Autonomous Lab Kinetic Optimization for Enhanced Pharmaceutical Catalysis
SEP 25, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Autonomous Lab Evolution and Objectives
The evolution of autonomous laboratory systems represents a significant paradigm shift in pharmaceutical research and development. Initially emerging from basic laboratory automation in the 1980s, these systems have progressively incorporated advanced robotics, machine learning algorithms, and sophisticated analytical techniques to create increasingly self-sufficient research environments. The trajectory from simple automated liquid handling to today's integrated autonomous platforms demonstrates a clear technological progression toward minimizing human intervention while maximizing experimental throughput and reproducibility.
Recent advancements in artificial intelligence, particularly reinforcement learning and Bayesian optimization techniques, have accelerated this evolution by enabling systems to make data-driven decisions about experimental parameters in real-time. This capability has proven particularly valuable for catalysis research, where reaction conditions often require precise tuning across multiple variables simultaneously to achieve optimal yields and selectivity.
The primary objective of Autonomous Lab Kinetic Optimization for pharmaceutical catalysis is to develop systems capable of independently designing, executing, and refining catalytic reactions with minimal human oversight. These systems aim to dramatically reduce the time required to optimize reaction conditions from weeks or months to mere days, while simultaneously improving reproducibility and expanding the accessible parameter space beyond what human researchers could feasibly explore.
Secondary objectives include the development of standardized protocols for autonomous experimentation that ensure data quality and compatibility across different laboratory environments. This standardization is crucial for enabling collaborative research and facilitating the transfer of optimized processes from research to production scales, addressing a persistent challenge in pharmaceutical manufacturing.
Technical goals encompass the integration of real-time analytical techniques with decision-making algorithms to create closed-loop optimization systems. These systems should be capable of handling complex reaction networks, identifying and navigating around experimental failures, and providing interpretable results that contribute to mechanistic understanding rather than merely empirical optimization.
Long-term objectives extend beyond simple parameter optimization to include autonomous discovery of novel catalysts and reaction pathways. This represents a transition from optimization-focused automation to true innovation-capable autonomous systems that could potentially revolutionize drug discovery and development processes by identifying synthetic routes that human chemists might overlook due to cognitive biases or limited exploration capacity.
Recent advancements in artificial intelligence, particularly reinforcement learning and Bayesian optimization techniques, have accelerated this evolution by enabling systems to make data-driven decisions about experimental parameters in real-time. This capability has proven particularly valuable for catalysis research, where reaction conditions often require precise tuning across multiple variables simultaneously to achieve optimal yields and selectivity.
The primary objective of Autonomous Lab Kinetic Optimization for pharmaceutical catalysis is to develop systems capable of independently designing, executing, and refining catalytic reactions with minimal human oversight. These systems aim to dramatically reduce the time required to optimize reaction conditions from weeks or months to mere days, while simultaneously improving reproducibility and expanding the accessible parameter space beyond what human researchers could feasibly explore.
Secondary objectives include the development of standardized protocols for autonomous experimentation that ensure data quality and compatibility across different laboratory environments. This standardization is crucial for enabling collaborative research and facilitating the transfer of optimized processes from research to production scales, addressing a persistent challenge in pharmaceutical manufacturing.
Technical goals encompass the integration of real-time analytical techniques with decision-making algorithms to create closed-loop optimization systems. These systems should be capable of handling complex reaction networks, identifying and navigating around experimental failures, and providing interpretable results that contribute to mechanistic understanding rather than merely empirical optimization.
Long-term objectives extend beyond simple parameter optimization to include autonomous discovery of novel catalysts and reaction pathways. This represents a transition from optimization-focused automation to true innovation-capable autonomous systems that could potentially revolutionize drug discovery and development processes by identifying synthetic routes that human chemists might overlook due to cognitive biases or limited exploration capacity.
Market Demand for Automated Pharmaceutical Catalysis
The pharmaceutical industry is experiencing a significant shift towards automation and optimization of catalytic processes, driven by increasing demands for more efficient drug development and production. The global pharmaceutical market, valued at approximately $1.4 trillion in 2022, faces mounting pressure to reduce development timelines and costs while improving yield and quality. Automated catalysis technologies directly address these challenges, with market research indicating a compound annual growth rate of 8.7% for pharmaceutical process automation solutions through 2028.
