Patent Integration of Autonomous Lab in Advanced Analytical Instruments
SEP 25, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Autonomous Lab Integration Background and Objectives
The integration of autonomous laboratory capabilities into advanced analytical instruments represents a significant technological evolution in scientific research and industrial applications. This convergence has emerged from decades of separate development in laboratory automation, robotics, artificial intelligence, and analytical instrumentation. The trajectory began with simple automated sample handling systems in the 1980s, progressing through increasingly sophisticated robotic systems, and now culminating in fully integrated autonomous laboratories capable of designing, executing, and optimizing experimental workflows with minimal human intervention.
The primary objective of autonomous lab integration is to revolutionize the analytical process by creating self-operating systems that can perform complex analytical procedures with enhanced precision, reproducibility, and efficiency. These systems aim to eliminate human error, reduce experimental variability, and dramatically accelerate the pace of scientific discovery and product development across multiple industries including pharmaceuticals, materials science, and biotechnology.
Current technological goals include developing seamless integration protocols between robotic systems and analytical instruments, creating standardized interfaces for instrument communication, and implementing advanced AI algorithms capable of experimental design and result interpretation. The ultimate vision encompasses closed-loop systems that can autonomously formulate hypotheses, design and execute experiments, analyze results, and refine subsequent experimental approaches based on accumulated data.
Market drivers for this technology include increasing pressure for research efficiency, growing complexity of analytical challenges, and the need to maintain consistent quality in regulated industries. The COVID-19 pandemic has further accelerated interest in autonomous laboratory capabilities, highlighting the value of remote operation and reduced human intervention in critical research environments.
Technical evolution in this field is characterized by convergence of multiple disciplines, including robotics, machine learning, cloud computing, and specialized analytical techniques. The integration challenge lies in harmonizing these diverse technologies into cohesive systems that maintain the analytical integrity of sophisticated instruments while adding autonomous capabilities.
Regulatory considerations and validation requirements present additional complexity, particularly in highly regulated industries where analytical results must meet stringent compliance standards. This necessitates development of robust validation protocols and quality assurance mechanisms specifically designed for autonomous systems.
The long-term technological roadmap envisions fully autonomous discovery platforms capable of accelerating innovation across multiple scientific domains, potentially transforming how research is conducted and how analytical services are delivered in both academic and industrial settings.
The primary objective of autonomous lab integration is to revolutionize the analytical process by creating self-operating systems that can perform complex analytical procedures with enhanced precision, reproducibility, and efficiency. These systems aim to eliminate human error, reduce experimental variability, and dramatically accelerate the pace of scientific discovery and product development across multiple industries including pharmaceuticals, materials science, and biotechnology.
Current technological goals include developing seamless integration protocols between robotic systems and analytical instruments, creating standardized interfaces for instrument communication, and implementing advanced AI algorithms capable of experimental design and result interpretation. The ultimate vision encompasses closed-loop systems that can autonomously formulate hypotheses, design and execute experiments, analyze results, and refine subsequent experimental approaches based on accumulated data.
Market drivers for this technology include increasing pressure for research efficiency, growing complexity of analytical challenges, and the need to maintain consistent quality in regulated industries. The COVID-19 pandemic has further accelerated interest in autonomous laboratory capabilities, highlighting the value of remote operation and reduced human intervention in critical research environments.
Technical evolution in this field is characterized by convergence of multiple disciplines, including robotics, machine learning, cloud computing, and specialized analytical techniques. The integration challenge lies in harmonizing these diverse technologies into cohesive systems that maintain the analytical integrity of sophisticated instruments while adding autonomous capabilities.
Regulatory considerations and validation requirements present additional complexity, particularly in highly regulated industries where analytical results must meet stringent compliance standards. This necessitates development of robust validation protocols and quality assurance mechanisms specifically designed for autonomous systems.
The long-term technological roadmap envisions fully autonomous discovery platforms capable of accelerating innovation across multiple scientific domains, potentially transforming how research is conducted and how analytical services are delivered in both academic and industrial settings.
