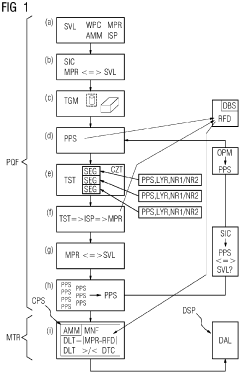
Regulatory Framework for Autonomous Lab Quality Assurance in Manufacturing
SEP 25, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Autonomous QA Regulatory Background and Objectives
The evolution of quality assurance in manufacturing has undergone significant transformation over the past decades, from manual inspection processes to increasingly automated systems. The emergence of autonomous laboratory quality assurance represents the latest frontier in this progression, driven by advancements in artificial intelligence, machine learning, and robotics. This technological shift necessitates a corresponding evolution in regulatory frameworks to ensure that autonomous QA systems maintain compliance with established quality standards while enabling innovation.
Historically, quality assurance regulations in manufacturing have been developed within industry-specific contexts, such as pharmaceutical GMP (Good Manufacturing Practice), ISO standards for various industries, and FDA regulations for medical devices and food products. These frameworks were primarily designed for human-operated quality control processes, creating a regulatory gap as autonomous systems become more prevalent.
The primary objective of developing a regulatory framework for autonomous lab quality assurance is to establish clear guidelines that ensure the reliability, accuracy, and compliance of AI-driven quality control systems. This framework must address the unique challenges posed by autonomous systems, including validation of AI algorithms, data integrity, system transparency, and the establishment of appropriate human oversight mechanisms.
Current regulatory landscapes across major manufacturing regions—North America, Europe, and Asia—show varying approaches to autonomous systems in quality control. The EU has taken steps toward addressing AI systems through its AI Act, while the FDA in the United States has begun exploring regulatory pathways for AI/ML-based technologies in healthcare manufacturing. However, comprehensive frameworks specifically addressing autonomous laboratory QA remain underdeveloped globally.
The technological trajectory indicates a need for regulations that can adapt to rapidly evolving capabilities in machine learning, computer vision, and automated decision-making. These regulations must balance innovation enablement with risk management, particularly in critical industries where quality assurance directly impacts public safety.
Key regulatory considerations include validation protocols for autonomous QA systems, standards for algorithm transparency and explainability, requirements for continuous monitoring and performance verification, and frameworks for establishing the appropriate level of human supervision. Additionally, data governance standards must address the collection, storage, and processing of the vast datasets used to train and operate these systems.
The development of this regulatory framework aims to create a harmonized approach that can be adopted across different manufacturing sectors and geographical regions, facilitating global trade while maintaining stringent quality standards in an increasingly automated manufacturing ecosystem.
Historically, quality assurance regulations in manufacturing have been developed within industry-specific contexts, such as pharmaceutical GMP (Good Manufacturing Practice), ISO standards for various industries, and FDA regulations for medical devices and food products. These frameworks were primarily designed for human-operated quality control processes, creating a regulatory gap as autonomous systems become more prevalent.
The primary objective of developing a regulatory framework for autonomous lab quality assurance is to establish clear guidelines that ensure the reliability, accuracy, and compliance of AI-driven quality control systems. This framework must address the unique challenges posed by autonomous systems, including validation of AI algorithms, data integrity, system transparency, and the establishment of appropriate human oversight mechanisms.
Current regulatory landscapes across major manufacturing regions—North America, Europe, and Asia—show varying approaches to autonomous systems in quality control. The EU has taken steps toward addressing AI systems through its AI Act, while the FDA in the United States has begun exploring regulatory pathways for AI/ML-based technologies in healthcare manufacturing. However, comprehensive frameworks specifically addressing autonomous laboratory QA remain underdeveloped globally.
The technological trajectory indicates a need for regulations that can adapt to rapidly evolving capabilities in machine learning, computer vision, and automated decision-making. These regulations must balance innovation enablement with risk management, particularly in critical industries where quality assurance directly impacts public safety.
Key regulatory considerations include validation protocols for autonomous QA systems, standards for algorithm transparency and explainability, requirements for continuous monitoring and performance verification, and frameworks for establishing the appropriate level of human supervision. Additionally, data governance standards must address the collection, storage, and processing of the vast datasets used to train and operate these systems.
