased labor requirements, minimized protocol deviations, and accelerated timelines. Market analysis suggests that implementation of autonomous technologies could reduce clinical trial costs by up to 15-20% while simultaneously improving data quality and reproducibility.
Patient-centricity has emerged as a key focus in clinical trial design, with increasing emphasis on reducing participant burden and improving the trial experience. Autonomous labs can support this trend by enabling more flexible sample collection schedules, reducing the need for site visits, and providing faster results. This alignment with patient-centric approaches represents a significant market opportunity for autonomous lab technologies.
Autonomous laboratories represent a transformative approach to clinical trials, offering potential solutions to longstanding challenges in the industry. Market research indicates that approximately 80% of clinical trials fail to meet enrollment timelines, and nearly 30% of phase III clinical trials fail due to issues with data collection and management. These inefficiencies create a compelling market need for autonomous lab technologies that can streamline processes and minimize human error.
The COVID-19 pandemic has accelerated the adoption of decentralized and virtual clinical trial models, with 76% of research organizations implementing some form of remote monitoring during the pandemic. This shift has created an environment more receptive to autonomous lab technologies that can support remote sample processing, analysis, and data management. Industry surveys reveal that 64% of clinical trial sponsors are actively exploring automation technologies to enhance trial operations.
Regulatory bodies are increasingly supportive of innovative approaches in clinical trials. The FDA's Clinical Trial Transformation Initiative and the EMA's Regulatory Science Strategy both emphasize the importance of modernizing clinical trials through technology adoption. This regulatory environment creates favorable conditions for autonomous lab implementation, particularly as these technologies demonstrate their ability to maintain or improve data integrity and patient safety.
Cost considerations remain a significant market driver, with the average cost of bringing a drug to market exceeding $2.6 billion. Autonomous labs offer potential cost reductions through decreased labor requirements, minimized protocol deviations, and accelerated timelines. Market analysis suggests that implementation of autonomous technologies could reduce clinical trial costs by up to 15-20% while simultaneously improving data quality and reproducibility.
Patient-centricity has emerged as a key focus in clinical trial design, with increasing emphasis on reducing participant burden and improving the trial experience. Autonomous labs can support this trend by enabling more flexible sample collection schedules, reducing the need for site visits, and providing faster results. This alignment with patient-centric approaches represents a significant market opportunity for autonomous lab technologies.
Current Autonomous Lab Technologies and Barriers
The autonomous laboratory landscape in clinical trials is currently dominated by several key technologies. Robotic automation systems form the backbone of these labs, with articulated robotic arms and specialized instruments performing precise sample handling, preparation, and analysis tasks. These systems can operate continuously without human intervention, significantly reducing manual labor and human error while increasing throughput.
Advanced analytical instruments with high-throughput capabilities represent another critical component. Mass spectrometers, next-generation sequencing platforms, and high-content imaging systems can process large sample volumes rapidly while maintaining precision. These instruments often feature auto-calibration and self-diagnostic capabilities to ensure data reliability.
Laboratory information management systems (LIMS) and sophisticated software platforms coordinate operations across the autonomous lab ecosystem. These systems integrate instrument control, data acquisition, analysis pipelines, and quality control processes. Machine learning algorithms increasingly enhance these platforms by optimizing experimental workflows and predicting maintenance needs.
Despite these technological advances, significant barriers impede widespread adoption of autonomous labs in clinical trials. High initial capital investment represents a primary obstacle, with comprehensive autonomous lab setups potentially costing millions of dollars. This creates a substantial financial barrier, particularly for smaller research organizations and contract research organizations (CROs).
Regulatory compliance presents another major challenge. Autonomous systems must meet stringent validation requirements for clinical trial applications, including 21 CFR Part 11 compliance for electronic records. The regulatory framework for fully autonomous testing in clinical settings remains underdeveloped in many jurisdictions, creating uncertainty for implementers.