Market Analysis for Automated Analytical Instrumentation
The global market for automated analytical instrumentation is experiencing robust growth, driven by increasing demand for high-throughput analysis across pharmaceutical, biotechnology, environmental, and industrial sectors. Current market valuations indicate the automated analytical instruments market reached approximately 4.9 billion USD in 2022, with projections suggesting a compound annual growth rate (CAGR) of 7.2% through 2030, potentially reaching 8.5 billion USD by the end of the forecast period.
Key market drivers include the rising need for reproducibility in analytical results, increasing sample volumes in research and quality control applications, and growing pressure to reduce operational costs while improving efficiency. The integration of autonomous laboratory capabilities within advanced analytical instruments represents a significant value proposition, addressing these market demands through enhanced automation, reduced human intervention, and improved data quality.
Regionally, North America currently dominates the market share at approximately 38%, followed by Europe at 29% and Asia-Pacific at 24%. However, the Asia-Pacific region is demonstrating the fastest growth rate, particularly in China, India, and South Korea, where significant investments in pharmaceutical research, environmental monitoring, and industrial quality control are creating expanded market opportunities.
By application segment, pharmaceutical and biotechnology applications account for the largest market share (approximately 35%), followed by environmental testing (22%), food and beverage analysis (18%), and industrial quality control (15%). The remaining market share is distributed across academic research, forensic analysis, and other specialized applications.
Customer demand patterns reveal increasing preference for integrated systems that combine multiple analytical techniques, offer enhanced data management capabilities, and provide remote operation features. End-users are particularly interested in solutions that reduce training requirements, minimize human error, and enable 24/7 operation capabilities – all attributes directly addressed by autonomous lab integration.
Market challenges include high initial investment costs, concerns regarding system validation and regulatory compliance, and resistance to workflow changes in established laboratories. Additionally, there exists a significant knowledge gap regarding the full capabilities and return on investment potential of autonomous systems among potential customers.
Competitive analysis reveals that major analytical instrument manufacturers are increasingly incorporating autonomous features into their product roadmaps, with several strategic acquisitions occurring between instrument manufacturers and laboratory automation specialists. This trend indicates market recognition of autonomous capabilities as a critical differentiator in future product offerings.
Key market drivers include the rising need for reproducibility in analytical results, increasing sample volumes in research and quality control applications, and growing pressure to reduce operational costs while improving efficiency. The integration of autonomous laboratory capabilities within advanced analytical instruments represents a significant value proposition, addressing these market demands through enhanced automation, reduced human intervention, and improved data quality.
Regionally, North America currently dominates the market share at approximately 38%, followed by Europe at 29% and Asia-Pacific at 24%. However, the Asia-Pacific region is demonstrating the fastest growth rate, particularly in China, India, and South Korea, where significant investments in pharmaceutical research, environmental monitoring, and industrial quality control are creating expanded market opportunities.
By application segment, pharmaceutical and biotechnology applications account for the largest market share (approximately 35%), followed by environmental testing (22%), food and beverage analysis (18%), and industrial quality control (15%). The remaining market share is distributed across academic research, forensic analysis, and other specialized applications.
Customer demand patterns reveal increasing preference for integrated systems that combine multiple analytical techniques, offer enhanced data management capabilities, and provide remote operation features. End-users are particularly interested in solutions that reduce training requirements, minimize human error, and enable 24/7 operation capabilities – all attributes directly addressed by autonomous lab integration.
Market challenges include high initial investment costs, concerns regarding system validation and regulatory compliance, and resistance to workflow changes in established laboratories. Additionally, there exists a significant knowledge gap regarding the full capabilities and return on investment potential of autonomous systems among potential customers.
Competitive analysis reveals that major analytical instrument manufacturers are increasingly incorporating autonomous features into their product roadmaps, with several strategic acquisitions occurring between instrument manufacturers and laboratory automation specialists. This trend indicates market recognition of autonomous capabilities as a critical differentiator in future product offerings.
Current Challenges in Autonomous Lab Implementation
Despite the promising potential of autonomous laboratories in analytical instrumentation, several significant challenges currently impede their widespread implementation and integration. These challenges span technical, operational, and regulatory domains, creating a complex landscape for innovators and manufacturers to navigate.