The development of this regulatory framework aims to create a harmonized approach that can be adopted across different manufacturing sectors and geographical regions, facilitating global trade while maintaining stringent quality standards in an increasingly automated manufacturing ecosystem.
Market Demand Analysis for Automated Lab QA Systems
The global market for automated laboratory quality assurance systems in manufacturing is experiencing robust growth, driven by increasing regulatory pressures and the need for consistent product quality. Current market estimates value this sector at approximately 3.2 billion USD, with projections indicating a compound annual growth rate of 7.8% through 2028. This growth trajectory reflects the manufacturing industry's accelerating adoption of autonomous quality control technologies to meet stringent compliance requirements.
Manufacturing companies across pharmaceuticals, food and beverage, chemicals, and electronics sectors are demonstrating heightened demand for autonomous lab QA systems. This demand stems primarily from regulatory bodies worldwide implementing more rigorous quality standards that traditional manual testing processes struggle to satisfy consistently. The FDA in the United States, the EMA in Europe, and similar regulatory authorities in Asia have all strengthened compliance requirements, creating substantial market pull for automated solutions.
Cost reduction represents another significant market driver, with manufacturers reporting 30-45% decreases in quality-related expenses after implementing autonomous lab QA systems. These savings derive from reduced labor costs, minimized product recalls, decreased waste from quality failures, and optimized resource allocation. The return on investment period for such systems has shortened from 3-4 years to 18-24 months in many implementation cases, further accelerating market adoption.
The COVID-19 pandemic has markedly accelerated market demand, as manufacturing facilities faced unprecedented challenges maintaining quality standards with reduced on-site personnel. This catalyzed a 22% increase in inquiries for autonomous lab systems during 2020-2021, according to industry surveys. The resulting operational resilience demonstrated by early adopters has convinced many previously hesitant manufacturers to pursue implementation plans.
Regional analysis reveals North America currently leads market consumption at 38% of global demand, followed by Europe (29%) and Asia-Pacific (24%). However, the highest growth rates are observed in emerging markets, particularly in India, China, and Brazil, where manufacturing sectors are rapidly modernizing quality infrastructure to meet international export standards.
By industry vertical, pharmaceutical manufacturing represents the largest market segment (41%), followed by food and beverage (23%), chemicals (17%), and electronics (12%). The pharmaceutical sector's dominance stems from exceptionally stringent regulatory requirements and the critical nature of product quality for patient safety, creating compelling incentives for automation investment.
Manufacturing companies across pharmaceuticals, food and beverage, chemicals, and electronics sectors are demonstrating heightened demand for autonomous lab QA systems. This demand stems primarily from regulatory bodies worldwide implementing more rigorous quality standards that traditional manual testing processes struggle to satisfy consistently. The FDA in the United States, the EMA in Europe, and similar regulatory authorities in Asia have all strengthened compliance requirements, creating substantial market pull for automated solutions.
Cost reduction represents another significant market driver, with manufacturers reporting 30-45% decreases in quality-related expenses after implementing autonomous lab QA systems. These savings derive from reduced labor costs, minimized product recalls, decreased waste from quality failures, and optimized resource allocation. The return on investment period for such systems has shortened from 3-4 years to 18-24 months in many implementation cases, further accelerating market adoption.
The COVID-19 pandemic has markedly accelerated market demand, as manufacturing facilities faced unprecedented challenges maintaining quality standards with reduced on-site personnel. This catalyzed a 22% increase in inquiries for autonomous lab systems during 2020-2021, according to industry surveys. The resulting operational resilience demonstrated by early adopters has convinced many previously hesitant manufacturers to pursue implementation plans.
Regional analysis reveals North America currently leads market consumption at 38% of global demand, followed by Europe (29%) and Asia-Pacific (24%). However, the highest growth rates are observed in emerging markets, particularly in India, China, and Brazil, where manufacturing sectors are rapidly modernizing quality infrastructure to meet international export standards.
By industry vertical, pharmaceutical manufacturing represents the largest market segment (41%), followed by food and beverage (23%), chemicals (17%), and electronics (12%). The pharmaceutical sector's dominance stems from exceptionally stringent regulatory requirements and the critical nature of product quality for patient safety, creating compelling incentives for automation investment.