Technical integration difficulties also hinder adoption. Many existing laboratory facilities face challenges incorporating autonomous systems into established workflows and infrastructure. Legacy systems often lack standardized interfaces for seamless integration, requiring custom solutions that increase implementation complexity and cost.
Workforce concerns constitute another barrier, with laboratory professionals expressing apprehension about job displacement. Organizations must manage this transition carefully, focusing on reskilling staff for oversight and specialized roles within the autonomous lab environment. Additionally, a shortage of personnel with expertise in both clinical research and autonomous systems operation creates staffing challenges.
Data security and privacy considerations add further complexity, particularly for clinical trial data subject to regulations like HIPAA and GDPR. Autonomous systems that generate, process, and store sensitive patient information require robust security protocols and compliance frameworks that can be difficult to implement comprehensively.
Advanced analytical instruments with high-throughput capabilities represent another critical component. Mass spectrometers, next-generation sequencing platforms, and high-content imaging systems can process large sample volumes rapidly while maintaining precision. These instruments often feature auto-calibration and self-diagnostic capabilities to ensure data reliability.
Laboratory information management systems (LIMS) and sophisticated software platforms coordinate operations across the autonomous lab ecosystem. These systems integrate instrument control, data acquisition, analysis pipelines, and quality control processes. Machine learning algorithms increasingly enhance these platforms by optimizing experimental workflows and predicting maintenance needs.
Despite these technological advances, significant barriers impede widespread adoption of autonomous labs in clinical trials. High initial capital investment represents a primary obstacle, with comprehensive autonomous lab setups potentially costing millions of dollars. This creates a substantial financial barrier, particularly for smaller research organizations and contract research organizations (CROs).
Regulatory compliance presents another major challenge. Autonomous systems must meet stringent validation requirements for clinical trial applications, including 21 CFR Part 11 compliance for electronic records. The regulatory framework for fully autonomous testing in clinical settings remains underdeveloped in many jurisdictions, creating uncertainty for implementers.
Technical integration difficulties also hinder adoption. Many existing laboratory facilities face challenges incorporating autonomous systems into established workflows and infrastructure. Legacy systems often lack standardized interfaces for seamless integration, requiring custom solutions that increase implementation complexity and cost.
Workforce concerns constitute another barrier, with laboratory professionals expressing apprehension about job displacement. Organizations must manage this transition carefully, focusing on reskilling staff for oversight and specialized roles within the autonomous lab environment. Additionally, a shortage of personnel with expertise in both clinical research and autonomous systems operation creates staffing challenges.
Data security and privacy considerations add further complexity, particularly for clinical trial data subject to regulations like HIPAA and GDPR. Autonomous systems that generate, process, and store sensitive patient information require robust security protocols and compliance frameworks that can be difficult to implement comprehensively.
Existing Autonomous Solutions in Clinical Trials
01 Automated laboratory systems and workflows
Autonomous laboratory systems integrate robotics, AI, and automation to streamline research workflows. These systems can perform experiments with minimal human intervention, handling sample preparation, testing, and analysis. The automation includes intelligent scheduling of laboratory tasks, resource management, and coordination of multiple instruments to maximize efficiency and reproducibility in scientific research.- Automated laboratory systems and workflows: Autonomous laboratory systems incorporate robotics and automation to streamline research workflows. These systems can perform experiments with minimal human intervention, handling sample preparation, testing, and analysis. The integration of automated equipment improves efficiency, reduces human error, and enables continuous operation. These technologies allow for standardized protocols and higher throughput in research environments.
- AI and machine learning for laboratory decision-making: Artificial intelligence and machine learning algorithms enhance autonomous lab operations by optimizing experimental design and interpreting results. These systems can analyze complex datasets, identify patterns, and make real-time adjustments to experimental parameters. Machine learning models improve over time, learning from previous experiments to suggest more efficient protocols and predict outcomes, ultimately accelerating scientific discovery and innovation.
- Remote monitoring and control systems: Remote laboratory management systems enable researchers to monitor and control experiments from anywhere. These platforms provide real-time data access, visualization tools, and remote instrument control capabilities. Secure cloud-based interfaces allow multiple users to collaborate on experiments, review results, and make adjustments without being physically present in the laboratory, supporting distributed research teams and improving work flexibility.