Pharmaceutical companies are particularly interested in autonomous lab kinetic optimization systems due to their potential to dramatically accelerate catalyst discovery and optimization. Traditional manual methods for catalyst screening and optimization typically require 18-24 months of laboratory work, whereas automated systems can reduce this timeframe to 3-6 months while simultaneously exploring a broader parameter space.
Cost reduction represents another critical market driver. Development of a single pharmaceutical product can cost between $1-2 billion, with catalysis optimization accounting for approximately 15-20% of early-stage chemistry costs. Industry analyses suggest that autonomous optimization systems can reduce these specific costs by 30-45% while improving outcomes through more thorough exploration of reaction conditions.
Environmental sustainability requirements are also fueling demand for optimized catalytic processes. Regulatory bodies worldwide are implementing stricter environmental standards for pharmaceutical manufacturing, with the European Medicines Agency and FDA both releasing updated guidelines emphasizing green chemistry principles in 2021. Optimized catalysis directly supports these goals by reducing waste, energy consumption, and the use of harmful solvents.
The personalized medicine trend further amplifies market demand for flexible, efficient catalytic processes. As treatments become increasingly tailored to specific patient populations, manufacturing must adapt to smaller batch sizes and more diverse product portfolios. Autonomous optimization systems enable rapid reconfiguration of catalytic processes to accommodate these changing requirements.
Contract Development and Manufacturing Organizations (CDMOs) represent a particularly strong market segment, with 68% of surveyed CDMOs indicating plans to invest in advanced catalysis automation within the next three years. This reflects the competitive advantage these technologies provide in an increasingly crowded market where speed and efficiency differentiate service providers.
Regional analysis reveals particularly strong demand growth in Asia-Pacific markets, where pharmaceutical manufacturing capacity is expanding rapidly, and in North America, where specialty and biologics production increasingly relies on complex catalytic processes requiring sophisticated optimization.
Pharmaceutical companies are particularly interested in autonomous lab kinetic optimization systems due to their potential to dramatically accelerate catalyst discovery and optimization. Traditional manual methods for catalyst screening and optimization typically require 18-24 months of laboratory work, whereas automated systems can reduce this timeframe to 3-6 months while simultaneously exploring a broader parameter space.
Cost reduction represents another critical market driver. Development of a single pharmaceutical product can cost between $1-2 billion, with catalysis optimization accounting for approximately 15-20% of early-stage chemistry costs. Industry analyses suggest that autonomous optimization systems can reduce these specific costs by 30-45% while improving outcomes through more thorough exploration of reaction conditions.
Environmental sustainability requirements are also fueling demand for optimized catalytic processes. Regulatory bodies worldwide are implementing stricter environmental standards for pharmaceutical manufacturing, with the European Medicines Agency and FDA both releasing updated guidelines emphasizing green chemistry principles in 2021. Optimized catalysis directly supports these goals by reducing waste, energy consumption, and the use of harmful solvents.
The personalized medicine trend further amplifies market demand for flexible, efficient catalytic processes. As treatments become increasingly tailored to specific patient populations, manufacturing must adapt to smaller batch sizes and more diverse product portfolios. Autonomous optimization systems enable rapid reconfiguration of catalytic processes to accommodate these changing requirements.
Contract Development and Manufacturing Organizations (CDMOs) represent a particularly strong market segment, with 68% of surveyed CDMOs indicating plans to invest in advanced catalysis automation within the next three years. This reflects the competitive advantage these technologies provide in an increasingly crowded market where speed and efficiency differentiate service providers.
Regional analysis reveals particularly strong demand growth in Asia-Pacific markets, where pharmaceutical manufacturing capacity is expanding rapidly, and in North America, where specialty and biologics production increasingly relies on complex catalytic processes requiring sophisticated optimization.
Current Challenges in Kinetic Optimization Technologies
Despite significant advancements in laboratory automation, current kinetic optimization technologies for pharmaceutical catalysis face several critical challenges. The primary limitation lies in the integration of real-time data analysis with experimental execution. Most systems operate in a semi-autonomous fashion, requiring human intervention at decision points, which creates bottlenecks in the optimization workflow and reduces overall efficiency.
Data quality and reproducibility present another significant hurdle. Kinetic measurements often suffer from inconsistencies due to variations in experimental conditions, catalyst degradation, and instrument drift. These inconsistencies compromise the reliability of optimization algorithms and lead to suboptimal catalytic processes, particularly when scaling from laboratory to production environments.