Hardware integration presents a formidable obstacle, as autonomous lab systems require seamless communication between diverse analytical instruments, robotic components, and sample handling mechanisms. The lack of standardized interfaces and communication protocols between equipment from different manufacturers creates compatibility issues that hinder system cohesion. Additionally, the physical space constraints in traditional laboratories often complicate the installation of robotic systems that require specific operational clearances.
Software interoperability remains equally problematic. Current analytical instruments utilize proprietary software systems that rarely communicate effectively with each other or with overarching laboratory management systems. This fragmentation necessitates complex middleware solutions and custom integration efforts that increase implementation costs and technical complexity. The absence of universal data formats further complicates data transfer and analysis across different platforms.
Reliability and robustness concerns persist in autonomous systems operating with minimal human supervision. Error handling mechanisms must be sophisticated enough to address unexpected situations without human intervention, yet current systems often struggle with edge cases and unforeseen scenarios. The complexity increases exponentially when dealing with delicate or hazardous materials that require precise handling under variable conditions.
Validation and regulatory compliance represent another significant hurdle, particularly in highly regulated industries such as pharmaceuticals and healthcare. Regulatory frameworks have not fully adapted to autonomous laboratory technologies, creating uncertainty regarding validation requirements, data integrity standards, and quality assurance protocols for automated processes. This regulatory ambiguity often delays implementation and increases compliance costs.
Cost justification remains challenging for many organizations. The substantial initial investment in autonomous lab equipment, integration services, and staff training must be balanced against projected efficiency gains and error reduction. Without clear ROI models specific to different laboratory environments, decision-makers often hesitate to commit resources to these transformative technologies.
Workforce adaptation issues further complicate implementation. Laboratory professionals may resist automation due to concerns about job security or may lack the technical skills needed to operate and troubleshoot sophisticated autonomous systems. This skills gap necessitates comprehensive training programs and organizational change management strategies that many institutions are ill-equipped to provide.
Hardware integration presents a formidable obstacle, as autonomous lab systems require seamless communication between diverse analytical instruments, robotic components, and sample handling mechanisms. The lack of standardized interfaces and communication protocols between equipment from different manufacturers creates compatibility issues that hinder system cohesion. Additionally, the physical space constraints in traditional laboratories often complicate the installation of robotic systems that require specific operational clearances.
Software interoperability remains equally problematic. Current analytical instruments utilize proprietary software systems that rarely communicate effectively with each other or with overarching laboratory management systems. This fragmentation necessitates complex middleware solutions and custom integration efforts that increase implementation costs and technical complexity. The absence of universal data formats further complicates data transfer and analysis across different platforms.
Reliability and robustness concerns persist in autonomous systems operating with minimal human supervision. Error handling mechanisms must be sophisticated enough to address unexpected situations without human intervention, yet current systems often struggle with edge cases and unforeseen scenarios. The complexity increases exponentially when dealing with delicate or hazardous materials that require precise handling under variable conditions.
Validation and regulatory compliance represent another significant hurdle, particularly in highly regulated industries such as pharmaceuticals and healthcare. Regulatory frameworks have not fully adapted to autonomous laboratory technologies, creating uncertainty regarding validation requirements, data integrity standards, and quality assurance protocols for automated processes. This regulatory ambiguity often delays implementation and increases compliance costs.
Cost justification remains challenging for many organizations. The substantial initial investment in autonomous lab equipment, integration services, and staff training must be balanced against projected efficiency gains and error reduction. Without clear ROI models specific to different laboratory environments, decision-makers often hesitate to commit resources to these transformative technologies.
Workforce adaptation issues further complicate implementation. Laboratory professionals may resist automation due to concerns about job security or may lack the technical skills needed to operate and troubleshoot sophisticated autonomous systems. This skills gap necessitates comprehensive training programs and organizational change management strategies that many institutions are ill-equipped to provide.