- Laboratory information management systems (LIMS): Specialized software platforms manage laboratory data, workflows, and resources in autonomous lab environments. These systems track samples, equipment usage, and experimental results while ensuring regulatory compliance and data integrity. Advanced LIMS integrate with laboratory instruments, automate data collection, and provide analytical tools for result interpretation, creating a comprehensive digital ecosystem for modern research facilities.
- IoT and sensor networks for laboratory environments: Internet of Things (IoT) devices and sensor networks create connected laboratory environments that continuously monitor experimental conditions and equipment status. These systems collect data on temperature, humidity, pressure, and other critical parameters to ensure experimental consistency. Automated alerts notify researchers of deviations or equipment failures, while integrated analytics provide insights for process optimization and preventive maintenance.
02 AI and machine learning for laboratory decision making
Artificial intelligence and machine learning algorithms are being implemented in laboratory environments to enhance decision-making processes. These technologies enable autonomous experiment design, predictive maintenance of equipment, and adaptive optimization of research protocols. The systems can analyze experimental results in real-time, make adjustments to parameters, and suggest follow-up experiments, significantly accelerating the research and development cycle.Expand Specific Solutions03 Remote laboratory monitoring and control systems
Remote monitoring and control technologies allow researchers to operate laboratory equipment from anywhere. These systems provide real-time data access, remote instrument operation, and virtual collaboration capabilities. They incorporate secure communication protocols, video monitoring, and digital interfaces that enable scientists to conduct experiments, analyze results, and make adjustments without being physically present in the laboratory.Expand Specific Solutions04 Laboratory data management and integration platforms
Comprehensive data management systems are essential for autonomous laboratory adoption. These platforms integrate data from various instruments and experiments, providing unified storage, analysis, and visualization capabilities. They incorporate electronic laboratory notebooks, automated data validation, and standardized formats to ensure data integrity and facilitate knowledge sharing across research teams.Expand Specific Solutions05 Quality control and validation in autonomous laboratories
Quality control mechanisms are critical for ensuring reliability in autonomous laboratory operations. These systems include automated calibration routines, error detection algorithms, and validation protocols that verify experimental results. They implement continuous monitoring of system performance, automated documentation of procedures, and compliance with regulatory standards to maintain scientific rigor in automated research environments.Expand Specific Solutions
Key Industry Players and Competitive Landscape
The autonomous lab adoption in clinical trials is currently in an early growth phase, with the market expected to expand significantly due to increasing demand for efficiency and reproducibility. Key players like F. Hoffmann-La Roche, Abbott Laboratories, and IBM are driving technological innovation through advanced automation platforms. Companies including Beckman Coulter and Eppendorf are developing specialized instrumentation, while academic institutions such as MIT and Duke University contribute fundamental research. The technology's maturity varies across applications, with basic automation well-established but AI-driven fully autonomous systems still emerging. Integration challenges and regulatory considerations remain significant barriers to widespread adoption, though collaborative efforts between pharmaceutical companies and technology providers are accelerating development.
F. Hoffmann-La Roche Ltd.
Technical Solution: Roche has developed an integrated autonomous lab solution for clinical trials called "NAVIFY Clinical Trial Management" that combines digital technologies, automation, and data analytics. Their approach focuses on decentralized clinical trials (DCTs) where remote patient monitoring and automated sample collection are key components. The system incorporates AI-driven predictive analytics to optimize trial protocols and patient recruitment, while their cobas® laboratory instruments provide automated testing with minimal human intervention. Roche has implemented a cloud-based infrastructure that enables real-time data sharing between trial sites, central laboratories, and investigators, significantly reducing manual data entry and transcription errors. Their autonomous lab solutions have demonstrated up to 30% reduction in trial duration and approximately 25% cost savings in large-scale clinical studies through improved operational efficiency and reduced protocol deviations.