Current Regulatory Landscape and Technical Challenges
The regulatory landscape for autonomous lab quality assurance in manufacturing is currently fragmented across different jurisdictions, with varying degrees of specificity regarding AI-driven quality control systems. In the United States, the FDA's framework for Computer Software Assurance (CSA) represents a shift toward risk-based validation approaches that could accommodate autonomous systems, though explicit guidance for laboratory automation remains limited. The EU's regulatory approach emphasizes human oversight through the AI Act, which categorizes quality assurance systems as "high-risk" applications requiring rigorous validation and transparency.
ISO/IEC standards, particularly ISO 9001:2015 for quality management systems and ISO/IEC 17025 for testing and calibration laboratories, provide general frameworks but lack specific provisions for autonomous decision-making in quality assurance. This regulatory gap creates uncertainty for manufacturers implementing advanced AI-driven laboratory systems, as compliance pathways remain unclear.
Technical challenges compound these regulatory uncertainties. Data integrity represents a primary concern, as autonomous quality assurance systems must maintain unimpeachable audit trails while processing vast quantities of testing data. Current validation methodologies struggle to accommodate the dynamic nature of machine learning algorithms that continuously evolve through operational data, creating a fundamental tension with traditional validation approaches that assume static system behavior.
Interoperability presents another significant hurdle, as laboratory information management systems (LIMS), manufacturing execution systems (MES), and enterprise resource planning (ERP) platforms often utilize proprietary data formats and communication protocols. This fragmentation impedes the seamless integration necessary for autonomous quality assurance systems to access comprehensive production and testing data across the manufacturing ecosystem.
Cybersecurity vulnerabilities introduce additional complexity, as autonomous laboratory systems represent potential attack vectors that could compromise product quality or intellectual property. Current regulatory frameworks inadequately address the intersection of cybersecurity and quality assurance, leaving manufacturers without clear guidance on securing these critical systems against emerging threats.
The validation of AI decision-making algorithms represents perhaps the most formidable technical challenge. Traditional validation approaches rely on predetermined test cases with known outcomes, but autonomous systems making complex quality decisions based on multidimensional data analysis resist such simplistic validation methods. Regulators and industry stakeholders alike struggle to define appropriate validation methodologies that can provide reasonable assurance of system performance without stifling innovation through excessive documentation requirements.
ISO/IEC standards, particularly ISO 9001:2015 for quality management systems and ISO/IEC 17025 for testing and calibration laboratories, provide general frameworks but lack specific provisions for autonomous decision-making in quality assurance. This regulatory gap creates uncertainty for manufacturers implementing advanced AI-driven laboratory systems, as compliance pathways remain unclear.
Technical challenges compound these regulatory uncertainties. Data integrity represents a primary concern, as autonomous quality assurance systems must maintain unimpeachable audit trails while processing vast quantities of testing data. Current validation methodologies struggle to accommodate the dynamic nature of machine learning algorithms that continuously evolve through operational data, creating a fundamental tension with traditional validation approaches that assume static system behavior.
Interoperability presents another significant hurdle, as laboratory information management systems (LIMS), manufacturing execution systems (MES), and enterprise resource planning (ERP) platforms often utilize proprietary data formats and communication protocols. This fragmentation impedes the seamless integration necessary for autonomous quality assurance systems to access comprehensive production and testing data across the manufacturing ecosystem.
Cybersecurity vulnerabilities introduce additional complexity, as autonomous laboratory systems represent potential attack vectors that could compromise product quality or intellectual property. Current regulatory frameworks inadequately address the intersection of cybersecurity and quality assurance, leaving manufacturers without clear guidance on securing these critical systems against emerging threats.
The validation of AI decision-making algorithms represents perhaps the most formidable technical challenge. Traditional validation approaches rely on predetermined test cases with known outcomes, but autonomous systems making complex quality decisions based on multidimensional data analysis resist such simplistic validation methods. Regulators and industry stakeholders alike struggle to define appropriate validation methodologies that can provide reasonable assurance of system performance without stifling innovation through excessive documentation requirements.
