Challenges and Solutions for Integrating Autonomous Lab in Biotechnology
SEP 25, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Autonomous Lab Integration Background and Objectives
The integration of autonomous laboratories in biotechnology represents a transformative shift in how scientific research and development are conducted. Originating from the convergence of robotics, artificial intelligence, and biotechnology, autonomous labs have evolved from simple automated systems to sophisticated platforms capable of designing, executing, and analyzing experiments with minimal human intervention. This technological evolution has been accelerated by advancements in machine learning algorithms, robotics precision, and cloud computing infrastructure over the past decade.
The primary objective of autonomous lab integration is to overcome the inherent limitations of traditional laboratory workflows, which are often characterized by labor-intensive processes, human error, and limited throughput. By automating routine tasks and decision-making processes, autonomous labs aim to dramatically increase experimental efficiency, reproducibility, and data quality while reducing operational costs and time-to-discovery in biotechnology research.
Current technological trajectories suggest that autonomous labs will continue to evolve toward greater intelligence and autonomy, with systems increasingly capable of self-optimization and novel discovery. The integration of these technologies is expected to fundamentally reshape drug discovery, synthetic biology, and materials science by enabling exploration of experimental spaces that would be impractical with conventional approaches.
Market analyses indicate growing demand for autonomous lab solutions across pharmaceutical, agricultural biotechnology, and academic research sectors, driven by pressures to accelerate innovation cycles and reduce R&D costs. This demand is further amplified by the increasing complexity of biological research questions and the exponential growth in available biological data that requires automated processing and analysis.
The successful integration of autonomous labs in biotechnology necessitates addressing several key objectives: developing standardized interfaces between diverse laboratory equipment, creating robust software architectures that can coordinate complex experimental workflows, establishing validation protocols that ensure reliability of autonomous systems, and designing human-machine interfaces that enable effective oversight and intervention when necessary.
Additionally, autonomous lab integration must navigate the challenge of balancing automation with scientific creativity and intuition. The ultimate goal is not to replace human scientists but to augment their capabilities, allowing researchers to focus on hypothesis generation, experimental design, and interpretation of results while automated systems handle execution and data collection with unprecedented speed and precision.
The primary objective of autonomous lab integration is to overcome the inherent limitations of traditional laboratory workflows, which are often characterized by labor-intensive processes, human error, and limited throughput. By automating routine tasks and decision-making processes, autonomous labs aim to dramatically increase experimental efficiency, reproducibility, and data quality while reducing operational costs and time-to-discovery in biotechnology research.
Current technological trajectories suggest that autonomous labs will continue to evolve toward greater intelligence and autonomy, with systems increasingly capable of self-optimization and novel discovery. The integration of these technologies is expected to fundamentally reshape drug discovery, synthetic biology, and materials science by enabling exploration of experimental spaces that would be impractical with conventional approaches.
Market analyses indicate growing demand for autonomous lab solutions across pharmaceutical, agricultural biotechnology, and academic research sectors, driven by pressures to accelerate innovation cycles and reduce R&D costs. This demand is further amplified by the increasing complexity of biological research questions and the exponential growth in available biological data that requires automated processing and analysis.
The successful integration of autonomous labs in biotechnology necessitates addressing several key objectives: developing standardized interfaces between diverse laboratory equipment, creating robust software architectures that can coordinate complex experimental workflows, establishing validation protocols that ensure reliability of autonomous systems, and designing human-machine interfaces that enable effective oversight and intervention when necessary.
Additionally, autonomous lab integration must navigate the challenge of balancing automation with scientific creativity and intuition. The ultimate goal is not to replace human scientists but to augment their capabilities, allowing researchers to focus on hypothesis generation, experimental design, and interpretation of results while automated systems handle execution and data collection with unprecedented speed and precision.
Market Demand Analysis for Lab Automation in Biotechnology
The global market for laboratory automation in biotechnology has been experiencing robust growth, driven by increasing demand for precision, efficiency, and reproducibility in research and development processes. The current market size is estimated to exceed $5 billion, with projections indicating a compound annual growth rate of approximately 8-10% over the next five years. This growth trajectory is particularly pronounced in regions with established biotechnology hubs, including North America, Europe, and increasingly, Asia-Pacific.