The computational frameworks supporting kinetic optimization frequently struggle with complex reaction networks common in pharmaceutical synthesis. Current models often fail to accurately capture multistep reactions, competing pathways, and catalyst deactivation mechanisms. This modeling inadequacy results in optimization algorithms converging on local rather than global optima, limiting the discovery of truly innovative catalytic conditions.
Resource utilization efficiency remains problematic in existing systems. Autonomous laboratories typically employ brute-force approaches that consume excessive reagents and energy. The lack of intelligent experimental design strategies leads to redundant experiments and unnecessary consumption of precious catalysts and pharmaceutical intermediates, contradicting sustainability goals in modern pharmaceutical manufacturing.
Knowledge transfer between different catalytic systems represents another major challenge. Current technologies operate as isolated platforms with limited ability to leverage insights gained from previous optimization campaigns. The absence of standardized data formats and comprehensive knowledge bases prevents the development of transferable models that could accelerate optimization across different reaction classes.
Time constraints pose practical limitations for industrial implementation. Kinetic optimization often requires extended experimental campaigns, particularly for slow reactions or complex catalytic systems. The inability to effectively parallelize experiments or predict outcomes with sufficient accuracy extends development timelines, delaying the introduction of new pharmaceutical products to market.
Finally, existing technologies struggle with the integration of mechanistic understanding into optimization protocols. Most systems operate as "black boxes" that identify optimal conditions without providing insights into the underlying catalytic mechanisms. This lack of mechanistic transparency hinders the rational design of next-generation catalysts and limits the scientific value of autonomous optimization campaigns in advancing fundamental catalysis knowledge.
Data quality and reproducibility present another significant hurdle. Kinetic measurements often suffer from inconsistencies due to variations in experimental conditions, catalyst degradation, and instrument drift. These inconsistencies compromise the reliability of optimization algorithms and lead to suboptimal catalytic processes, particularly when scaling from laboratory to production environments.
The computational frameworks supporting kinetic optimization frequently struggle with complex reaction networks common in pharmaceutical synthesis. Current models often fail to accurately capture multistep reactions, competing pathways, and catalyst deactivation mechanisms. This modeling inadequacy results in optimization algorithms converging on local rather than global optima, limiting the discovery of truly innovative catalytic conditions.
Resource utilization efficiency remains problematic in existing systems. Autonomous laboratories typically employ brute-force approaches that consume excessive reagents and energy. The lack of intelligent experimental design strategies leads to redundant experiments and unnecessary consumption of precious catalysts and pharmaceutical intermediates, contradicting sustainability goals in modern pharmaceutical manufacturing.
Knowledge transfer between different catalytic systems represents another major challenge. Current technologies operate as isolated platforms with limited ability to leverage insights gained from previous optimization campaigns. The absence of standardized data formats and comprehensive knowledge bases prevents the development of transferable models that could accelerate optimization across different reaction classes.
Time constraints pose practical limitations for industrial implementation. Kinetic optimization often requires extended experimental campaigns, particularly for slow reactions or complex catalytic systems. The inability to effectively parallelize experiments or predict outcomes with sufficient accuracy extends development timelines, delaying the introduction of new pharmaceutical products to market.
Finally, existing technologies struggle with the integration of mechanistic understanding into optimization protocols. Most systems operate as "black boxes" that identify optimal conditions without providing insights into the underlying catalytic mechanisms. This lack of mechanistic transparency hinders the rational design of next-generation catalysts and limits the scientific value of autonomous optimization campaigns in advancing fundamental catalysis knowledge.
Existing Kinetic Optimization Methodologies
01 Autonomous laboratory systems for reaction kinetics optimization
Autonomous laboratory systems that can automatically optimize reaction kinetics without human intervention. These systems use machine learning algorithms to design experiments, analyze results, and make decisions about subsequent experiments to optimize reaction conditions and improve yields. The systems can continuously monitor reaction parameters and adjust conditions in real-time to achieve optimal kinetic performance.- Autonomous laboratory systems for reaction kinetics optimization: Autonomous laboratory systems that can automatically optimize reaction kinetics without human intervention. These systems use machine learning algorithms to design experiments, analyze results, and make decisions about subsequent experiments to optimize reaction conditions. They can continuously monitor reaction parameters and adjust conditions in real-time to achieve optimal kinetic performance, significantly reducing the time and resources required for chemical process development.