Patent-Protected Integration Solutions for Autonomous Labs
01 Automated laboratory systems and equipment
Autonomous laboratory systems incorporate robotic equipment and automated processes to conduct experiments with minimal human intervention. These systems include automated sample handling, preparation, and analysis capabilities. They can perform repetitive tasks with high precision, reducing human error and increasing throughput in scientific research and testing environments.- Automated laboratory systems for scientific research: Autonomous laboratory systems that integrate robotics, AI, and automation to conduct scientific experiments with minimal human intervention. These systems can handle sample preparation, testing, data collection, and analysis, significantly increasing throughput and reproducibility in research environments. They typically incorporate machine learning algorithms to optimize experimental parameters and interpret results.
- Cloud-based laboratory management and control systems: Networked laboratory systems that leverage cloud computing for remote operation, monitoring, and data management of laboratory equipment. These platforms enable researchers to design experiments, control instruments, and analyze results from anywhere. The cloud infrastructure facilitates collaboration between distributed teams, provides scalable computing resources for data processing, and supports integration with various laboratory instruments and robots.
- AI-driven experimental design and optimization: Systems that employ artificial intelligence to design, optimize, and execute laboratory experiments autonomously. These technologies use machine learning algorithms to analyze experimental data, identify patterns, and make decisions about subsequent experiments without human intervention. They can significantly reduce the number of experiments needed to achieve research goals by intelligently exploring experimental parameters and adapting based on real-time results.
- Robotic laboratory automation and hardware integration: Physical robotic systems designed to automate laboratory workflows by integrating various instruments and handling equipment. These systems include robotic arms, liquid handlers, sample transporters, and specialized instruments that work together to perform complex laboratory procedures. The hardware components are coordinated through sophisticated control software that ensures precise timing and positioning for reliable experimental execution.
- Quality control and error detection in autonomous labs: Systems and methods for ensuring reliability and accuracy in autonomous laboratory operations through continuous monitoring and error detection. These technologies incorporate sensors, computer vision, and statistical analysis to identify anomalies, equipment malfunctions, or experimental deviations in real-time. They can automatically implement corrective actions or alert operators when issues are detected, maintaining experimental integrity and data quality in automated environments.
02 AI and machine learning for laboratory automation
Artificial intelligence and machine learning algorithms are integrated into autonomous lab systems to optimize experimental design, predict outcomes, and analyze results. These technologies enable self-learning capabilities where the system can adapt protocols based on previous experimental results. AI-driven autonomous labs can identify patterns in data, suggest new experimental approaches, and accelerate scientific discovery through intelligent decision-making processes.Expand Specific Solutions03 Laboratory management and control systems
Comprehensive software platforms manage and control autonomous laboratory operations, coordinating multiple instruments and processes. These systems provide centralized monitoring, scheduling, and data management capabilities. They enable remote operation of laboratory equipment, real-time tracking of experiments, and integration of various laboratory instruments into a cohesive workflow system.Expand Specific Solutions04 IoT and connectivity in autonomous labs
Internet of Things (IoT) technology connects laboratory instruments, sensors, and data systems to create a networked autonomous laboratory environment. These connected systems enable real-time data collection, monitoring, and analysis across multiple devices. IoT integration allows for remote access to laboratory equipment, automated alerts and notifications, and seamless data transfer between instruments and analysis platforms.Expand Specific Solutions05 Quality control and validation systems
Autonomous laboratories incorporate automated quality control and validation processes to ensure experimental reliability and reproducibility. These systems include self-diagnostic capabilities, automated calibration procedures, and error detection mechanisms. They continuously monitor operational parameters, validate experimental results, and maintain compliance with regulatory standards through automated documentation and verification processes.Expand Specific Solutions
Key Industry Players in Autonomous Analytical Instruments
The patent integration of autonomous labs in advanced analytical instruments is in an early growth phase, characterized by increasing adoption across pharmaceutical, healthcare, and research sectors. The market is projected to expand significantly due to rising demand for automated, high-throughput analytical solutions. While the technology shows promising development, it remains in transition from experimental to commercial applications. Leading players like Roche Diagnostics, Abbott Laboratories, and Hitachi demonstrate advanced capabilities in autonomous lab integration, with Siemens Healthcare Diagnostics and Agilent Technologies making significant investments. Emerging competitors from China, including Mindray and Dymind Biotechnology, are rapidly advancing their technological offerings, while established instrument manufacturers like JEOL and Waters Technology are incorporating AI and robotics to enhance analytical capabilities.