Strengths: Extensive global laboratory network and established regulatory compliance expertise; comprehensive end-to-end solution integrating both hardware and software components. Weaknesses: High implementation costs may limit adoption by smaller research organizations; system requires significant training and organizational change management.
Siemens Healthcare Diagnostics, Inc.
Technical Solution: Siemens has developed the Aptio Automation system specifically adapted for clinical trial applications, featuring a modular design that allows for customization based on specific trial requirements. Their autonomous lab solution incorporates track-based transportation systems that connect multiple analytical instruments, centrifuges, and storage modules to create a fully automated workflow. Siemens' CentraLink Data Management System serves as the central control platform, orchestrating sample movement and testing while capturing comprehensive audit trails for regulatory compliance. Their technology implements sophisticated sample prioritization algorithms that can adapt to changing trial protocols and urgent testing needs. Siemens has also developed remote monitoring capabilities that allow trial sponsors to observe testing processes in real-time without being physically present at the laboratory. Their system includes automated quality control verification and calibration procedures that ensure consistent analytical performance across multiple trial sites, addressing one of the key challenges in multi-center clinical studies.
Strengths: Highly flexible modular design allows for customization to specific trial needs; excellent track record in laboratory automation reliability. Weaknesses: Integration with non-Siemens instruments can be challenging; significant physical space requirements for full implementation.
Critical Technologies Enabling Autonomous Labs
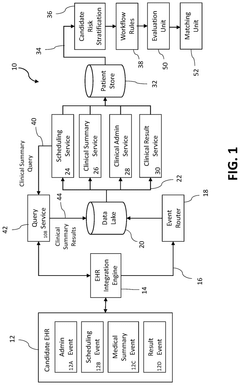
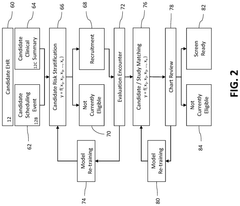
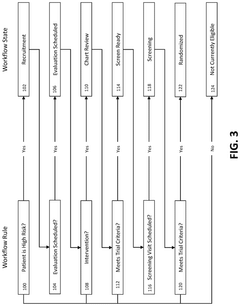
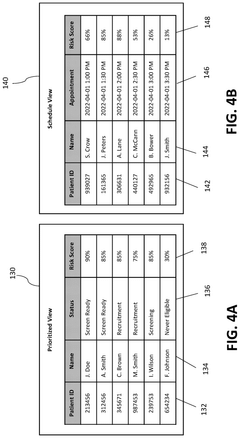
System and method for identifying candidates for clinical trials
PatentPendingUS20250125022A1
Innovation
- A computerized system and method that risk stratifies and prioritizes potential clinical trial participants using real-time electronic health record data, applying predictive risk models and machine learning techniques to match candidates to relevant clinical trials based on their similarity to enrolled participants.
Automated testing with improved scalability and compatibility
PatentWO2023096690A1
Innovation
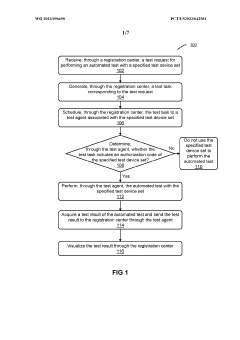
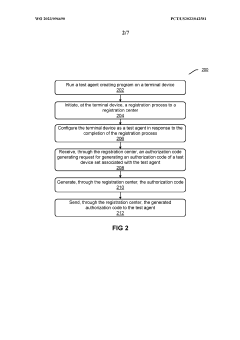
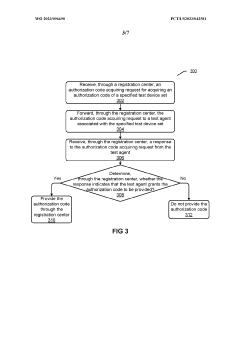
- The system decouples automated testing into a registration center and multiple test agents, allowing for distributed deployment, failover capabilities, and the creation of test agents from various terminal devices, enabling scalable and secure execution of automated tests across diverse device sets and frameworks.