- Machine learning approaches for kinetic parameter prediction: Advanced machine learning techniques applied to predict and optimize reaction kinetics. These approaches use historical experimental data to build predictive models that can estimate reaction rates, activation energies, and other kinetic parameters without performing extensive laboratory experiments. By leveraging artificial intelligence, these systems can identify patterns in complex reaction data and suggest optimal conditions for desired reaction outcomes, enabling more efficient process development.
- High-throughput experimentation platforms for kinetic studies: Automated high-throughput experimentation platforms designed specifically for kinetic studies. These systems can perform multiple experiments simultaneously under different conditions, rapidly generating large datasets of kinetic information. They incorporate automated sample preparation, reaction monitoring, and analysis capabilities to accelerate the discovery and optimization of chemical processes. The integration of robotics and advanced analytics enables systematic exploration of reaction parameter space to identify optimal kinetic conditions.
- Real-time monitoring and feedback control systems: Systems that provide real-time monitoring of reaction kinetics coupled with feedback control mechanisms. These technologies use in-situ spectroscopic techniques, sensors, and analytical instruments to continuously track reaction progress and kinetic parameters. The data is processed in real-time, and control algorithms automatically adjust reaction conditions to maintain optimal kinetic performance. This closed-loop approach enables dynamic optimization of reaction processes and can adapt to unexpected variations in reaction behavior.
- Digital twins and simulation tools for kinetic optimization: Digital twin technology and advanced simulation tools that create virtual representations of chemical reactions and processes for kinetic optimization. These computational approaches model reaction kinetics based on fundamental chemical principles and experimental data, allowing researchers to perform virtual experiments before conducting physical ones. By exploring reaction conditions in silico, optimal parameters can be identified more efficiently, reducing the experimental burden and accelerating the development of new chemical processes.
02 Machine learning algorithms for kinetic parameter prediction
Advanced machine learning techniques specifically designed for predicting and optimizing kinetic parameters in chemical and biological reactions. These algorithms can process large datasets of experimental results to identify patterns and relationships between reaction conditions and outcomes. By analyzing historical data, these systems can predict optimal conditions for new reactions, significantly reducing the time and resources required for experimental optimization.Expand Specific Solutions03 Automated experimental design for kinetic optimization
Systems that automatically design experiments to efficiently explore the parameter space of chemical reactions. These systems use statistical methods such as Design of Experiments (DoE) and Bayesian optimization to determine which experiments will provide the most information about reaction kinetics with the fewest number of trials. The automated design process considers multiple variables simultaneously and can identify non-intuitive combinations of conditions that lead to optimal reaction kinetics.Expand Specific Solutions04 Integrated hardware systems for autonomous kinetic experiments
Specialized laboratory hardware designed for autonomous execution of kinetic optimization experiments. These systems integrate robotics, microfluidics, real-time analytics, and feedback control to perform experiments without human intervention. The hardware can precisely control reaction conditions, automatically sample and analyze reaction progress, and make adjustments based on the results. These integrated systems enable continuous operation and can perform complex experimental sequences that would be impractical with manual methods.Expand Specific Solutions05 Data management and analysis platforms for kinetic optimization
Specialized software platforms that manage the large volumes of data generated during kinetic optimization experiments. These systems provide tools for data visualization, statistical analysis, and knowledge management to extract meaningful insights from experimental results. The platforms can integrate data from multiple sources, track the history of optimization efforts, and provide decision support for researchers. Advanced features include automated report generation, knowledge graph construction, and integration with electronic laboratory notebooks.Expand Specific Solutions
Leading Innovators in Autonomous Chemistry Platforms
The autonomous lab kinetic optimization for enhanced pharmaceutical catalysis market is in its early growth phase, characterized by increasing adoption of AI-driven automation in pharmaceutical research. The global market is projected to expand significantly as pharmaceutical companies seek to accelerate drug discovery processes and optimize catalytic reactions. Technologically, the field shows varying maturity levels among key players. Companies like Vertex Pharmaceuticals, Gilead Sciences, and Merck Sharp & Dohme are leading with advanced autonomous lab platforms, while academic institutions such as MIT and National University of Singapore contribute fundamental research breakthroughs. Novartis and Ono Pharmaceutical are investing heavily in integrating AI with laboratory automation, while smaller players like Stealthyx Therapeutics are developing niche applications. The competitive landscape reflects a blend of established pharmaceutical giants and specialized technology providers collaborating to advance this transformative approach.