Hitachi Ltd.
Technical Solution: Hitachi's autonomous lab integration platform for analytical instruments combines robotics, AI-driven decision making, and IoT connectivity to create self-operating laboratory environments. Their system incorporates automated sample preparation, intelligent scheduling algorithms, and real-time data analysis capabilities. The platform features a modular architecture allowing seamless integration with existing laboratory information management systems (LIMS) and instrument ecosystems. Hitachi's solution employs machine learning algorithms that continuously optimize analytical workflows based on historical performance data, reducing human intervention while maintaining analytical precision. Their patent portfolio includes technologies for automated calibration, predictive maintenance, and cross-instrument synchronization protocols that enable complex multi-step analytical procedures to be executed autonomously[1][3]. The system also incorporates advanced error detection and recovery mechanisms to ensure reliability in high-throughput environments.
Strengths: Extensive experience in automation and robotics integration; strong existing relationships with clinical laboratories; comprehensive IoT ecosystem that facilitates instrument connectivity. Weaknesses: Higher implementation costs compared to competitors; complex integration requirements may limit adoption in smaller laboratories; relatively steep learning curve for laboratory staff transitioning to the autonomous platform.
F. Hoffmann-La Roche Ltd.
Technical Solution: Roche's autonomous laboratory integration technology centers on their cobas® infinity laboratory solution, which creates a fully connected ecosystem of analytical instruments with autonomous operation capabilities. Their patented approach incorporates a central orchestration engine that coordinates multiple analytical platforms through standardized communication protocols. The system features dynamic resource allocation, allowing instruments to automatically redistribute workloads based on current demand and instrument availability. Roche's technology implements sophisticated sample tracking using RFID and computer vision, enabling autonomous routing of specimens through complex analytical workflows. Their solution incorporates predictive analytics for reagent management, automatically triggering orders and optimizing inventory levels based on usage patterns[2][5]. The platform also features self-learning quality control systems that can detect subtle shifts in instrument performance before they affect analytical results, automatically initiating calibration or maintenance procedures when necessary.
Strengths: Comprehensive integration with clinical decision support systems; extensive installed base of compatible instruments; strong regulatory compliance features built into autonomous operations. Weaknesses: Relatively closed ecosystem that works best within Roche's instrument portfolio; higher initial investment compared to manual systems; dependency on Roche's proprietary middleware for full functionality.
Critical Patent Analysis for Autonomous Lab Technologies
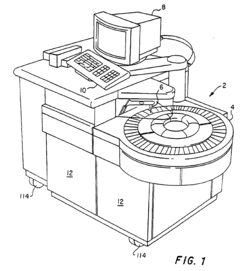
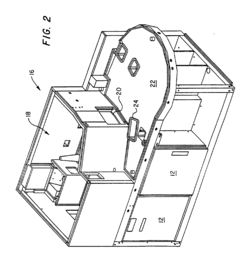
Integrated, automated system for the study of cell and tissue function
PatentWO2010022391A2
Innovation
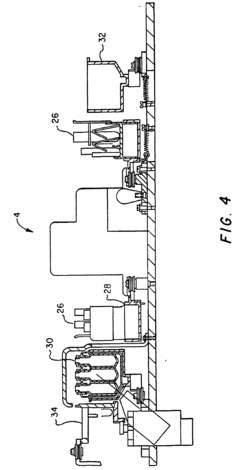
- A modular, extensible, and flexible automated system architecture that enables high-throughput analysis of single cells and small populations using microfluidics, optics, and robotics, allowing for precise control of fluidic stimuli, data acquisition, and end-point analyses, with closed-loop feedback control and minimal operator interaction.