The primary market drivers for autonomous lab integration in biotechnology stem from several converging factors. Pharmaceutical and biotechnology companies face mounting pressure to accelerate drug discovery timelines while simultaneously reducing development costs. Traditional manual laboratory processes are increasingly viewed as bottlenecks in the R&D pipeline, creating substantial demand for automated solutions that can operate continuously and with minimal human intervention.
High-throughput screening and large-scale data generation have become essential components of modern biotechnology research, necessitating automation systems capable of handling complex experimental workflows. The industry's shift toward personalized medicine and biologics has further intensified the need for flexible automation platforms that can accommodate diverse protocols and sample types.
Market segmentation reveals distinct demand patterns across different biotechnology sectors. Clinical diagnostics laboratories represent a significant market segment, driven by the need to process large volumes of samples with consistent quality and rapid turnaround times. Research institutions and academic laboratories constitute another key segment, although their adoption is often constrained by budget limitations and the need for highly customizable solutions.
Contract research organizations (CROs) and contract development and manufacturing organizations (CDMOs) form a rapidly growing market segment, as these entities increasingly leverage automation to enhance their service offerings and operational efficiency. The biopharmaceutical manufacturing sector also demonstrates strong demand for automation solutions that can ensure compliance with stringent regulatory requirements while maintaining production consistency.
End-user feedback indicates several critical requirements for autonomous lab systems. Interoperability with existing laboratory information management systems (LIMS) and electronic laboratory notebooks (ELNs) ranks as a top priority. Scalability and modularity are equally important, allowing organizations to expand their automation capabilities incrementally. Additionally, there is growing demand for systems incorporating artificial intelligence and machine learning capabilities to optimize experimental design and interpret complex datasets.
Market barriers include significant initial capital investment requirements, concerns regarding system reliability and maintenance, and challenges related to staff training and adaptation. Despite these obstacles, the fundamental value proposition of autonomous labs—enhanced productivity, improved data quality, and reduced operational costs—continues to drive market expansion across the biotechnology landscape.
The primary market drivers for autonomous lab integration in biotechnology stem from several converging factors. Pharmaceutical and biotechnology companies face mounting pressure to accelerate drug discovery timelines while simultaneously reducing development costs. Traditional manual laboratory processes are increasingly viewed as bottlenecks in the R&D pipeline, creating substantial demand for automated solutions that can operate continuously and with minimal human intervention.
High-throughput screening and large-scale data generation have become essential components of modern biotechnology research, necessitating automation systems capable of handling complex experimental workflows. The industry's shift toward personalized medicine and biologics has further intensified the need for flexible automation platforms that can accommodate diverse protocols and sample types.
Market segmentation reveals distinct demand patterns across different biotechnology sectors. Clinical diagnostics laboratories represent a significant market segment, driven by the need to process large volumes of samples with consistent quality and rapid turnaround times. Research institutions and academic laboratories constitute another key segment, although their adoption is often constrained by budget limitations and the need for highly customizable solutions.
Contract research organizations (CROs) and contract development and manufacturing organizations (CDMOs) form a rapidly growing market segment, as these entities increasingly leverage automation to enhance their service offerings and operational efficiency. The biopharmaceutical manufacturing sector also demonstrates strong demand for automation solutions that can ensure compliance with stringent regulatory requirements while maintaining production consistency.
End-user feedback indicates several critical requirements for autonomous lab systems. Interoperability with existing laboratory information management systems (LIMS) and electronic laboratory notebooks (ELNs) ranks as a top priority. Scalability and modularity are equally important, allowing organizations to expand their automation capabilities incrementally. Additionally, there is growing demand for systems incorporating artificial intelligence and machine learning capabilities to optimize experimental design and interpret complex datasets.
Market barriers include significant initial capital investment requirements, concerns regarding system reliability and maintenance, and challenges related to staff training and adaptation. Despite these obstacles, the fundamental value proposition of autonomous labs—enhanced productivity, improved data quality, and reduced operational costs—continues to drive market expansion across the biotechnology landscape.