Current Autonomous QA Implementation Approaches
01 Automated quality control systems for laboratory environments
Automated systems that monitor and control quality in laboratory settings, ensuring compliance with regulatory standards. These systems use sensors, data analytics, and AI to continuously monitor lab conditions, equipment performance, and test results. They can automatically detect anomalies, trigger alerts, and document quality metrics for regulatory reporting purposes, reducing human error and increasing reliability in quality assurance processes.- Automated quality control systems for laboratory environments: Autonomous laboratory quality assurance systems that incorporate automated monitoring and testing procedures to ensure compliance with regulatory standards. These systems utilize sensors, cameras, and other monitoring devices to continuously track laboratory conditions, equipment performance, and test results. The automation reduces human error, increases testing consistency, and provides real-time data for regulatory compliance documentation.
- AI-driven regulatory compliance frameworks: Artificial intelligence and machine learning technologies applied to laboratory quality assurance to interpret regulatory requirements, predict compliance issues, and recommend corrective actions. These systems analyze historical data, current regulations, and laboratory operations to identify potential regulatory gaps and suggest improvements. The AI frameworks continuously learn from new regulatory updates and laboratory performance data to enhance compliance strategies.
- Digital validation and verification protocols: Digital systems for validating laboratory processes, equipment calibration, and test results to meet regulatory requirements. These protocols include electronic signatures, audit trails, and automated documentation to ensure data integrity and traceability. The digital validation frameworks streamline the quality assurance process while maintaining compliance with regulations such as GLP, GMP, and ISO standards.
- Remote monitoring and distributed quality management systems: Networked quality assurance systems that enable remote monitoring, management, and auditing of laboratory operations across multiple facilities. These systems utilize cloud computing, IoT devices, and secure communication protocols to maintain consistent quality standards across distributed laboratory environments. Remote capabilities allow for real-time oversight by quality assurance personnel and regulatory authorities without physical presence.
- Risk-based quality assurance methodologies: Systematic approaches to laboratory quality assurance that prioritize resources based on risk assessment and critical control points. These methodologies identify high-risk processes, materials, or tests that require enhanced monitoring and validation. The risk-based frameworks adapt quality assurance intensity according to potential impact on results, patient safety, or regulatory compliance, optimizing resource allocation while maintaining quality standards.
02 Regulatory compliance management frameworks for autonomous laboratories
Comprehensive frameworks designed to ensure autonomous laboratories meet regulatory requirements across different jurisdictions. These systems track regulatory changes, manage documentation, and implement compliance protocols specific to laboratory operations. They include features for audit trails, electronic signatures, and validation processes that satisfy requirements from agencies like FDA, EPA, and international standards organizations, while maintaining operational efficiency in automated lab environments.Expand Specific Solutions03 AI-driven quality assurance for laboratory testing and analysis
Artificial intelligence systems that enhance quality assurance in laboratory testing procedures. These solutions use machine learning algorithms to validate test results, identify patterns in quality deviations, and predict potential issues before they affect test outcomes. The AI systems can analyze complex datasets from multiple instruments, standardize quality metrics across different testing protocols, and continuously improve through feedback loops, ensuring consistent and reliable laboratory results.Expand Specific Solutions04 Integrated laboratory information management systems with regulatory compliance features
Comprehensive laboratory information management systems (LIMS) that incorporate regulatory compliance features for autonomous lab environments. These platforms integrate data from laboratory instruments, quality control processes, and regulatory documentation into a unified system. They provide automated workflows for sample tracking, test scheduling, result validation, and compliance reporting, while maintaining data integrity and security required by various regulatory frameworks.Expand Specific Solutions05 Remote monitoring and validation systems for autonomous laboratory operations
Systems that enable remote monitoring, control, and validation of autonomous laboratory operations to ensure quality standards and regulatory compliance. These solutions provide real-time visibility into laboratory processes, equipment status, and environmental conditions from off-site locations. They include secure communication protocols, remote authorization mechanisms, and digital validation tools that allow quality assurance personnel and regulatory authorities to oversee operations, conduct virtual inspections, and approve processes without physical presence in the laboratory.Expand Specific Solutions
Key Regulatory Bodies and Industry Stakeholders
The regulatory framework for autonomous lab quality assurance in manufacturing is evolving rapidly as the industry transitions from early adoption to growth phase. The market is projected to reach significant scale as manufacturing sectors increasingly embrace automation for quality control processes. From a technological maturity perspective, established industrial leaders like Siemens AG and Bio-Rad Laboratories are driving standardization efforts, while specialized quality assurance firms such as MasterControl Solutions and Beijing Quality Technology are developing tailored solutions. Academic institutions like Zhejiang University are contributing fundamental research. The competitive landscape features a mix of traditional industrial automation companies, specialized quality assurance providers, and research institutions collaborating to establish regulatory frameworks that balance innovation with compliance requirements in autonomous laboratory environments.