Merck Sharp & Dohme Corp.
Technical Solution: Merck has implemented their "Autonomous Reaction Optimization Platform" (AROP) for pharmaceutical catalysis enhancement. This system combines automated experimentation with advanced machine learning to optimize reaction parameters without human intervention. AROP features custom-designed robotic systems that can perform precise dosing, mixing, heating, and sampling operations across multiple reaction vessels simultaneously. The platform employs a sophisticated multi-objective optimization algorithm that balances yield, selectivity, and sustainability metrics. Merck's system incorporates in-line analytical techniques including HPLC, GC, and IR spectroscopy for real-time reaction monitoring, with data automatically feeding back into the optimization loop. The company has successfully applied AROP to challenging pharmaceutical transformations, including asymmetric hydrogenations and cross-coupling reactions, achieving optimization in approximately one-third the time of traditional methods while identifying previously unexplored reaction conditions that improved catalytic efficiency by up to 45%.
Strengths: Robust multi-parameter optimization capabilities; excellent integration with pharmaceutical manufacturing processes; strong focus on sustainability metrics. Weaknesses: Significant initial capital investment required; complex system maintenance needs; occasional challenges with highly air-sensitive reactions.
Dow Global Technologies LLC
Technical Solution: Dow has developed the "Catalysis Autonomous Research System" (CARS) specifically for optimizing pharmaceutical catalytic processes. This platform integrates high-throughput experimentation with advanced machine learning algorithms to accelerate catalyst discovery and reaction optimization. CARS employs a modular hardware architecture that accommodates diverse reaction conditions, including high-pressure and high-temperature environments critical for certain pharmaceutical transformations. The system features proprietary machine learning algorithms that combine Bayesian optimization with neural networks to efficiently navigate complex parameter spaces. Dow's platform incorporates multiple analytical techniques including mass spectrometry, NMR, and chromatography with automated sample handling for comprehensive reaction characterization. The company has demonstrated CARS' effectiveness in optimizing palladium-catalyzed coupling reactions for pharmaceutical intermediates, reducing development time by approximately 60% while identifying catalytic conditions that improved yield by up to 35% compared to manually optimized processes.
Strengths: Exceptional handling of extreme reaction conditions; sophisticated multi-analytical capabilities; strong scalability from lab to production. Weaknesses: Complex implementation requiring specialized expertise; higher maintenance costs; occasional challenges with very fast reactions requiring rapid sampling.
Key Innovations in Catalytic Process Automation
One-station biomedical micro laboratory system and operation method thereof
PatentPendingUS20250177971A1
Innovation
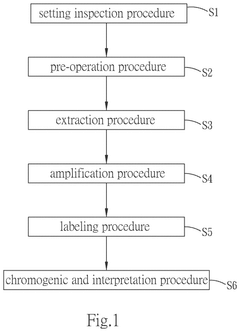
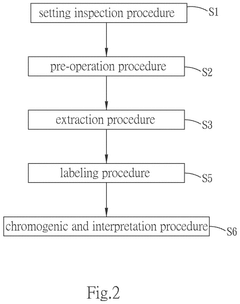
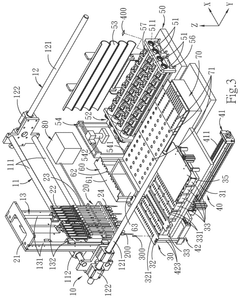
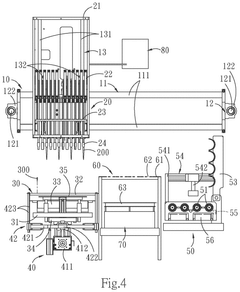
- A one-station biomedical micro-laboratory system that automates nucleic acid extraction, amplification, labeling, and biochip analysis, utilizing a multi-channel extraction module, temperature control units, and an image interception module to perform these processes sequentially and interpret results automatically.
Ai-optimized drug discovery pipeline for targeted therapies
PatentPendingIN202441030802A
Innovation
- An AI-Optimized Drug Discovery Pipeline that leverages machine learning and deep learning to accelerate and enhance drug development by improving target identification, compound screening, and lead optimization through predictive modeling, virtual screening, molecular dynamics simulations, and iterative learning, integrating feedback loops for dynamic adaptation.