Automated continuous and random access analytical method
PatentInactiveEP0746769B1
Innovation
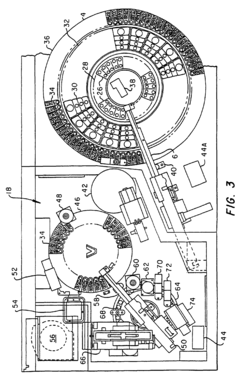
- An automated, continuous, and random access analytical system that schedules multiple assays for a plurality of liquid samples, allowing for independent preparation and incubation of reaction mixtures, and simultaneous analysis using a microprocessor-based system with robotic arm pipetters and bidirectional rotating carousels, enabling precise and efficient handling of reagents and samples.
Intellectual Property Strategy for Autonomous Lab Development
Developing a comprehensive intellectual property strategy is crucial for organizations investing in autonomous lab technologies within advanced analytical instruments. This strategy must balance protection of core innovations with the need for collaboration in this rapidly evolving field. A multi-layered approach to IP management begins with thorough patent landscape analysis to identify white spaces and potential infringement risks in the autonomous lab ecosystem.
Strategic patent filing should focus on protecting fundamental autonomous lab technologies that integrate with analytical instruments, including robotics interfaces, AI-driven experimental design algorithms, and automated sample handling systems. Organizations should prioritize patenting innovations at the intersection of laboratory automation and analytical instrumentation, as this convergence represents significant commercial value.
Defensive patenting strategies are equally important, creating protective barriers around core technologies while establishing freedom to operate. Companies should consider developing patent portfolios that cover not only hardware integration but also software systems that enable autonomous decision-making in analytical processes. These software patents should address data processing methods, machine learning algorithms for experimental optimization, and communication protocols between instruments.
Licensing strategies must be carefully crafted to maximize return on IP investments while fostering necessary collaborations. Cross-licensing agreements with complementary technology providers can accelerate development while reducing legal risks. For non-core technologies, open innovation approaches may be appropriate to stimulate ecosystem growth and establish technical standards.
Trade secret protection should complement patent strategies, particularly for proprietary calibration methods, specialized integration techniques, and complex AI training datasets that provide competitive advantages but may be difficult to reverse-engineer. Implementing robust internal IP management processes ensures consistent protection across global operations and research centers.
Geographic considerations are essential when developing the IP strategy, with different filing approaches needed for major markets including North America, Europe, China, and Japan. The strategy should account for varying patent enforcement environments and competitive landscapes in these regions, with particular attention to emerging markets where analytical instrument adoption is accelerating.
Regular IP portfolio reviews should be conducted to align protection with evolving business objectives and technological developments. This ensures resources are directed toward protecting innovations with the greatest strategic value as autonomous lab technologies mature and market applications expand.
Strategic patent filing should focus on protecting fundamental autonomous lab technologies that integrate with analytical instruments, including robotics interfaces, AI-driven experimental design algorithms, and automated sample handling systems. Organizations should prioritize patenting innovations at the intersection of laboratory automation and analytical instrumentation, as this convergence represents significant commercial value.
Defensive patenting strategies are equally important, creating protective barriers around core technologies while establishing freedom to operate. Companies should consider developing patent portfolios that cover not only hardware integration but also software systems that enable autonomous decision-making in analytical processes. These software patents should address data processing methods, machine learning algorithms for experimental optimization, and communication protocols between instruments.
Licensing strategies must be carefully crafted to maximize return on IP investments while fostering necessary collaborations. Cross-licensing agreements with complementary technology providers can accelerate development while reducing legal risks. For non-core technologies, open innovation approaches may be appropriate to stimulate ecosystem growth and establish technical standards.
Trade secret protection should complement patent strategies, particularly for proprietary calibration methods, specialized integration techniques, and complex AI training datasets that provide competitive advantages but may be difficult to reverse-engineer. Implementing robust internal IP management processes ensures consistent protection across global operations and research centers.
Geographic considerations are essential when developing the IP strategy, with different filing approaches needed for major markets including North America, Europe, China, and Japan. The strategy should account for varying patent enforcement environments and competitive landscapes in these regions, with particular attention to emerging markets where analytical instrument adoption is accelerating.