Siemens AG
Technical Solution: Siemens AG has developed a comprehensive Autonomous Lab Quality Assurance framework for manufacturing environments that integrates their Digital Enterprise portfolio with advanced regulatory compliance tools. Their solution implements a multi-layered approach combining Industrial IoT sensors, edge computing, and cloud-based analytics to create a closed-loop quality control system. The framework features real-time monitoring capabilities that automatically detect deviations from quality parameters and trigger corrective actions without human intervention. Siemens' system incorporates machine learning algorithms that continuously improve detection accuracy while maintaining detailed audit trails that satisfy FDA 21 CFR Part 11, EU GMP Annex 11, and ISO 13485 requirements. Their Digital Twin technology enables virtual validation of quality processes before physical implementation, significantly reducing compliance risks during actual production.
Strengths: Extensive integration with existing manufacturing systems; robust regulatory compliance documentation; proven scalability across multiple industries. Weaknesses: Higher implementation costs compared to standalone solutions; requires significant technical expertise for full deployment; potential vendor lock-in with proprietary protocols.
Bio-Rad Laboratories, Inc.
Technical Solution: Bio-Rad has pioneered an Autonomous Quality Management System (AQMS) specifically designed for life sciences manufacturing that addresses regulatory requirements while maximizing automation. Their framework incorporates continuous monitoring technologies with built-in compliance to FDA, EMA, and ISO standards. The system features autonomous decision-making capabilities based on predefined quality parameters, automatically triggering corrective actions when deviations occur. Bio-Rad's solution includes specialized modules for bioprocessing quality control with automated documentation generation that maintains GxP compliance. Their platform implements risk-based testing approaches that dynamically adjust sampling frequencies based on historical data and process stability metrics, optimizing resource allocation while maintaining regulatory compliance. The system also features automated validation protocols that significantly reduce the documentation burden associated with traditional quality assurance processes.
Strengths: Deep domain expertise in life sciences regulatory requirements; purpose-built for biological manufacturing processes; excellent documentation automation. Weaknesses: Limited application outside biotech/pharmaceutical industries; higher initial validation costs; requires specialized knowledge for configuration.
Critical Standards and Certification Requirements
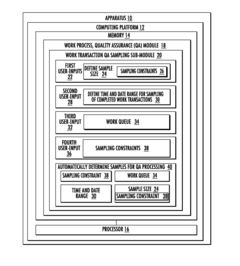
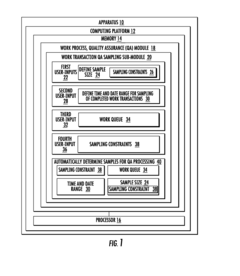
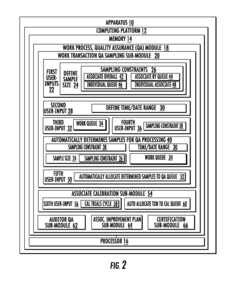
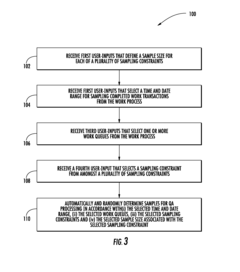
Systems for managing quality assurance of work assignments
PatentInactiveUS20160132813A1
Innovation
- An automated quality assurance system that assesses work performance, collects and tracks quality metrics, calibrates work transactions to standards, audits the auditors, and implements associate improvement plans, enabling real-time monitoring and systematic adjustments of quality scores.