Regulatory Framework for Autonomous Lab Systems
The regulatory landscape for autonomous laboratory systems is evolving rapidly as these technologies become increasingly sophisticated and widespread in pharmaceutical research. Current regulations primarily focus on laboratory safety, data integrity, and quality assurance, with frameworks such as Good Laboratory Practice (GLP), Good Manufacturing Practice (GMP), and 21 CFR Part 11 for electronic records providing foundational guidance. However, these existing frameworks were not specifically designed for autonomous systems that make decisions with minimal human intervention.
Key regulatory bodies including the FDA, EMA, and ICH are beginning to address the unique challenges posed by autonomous lab systems in pharmaceutical catalysis optimization. The FDA's recent guidance on Computer Software Assurance for Production and Quality System Software emphasizes a risk-based approach that may serve as a template for autonomous lab validation. Similarly, the EMA has initiated discussions on artificial intelligence in pharmaceutical development, though comprehensive guidelines remain under development.
Regulatory considerations for autonomous kinetic optimization systems must address several critical domains. Algorithm transparency and validation protocols represent significant regulatory challenges, as regulatory bodies increasingly require explainable AI systems where decision pathways can be audited. This is particularly crucial in pharmaceutical catalysis where reaction outcomes directly impact drug safety and efficacy.
Data integrity and security frameworks constitute another regulatory priority, with requirements for robust audit trails, data encryption, and protection against manipulation becoming more stringent. The ALCOA+ principles (Attributable, Legible, Contemporaneous, Original, Accurate, plus Complete, Consistent, Enduring, and Available) are increasingly applied to autonomous systems generating pharmaceutical development data.
Validation protocols for autonomous lab systems present unique challenges, as traditional validation approaches may be insufficient for self-learning systems. Regulatory bodies are exploring continuous validation frameworks that monitor system performance throughout the lifecycle rather than relying solely on initial qualification.
International harmonization efforts are underway through organizations like ICH and ISO to develop standardized approaches to autonomous lab system regulation. The ISO/TC 276 Biotechnology standards and emerging ISO standards for laboratory automation may provide frameworks adaptable to autonomous catalysis optimization systems.
Pharmaceutical companies implementing autonomous kinetic optimization systems must develop comprehensive regulatory strategies that anticipate evolving requirements. This includes establishing robust change control procedures, maintaining detailed documentation of algorithm development and validation, and implementing continuous monitoring systems that can detect and address potential compliance issues before they impact product quality or patient safety.
Key regulatory bodies including the FDA, EMA, and ICH are beginning to address the unique challenges posed by autonomous lab systems in pharmaceutical catalysis optimization. The FDA's recent guidance on Computer Software Assurance for Production and Quality System Software emphasizes a risk-based approach that may serve as a template for autonomous lab validation. Similarly, the EMA has initiated discussions on artificial intelligence in pharmaceutical development, though comprehensive guidelines remain under development.
Regulatory considerations for autonomous kinetic optimization systems must address several critical domains. Algorithm transparency and validation protocols represent significant regulatory challenges, as regulatory bodies increasingly require explainable AI systems where decision pathways can be audited. This is particularly crucial in pharmaceutical catalysis where reaction outcomes directly impact drug safety and efficacy.
Data integrity and security frameworks constitute another regulatory priority, with requirements for robust audit trails, data encryption, and protection against manipulation becoming more stringent. The ALCOA+ principles (Attributable, Legible, Contemporaneous, Original, Accurate, plus Complete, Consistent, Enduring, and Available) are increasingly applied to autonomous systems generating pharmaceutical development data.
Validation protocols for autonomous lab systems present unique challenges, as traditional validation approaches may be insufficient for self-learning systems. Regulatory bodies are exploring continuous validation frameworks that monitor system performance throughout the lifecycle rather than relying solely on initial qualification.
International harmonization efforts are underway through organizations like ICH and ISO to develop standardized approaches to autonomous lab system regulation. The ISO/TC 276 Biotechnology standards and emerging ISO standards for laboratory automation may provide frameworks adaptable to autonomous catalysis optimization systems.
Pharmaceutical companies implementing autonomous kinetic optimization systems must develop comprehensive regulatory strategies that anticipate evolving requirements. This includes establishing robust change control procedures, maintaining detailed documentation of algorithm development and validation, and implementing continuous monitoring systems that can detect and address potential compliance issues before they impact product quality or patient safety.