Regular IP portfolio reviews should be conducted to align protection with evolving business objectives and technological developments. This ensures resources are directed toward protecting innovations with the greatest strategic value as autonomous lab technologies mature and market applications expand.
Standardization Requirements for Autonomous Analytical Systems
The standardization of autonomous analytical systems represents a critical foundation for the successful integration of autonomous laboratory technologies within advanced analytical instruments. Current standardization efforts remain fragmented across different industries and applications, creating significant barriers to interoperability and widespread adoption. A comprehensive standardization framework must address multiple layers of the autonomous analytical ecosystem, including hardware interfaces, software protocols, data formats, and operational procedures.
Primary standardization requirements should focus on establishing universal communication protocols between diverse instrument components. This includes standardized APIs for instrument control, unified data exchange formats, and consistent command structures that enable seamless integration regardless of manufacturer or instrument type. The IEEE P2664 working group has begun addressing some of these needs, but industry-specific extensions are still required for analytical chemistry applications.
Safety and reliability standards constitute another crucial domain requiring immediate attention. Autonomous systems must operate within clearly defined safety parameters, particularly when handling hazardous materials or performing high-energy operations. Standards should define mandatory safety interlocks, error handling procedures, and fail-safe mechanisms. Additionally, validation protocols must be established to verify system performance against predetermined benchmarks.
Data integrity and security standards represent the third critical pillar of standardization requirements. As autonomous systems generate, process, and store increasingly sensitive analytical data, robust standards for data encryption, access control, audit trails, and long-term archiving become essential. These standards must align with existing regulatory frameworks such as 21 CFR Part 11 while addressing the unique challenges posed by autonomous operation.
Calibration and quality control standardization presents particular challenges in autonomous environments. Standards must define procedures for automated calibration, system suitability testing, and ongoing performance verification without human intervention. This includes specifications for reference materials, acceptance criteria, and corrective action protocols when deviations occur.
Finally, standardization efforts must address the ethical and regulatory dimensions of autonomous analytical systems. This includes guidelines for appropriate levels of autonomy in different analytical contexts, transparency requirements regarding algorithmic decision-making, and clear delineation of responsibilities between human operators and autonomous systems. International harmonization of these standards will be essential to prevent regulatory fragmentation and enable global deployment of autonomous laboratory technologies.
Primary standardization requirements should focus on establishing universal communication protocols between diverse instrument components. This includes standardized APIs for instrument control, unified data exchange formats, and consistent command structures that enable seamless integration regardless of manufacturer or instrument type. The IEEE P2664 working group has begun addressing some of these needs, but industry-specific extensions are still required for analytical chemistry applications.
Safety and reliability standards constitute another crucial domain requiring immediate attention. Autonomous systems must operate within clearly defined safety parameters, particularly when handling hazardous materials or performing high-energy operations. Standards should define mandatory safety interlocks, error handling procedures, and fail-safe mechanisms. Additionally, validation protocols must be established to verify system performance against predetermined benchmarks.
Data integrity and security standards represent the third critical pillar of standardization requirements. As autonomous systems generate, process, and store increasingly sensitive analytical data, robust standards for data encryption, access control, audit trails, and long-term archiving become essential. These standards must align with existing regulatory frameworks such as 21 CFR Part 11 while addressing the unique challenges posed by autonomous operation.
Calibration and quality control standardization presents particular challenges in autonomous environments. Standards must define procedures for automated calibration, system suitability testing, and ongoing performance verification without human intervention. This includes specifications for reference materials, acceptance criteria, and corrective action protocols when deviations occur.
Finally, standardization efforts must address the ethical and regulatory dimensions of autonomous analytical systems. This includes guidelines for appropriate levels of autonomy in different analytical contexts, transparency requirements regarding algorithmic decision-making, and clear delineation of responsibilities between human operators and autonomous systems. International harmonization of these standards will be essential to prevent regulatory fragmentation and enable global deployment of autonomous laboratory technologies.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!