Method for process qualification of a production process, method for production, production machine
PatentInactiveEP4361914A1
Innovation
- Implement an in-situ testing method using a manufacturing machine with an arrangement for measuring material properties like electrical conductivity during production, allowing for real-time monitoring and adjustment of process parameters, and integrating this with a computer system for process qualification and quality assurance.
Cross-Border Compliance Considerations
The implementation of autonomous lab quality assurance systems in manufacturing environments necessitates careful consideration of cross-border regulatory frameworks. Manufacturing operations spanning multiple countries face complex compliance challenges due to varying regulatory standards across jurisdictions. The European Union's approach to autonomous quality systems emphasizes human oversight through the AI Act, requiring manufacturers to maintain human verification of critical quality decisions even when using autonomous systems. This contrasts with the United States' more flexible, sector-specific regulatory approach that allows greater autonomy in certain manufacturing contexts.
Asian regulatory frameworks present additional complexity, with Japan's Society 5.0 initiative promoting integration of autonomous systems while maintaining strict quality control requirements. China's dual-focus approach emphasizes both technological advancement and stringent data security measures, particularly affecting cross-border data flows from quality assurance systems. These regional differences create significant compliance challenges for global manufacturers implementing unified autonomous lab quality assurance platforms.
Data sovereignty requirements represent a critical cross-border consideration, with many jurisdictions imposing restrictions on where manufacturing quality data can be stored and processed. The EU's GDPR and China's Personal Information Protection Law establish strict requirements for cross-border data transfers, necessitating careful architectural design of autonomous quality systems to accommodate regional data localization requirements while maintaining global operational consistency.
Certification and validation protocols also vary significantly across borders. The International Organization for Standardization (ISO) provides frameworks like ISO/IEC 27001 for information security and ISO 9001 for quality management, but implementation requirements differ regionally. Manufacturers must navigate these differences while ensuring their autonomous quality systems maintain consistent performance across all operational locations.
Harmonization efforts through international standards bodies offer potential pathways to simplify cross-border compliance. The International Medical Device Regulators Forum (IMDRF) and similar organizations are developing frameworks specifically addressing autonomous quality systems in regulated manufacturing. These initiatives aim to establish common validation protocols and interoperability standards that could reduce regulatory fragmentation, though full harmonization remains a distant goal.
Manufacturers implementing autonomous lab quality assurance systems must develop comprehensive regulatory mapping strategies that account for jurisdictional differences while maintaining operational efficiency. This typically involves creating modular system architectures that can adapt to local requirements while preserving core functionality and establishing clear documentation trails that satisfy diverse regulatory reporting requirements across all operational regions.
Asian regulatory frameworks present additional complexity, with Japan's Society 5.0 initiative promoting integration of autonomous systems while maintaining strict quality control requirements. China's dual-focus approach emphasizes both technological advancement and stringent data security measures, particularly affecting cross-border data flows from quality assurance systems. These regional differences create significant compliance challenges for global manufacturers implementing unified autonomous lab quality assurance platforms.
Data sovereignty requirements represent a critical cross-border consideration, with many jurisdictions imposing restrictions on where manufacturing quality data can be stored and processed. The EU's GDPR and China's Personal Information Protection Law establish strict requirements for cross-border data transfers, necessitating careful architectural design of autonomous quality systems to accommodate regional data localization requirements while maintaining global operational consistency.
Certification and validation protocols also vary significantly across borders. The International Organization for Standardization (ISO) provides frameworks like ISO/IEC 27001 for information security and ISO 9001 for quality management, but implementation requirements differ regionally. Manufacturers must navigate these differences while ensuring their autonomous quality systems maintain consistent performance across all operational locations.
Harmonization efforts through international standards bodies offer potential pathways to simplify cross-border compliance. The International Medical Device Regulators Forum (IMDRF) and similar organizations are developing frameworks specifically addressing autonomous quality systems in regulated manufacturing. These initiatives aim to establish common validation protocols and interoperability standards that could reduce regulatory fragmentation, though full harmonization remains a distant goal.