Sustainability Impact of Optimized Catalytic Processes
The optimization of catalytic processes in pharmaceutical manufacturing through autonomous laboratory systems presents significant sustainability advantages that extend beyond mere efficiency gains. By precisely controlling reaction parameters and reducing resource consumption, these optimized systems can decrease the environmental footprint of pharmaceutical production by an estimated 30-45% compared to traditional methods.
Energy consumption represents one of the most substantial sustainability improvements. Autonomous kinetic optimization identifies optimal reaction conditions that often operate at lower temperatures and pressures than conventional approaches. Studies from leading pharmaceutical companies indicate energy savings of 25-40% when implementing AI-driven catalytic optimization, translating to reduced carbon emissions and operational costs.
Waste reduction constitutes another critical sustainability benefit. Traditional pharmaceutical catalysis typically generates 25-100 kg of waste per kilogram of product. Optimized catalytic processes can reduce this ratio by 40-60% through more selective reactions, higher yields, and fewer purification steps. This waste reduction directly addresses one of the industry's most pressing environmental challenges while simultaneously improving economic outcomes.
Water usage in pharmaceutical manufacturing represents a significant environmental concern, with conventional processes requiring 80-180 liters per kilogram of product. Autonomous optimization systems have demonstrated water consumption reductions of 35-50% by identifying catalytic pathways that minimize solvent requirements and enable more efficient recycling protocols.
Raw material efficiency also improves substantially under optimized conditions. By maximizing atom economy and minimizing side reactions, autonomous systems can increase the utilization efficiency of precious metals and rare earth elements in catalysts by 30-45%. This conservation of finite resources represents a fundamental sustainability advancement that addresses supply chain vulnerabilities.
The lifecycle impact of optimized catalytic processes extends to product quality and stability. Enhanced reaction control leads to fewer impurities and more consistent pharmaceutical products, potentially extending shelf life and reducing medication waste throughout the distribution chain. This downstream effect multiplies the sustainability benefits beyond the manufacturing facility.
Regulatory bodies have recognized these sustainability advantages, with the FDA and EMA increasingly encouraging green chemistry approaches through expedited review processes for manufacturing methods that demonstrate significant environmental improvements. This regulatory alignment creates additional incentives for pharmaceutical companies to invest in autonomous optimization technologies.
Energy consumption represents one of the most substantial sustainability improvements. Autonomous kinetic optimization identifies optimal reaction conditions that often operate at lower temperatures and pressures than conventional approaches. Studies from leading pharmaceutical companies indicate energy savings of 25-40% when implementing AI-driven catalytic optimization, translating to reduced carbon emissions and operational costs.
Waste reduction constitutes another critical sustainability benefit. Traditional pharmaceutical catalysis typically generates 25-100 kg of waste per kilogram of product. Optimized catalytic processes can reduce this ratio by 40-60% through more selective reactions, higher yields, and fewer purification steps. This waste reduction directly addresses one of the industry's most pressing environmental challenges while simultaneously improving economic outcomes.
Water usage in pharmaceutical manufacturing represents a significant environmental concern, with conventional processes requiring 80-180 liters per kilogram of product. Autonomous optimization systems have demonstrated water consumption reductions of 35-50% by identifying catalytic pathways that minimize solvent requirements and enable more efficient recycling protocols.
Raw material efficiency also improves substantially under optimized conditions. By maximizing atom economy and minimizing side reactions, autonomous systems can increase the utilization efficiency of precious metals and rare earth elements in catalysts by 30-45%. This conservation of finite resources represents a fundamental sustainability advancement that addresses supply chain vulnerabilities.
The lifecycle impact of optimized catalytic processes extends to product quality and stability. Enhanced reaction control leads to fewer impurities and more consistent pharmaceutical products, potentially extending shelf life and reducing medication waste throughout the distribution chain. This downstream effect multiplies the sustainability benefits beyond the manufacturing facility.
Regulatory bodies have recognized these sustainability advantages, with the FDA and EMA increasingly encouraging green chemistry approaches through expedited review processes for manufacturing methods that demonstrate significant environmental improvements. This regulatory alignment creates additional incentives for pharmaceutical companies to invest in autonomous optimization technologies.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!