Regulatory Compliance Challenges
Regulatory compliance represents one of the most significant barriers to the widespread adoption of autonomous laboratories in clinical trials. The integration of AI-driven systems, robotics, and automated decision-making processes introduces complex regulatory challenges that span multiple jurisdictions and oversight bodies. Currently, the FDA, EMA, and other global regulatory authorities have not established comprehensive frameworks specifically addressing autonomous lab technologies in clinical settings, creating uncertainty for implementation.
The validation of autonomous systems presents a particular challenge, as regulatory bodies require evidence that these systems consistently produce reliable, accurate, and reproducible results. This validation process becomes exponentially more complex when machine learning algorithms continuously evolve based on new data inputs, raising questions about how to validate systems that inherently change over time. Organizations must develop robust validation protocols that can satisfy regulatory requirements while accommodating the dynamic nature of autonomous technologies.
Data integrity and security requirements introduce additional compliance hurdles. Clinical trial data must maintain ALCOA+ principles (Attributable, Legible, Contemporaneous, Original, Accurate, plus Complete, Consistent, Enduring, and Available), which becomes challenging in fully automated environments where human oversight is minimized. Regulatory bodies express particular concern about data provenance, audit trails, and the ability to reconstruct decision pathways in autonomous systems.
The question of liability and responsibility in autonomous lab settings remains largely unresolved from a regulatory perspective. When adverse events occur, determining accountability between technology providers, clinical trial sponsors, and healthcare institutions becomes problematic. This regulatory ambiguity creates hesitation among stakeholders who fear assuming undefined liability risks associated with autonomous technologies.
Privacy regulations such as GDPR in Europe and HIPAA in the United States impose strict requirements on patient data handling that autonomous labs must address. The continuous data collection and analysis inherent to autonomous systems may conflict with consent requirements and data minimization principles, requiring careful technical and procedural safeguards to ensure compliance.
Cross-border regulatory differences further complicate global clinical trials utilizing autonomous technologies. Harmonization efforts remain in early stages, forcing organizations to navigate a patchwork of requirements that vary by region. This regulatory fragmentation increases compliance costs and complexity, particularly for multi-center trials spanning multiple jurisdictions.
The validation of autonomous systems presents a particular challenge, as regulatory bodies require evidence that these systems consistently produce reliable, accurate, and reproducible results. This validation process becomes exponentially more complex when machine learning algorithms continuously evolve based on new data inputs, raising questions about how to validate systems that inherently change over time. Organizations must develop robust validation protocols that can satisfy regulatory requirements while accommodating the dynamic nature of autonomous technologies.
Data integrity and security requirements introduce additional compliance hurdles. Clinical trial data must maintain ALCOA+ principles (Attributable, Legible, Contemporaneous, Original, Accurate, plus Complete, Consistent, Enduring, and Available), which becomes challenging in fully automated environments where human oversight is minimized. Regulatory bodies express particular concern about data provenance, audit trails, and the ability to reconstruct decision pathways in autonomous systems.
The question of liability and responsibility in autonomous lab settings remains largely unresolved from a regulatory perspective. When adverse events occur, determining accountability between technology providers, clinical trial sponsors, and healthcare institutions becomes problematic. This regulatory ambiguity creates hesitation among stakeholders who fear assuming undefined liability risks associated with autonomous technologies.
Privacy regulations such as GDPR in Europe and HIPAA in the United States impose strict requirements on patient data handling that autonomous labs must address. The continuous data collection and analysis inherent to autonomous systems may conflict with consent requirements and data minimization principles, requiring careful technical and procedural safeguards to ensure compliance.
Cross-border regulatory differences further complicate global clinical trials utilizing autonomous technologies. Harmonization efforts remain in early stages, forcing organizations to navigate a patchwork of requirements that vary by region. This regulatory fragmentation increases compliance costs and complexity, particularly for multi-center trials spanning multiple jurisdictions.