Manufacturers implementing autonomous lab quality assurance systems must develop comprehensive regulatory mapping strategies that account for jurisdictional differences while maintaining operational efficiency. This typically involves creating modular system architectures that can adapt to local requirements while preserving core functionality and establishing clear documentation trails that satisfy diverse regulatory reporting requirements across all operational regions.
Risk Management Frameworks for Autonomous QA
Risk management frameworks for autonomous quality assurance systems in manufacturing environments must address the unique challenges posed by AI-driven decision-making processes. Traditional risk management approaches like ISO 31000 and COSO ERM provide foundational principles but require significant adaptation for autonomous systems. These frameworks must be enhanced to account for the dynamic nature of machine learning algorithms that continuously evolve based on new data inputs.
A comprehensive risk management framework for autonomous QA should incorporate tiered risk assessment methodologies. At the base level, potential failure modes must be identified through techniques such as Failure Mode and Effects Analysis (FMEA) specifically tailored to autonomous systems. This includes evaluating algorithm drift risks, data quality degradation scenarios, and potential false positive/negative outcomes in quality inspection processes.
Regulatory compliance represents a critical dimension within these frameworks. As autonomous systems increasingly make quality decisions without human intervention, frameworks must ensure alignment with industry standards such as ISO 9001:2015 for quality management, ISO/IEC 27001 for information security, and emerging standards specific to AI systems like IEEE P7000 series. The framework should establish clear audit trails that demonstrate regulatory compliance even when decisions are made autonomously.
Real-time risk monitoring constitutes another essential component, enabling continuous assessment of system performance against established risk thresholds. This includes implementing statistical process control methods adapted for autonomous systems that can detect anomalies in algorithm behavior before they impact product quality. Advanced frameworks incorporate digital twins that simulate potential risk scenarios without affecting actual production environments.
Governance structures within these frameworks must clearly delineate responsibility boundaries between human operators and autonomous systems. This includes establishing escalation protocols when risk levels exceed predetermined thresholds and defining human override mechanisms for high-consequence decisions. The most sophisticated frameworks implement "ethical guardrails" that prevent autonomous systems from making decisions that could compromise product safety or quality regardless of efficiency gains.
Resilience planning represents the final critical element, focusing on system recovery capabilities when failures occur. This includes redundancy mechanisms, fallback procedures to manual inspection when necessary, and continuous learning loops that incorporate incident data into future risk assessments. The framework should establish clear metrics for measuring risk management effectiveness, including false detection rates, algorithm confidence scores, and time-to-recovery measurements.
A comprehensive risk management framework for autonomous QA should incorporate tiered risk assessment methodologies. At the base level, potential failure modes must be identified through techniques such as Failure Mode and Effects Analysis (FMEA) specifically tailored to autonomous systems. This includes evaluating algorithm drift risks, data quality degradation scenarios, and potential false positive/negative outcomes in quality inspection processes.
Regulatory compliance represents a critical dimension within these frameworks. As autonomous systems increasingly make quality decisions without human intervention, frameworks must ensure alignment with industry standards such as ISO 9001:2015 for quality management, ISO/IEC 27001 for information security, and emerging standards specific to AI systems like IEEE P7000 series. The framework should establish clear audit trails that demonstrate regulatory compliance even when decisions are made autonomously.
Real-time risk monitoring constitutes another essential component, enabling continuous assessment of system performance against established risk thresholds. This includes implementing statistical process control methods adapted for autonomous systems that can detect anomalies in algorithm behavior before they impact product quality. Advanced frameworks incorporate digital twins that simulate potential risk scenarios without affecting actual production environments.
Governance structures within these frameworks must clearly delineate responsibility boundaries between human operators and autonomous systems. This includes establishing escalation protocols when risk levels exceed predetermined thresholds and defining human override mechanisms for high-consequence decisions. The most sophisticated frameworks implement "ethical guardrails" that prevent autonomous systems from making decisions that could compromise product safety or quality regardless of efficiency gains.
Resilience planning represents the final critical element, focusing on system recovery capabilities when failures occur. This includes redundancy mechanisms, fallback procedures to manual inspection when necessary, and continuous learning loops that incorporate incident data into future risk assessments. The framework should establish clear metrics for measuring risk management effectiveness, including false detection rates, algorithm confidence scores, and time-to-recovery measurements.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!