Data Security and Patient Privacy Considerations
Data security and patient privacy represent critical considerations in the adoption of autonomous laboratories for clinical trials. The implementation of AI-driven systems and automated processes introduces complex challenges regarding the protection of sensitive patient information. Regulatory frameworks such as GDPR in Europe, HIPAA in the United States, and similar regulations globally mandate strict compliance with data protection standards, requiring autonomous lab systems to incorporate robust security architectures from the design phase.
The autonomous nature of these systems presents unique vulnerabilities, as they often involve continuous data collection, storage, and transmission across multiple platforms and devices. Clinical trial data typically contains highly sensitive personal health information that requires enhanced protection measures. Organizations must implement end-to-end encryption, secure authentication protocols, and comprehensive access control systems to safeguard data throughout its lifecycle in autonomous lab environments.
Patient consent management becomes increasingly complex in autonomous settings. Traditional informed consent processes may be insufficient when data collection occurs continuously through automated systems. This necessitates the development of dynamic consent models that allow patients to maintain control over their data as it flows through various autonomous processes. The concept of "privacy by design" must be embedded within autonomous lab architectures to ensure patient rights are protected at every stage.
Data anonymization and pseudonymization techniques play vital roles in balancing research utility with privacy protection. However, the sophisticated data analytics capabilities of autonomous systems create re-identification risks that must be addressed through advanced privacy-preserving computational methods. Techniques such as differential privacy, federated learning, and homomorphic encryption are emerging as potential solutions that allow meaningful analysis while maintaining patient confidentiality.
Cross-border data transfers present additional challenges for multinational clinical trials utilizing autonomous labs. Varying regulatory requirements across jurisdictions necessitate careful planning and implementation of compliant data governance frameworks. Organizations must establish clear data localization strategies and international data transfer mechanisms that satisfy the most stringent regulatory requirements while enabling efficient operation of autonomous systems.
Audit trails and transparency mechanisms are essential components of trustworthy autonomous lab implementations. These systems must maintain comprehensive logs of all data access and processing activities, enabling verification of compliance and facilitating investigation of potential breaches. The ability to demonstrate responsible data stewardship through transparent practices significantly influences stakeholder trust and, consequently, adoption rates of autonomous lab technologies in clinical trial settings.
The autonomous nature of these systems presents unique vulnerabilities, as they often involve continuous data collection, storage, and transmission across multiple platforms and devices. Clinical trial data typically contains highly sensitive personal health information that requires enhanced protection measures. Organizations must implement end-to-end encryption, secure authentication protocols, and comprehensive access control systems to safeguard data throughout its lifecycle in autonomous lab environments.
Patient consent management becomes increasingly complex in autonomous settings. Traditional informed consent processes may be insufficient when data collection occurs continuously through automated systems. This necessitates the development of dynamic consent models that allow patients to maintain control over their data as it flows through various autonomous processes. The concept of "privacy by design" must be embedded within autonomous lab architectures to ensure patient rights are protected at every stage.
Data anonymization and pseudonymization techniques play vital roles in balancing research utility with privacy protection. However, the sophisticated data analytics capabilities of autonomous systems create re-identification risks that must be addressed through advanced privacy-preserving computational methods. Techniques such as differential privacy, federated learning, and homomorphic encryption are emerging as potential solutions that allow meaningful analysis while maintaining patient confidentiality.
Cross-border data transfers present additional challenges for multinational clinical trials utilizing autonomous labs. Varying regulatory requirements across jurisdictions necessitate careful planning and implementation of compliant data governance frameworks. Organizations must establish clear data localization strategies and international data transfer mechanisms that satisfy the most stringent regulatory requirements while enabling efficient operation of autonomous systems.
Audit trails and transparency mechanisms are essential components of trustworthy autonomous lab implementations. These systems must maintain comprehensive logs of all data access and processing activities, enabling verification of compliance and facilitating investigation of potential breaches. The ability to demonstrate responsible data stewardship through transparent practices significantly influences stakeholder trust and, consequently, adoption rates of autonomous lab technologies in clinical trial settings.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!