Current Autonomous Lab Technologies and Barriers
The autonomous laboratory landscape in biotechnology has evolved significantly over the past decade, with several key technologies now reaching maturity. Cloud-based laboratory management systems have become increasingly sophisticated, enabling remote experiment design, execution monitoring, and data analysis. These platforms typically integrate with laboratory hardware through standardized APIs, allowing for seamless communication between digital systems and physical equipment.
Robotic automation represents another cornerstone technology, with liquid handling robots, automated sample preparation systems, and high-throughput screening platforms becoming standard in advanced biotechnology operations. These systems can operate continuously with minimal human intervention, significantly increasing throughput while reducing experimental variability.
Machine learning and artificial intelligence algorithms have been successfully deployed for experimental design optimization, predictive modeling, and automated data interpretation. These systems can analyze complex biological datasets, identify patterns invisible to human researchers, and suggest optimal experimental parameters based on historical data.
Despite these advancements, significant barriers remain in achieving fully autonomous laboratory operations. Hardware integration challenges persist as a major obstacle, with many laboratory instruments using proprietary communication protocols and lacking standardized interfaces. This creates "islands of automation" rather than cohesive systems, requiring complex integration efforts and custom middleware development.
Data standardization represents another critical barrier, as biological data formats vary widely across instruments and analysis platforms. The absence of universally adopted data standards complicates data exchange between systems and limits the potential for cross-platform machine learning applications.
Regulatory compliance presents unique challenges for autonomous laboratories, particularly in pharmaceutical and clinical applications. Current regulatory frameworks were largely designed for traditional laboratory workflows with human oversight at critical decision points, creating uncertainty around validation requirements for AI-driven experimental decisions.
The complexity of biological systems themselves poses perhaps the most fundamental barrier. Unlike manufacturing automation, biological experiments often involve unpredictable living systems with high variability. This unpredictability makes it difficult to develop robust error-handling protocols and requires sophisticated adaptive algorithms capable of responding to unexpected experimental outcomes.
Cost barriers also remain significant, with comprehensive autonomous laboratory solutions requiring substantial capital investment in both hardware and software infrastructure. This creates accessibility challenges for smaller research organizations and limits widespread adoption across the biotechnology sector.
Robotic automation represents another cornerstone technology, with liquid handling robots, automated sample preparation systems, and high-throughput screening platforms becoming standard in advanced biotechnology operations. These systems can operate continuously with minimal human intervention, significantly increasing throughput while reducing experimental variability.
Machine learning and artificial intelligence algorithms have been successfully deployed for experimental design optimization, predictive modeling, and automated data interpretation. These systems can analyze complex biological datasets, identify patterns invisible to human researchers, and suggest optimal experimental parameters based on historical data.
Despite these advancements, significant barriers remain in achieving fully autonomous laboratory operations. Hardware integration challenges persist as a major obstacle, with many laboratory instruments using proprietary communication protocols and lacking standardized interfaces. This creates "islands of automation" rather than cohesive systems, requiring complex integration efforts and custom middleware development.
Data standardization represents another critical barrier, as biological data formats vary widely across instruments and analysis platforms. The absence of universally adopted data standards complicates data exchange between systems and limits the potential for cross-platform machine learning applications.
Regulatory compliance presents unique challenges for autonomous laboratories, particularly in pharmaceutical and clinical applications. Current regulatory frameworks were largely designed for traditional laboratory workflows with human oversight at critical decision points, creating uncertainty around validation requirements for AI-driven experimental decisions.
The complexity of biological systems themselves poses perhaps the most fundamental barrier. Unlike manufacturing automation, biological experiments often involve unpredictable living systems with high variability. This unpredictability makes it difficult to develop robust error-handling protocols and requires sophisticated adaptive algorithms capable of responding to unexpected experimental outcomes.
Cost barriers also remain significant, with comprehensive autonomous laboratory solutions requiring substantial capital investment in both hardware and software infrastructure. This creates accessibility challenges for smaller research organizations and limits widespread adoption across the biotechnology sector.
Current Integration Approaches for Autonomous Labs
01 Automated laboratory systems integration
Integration of automated laboratory systems involves connecting various laboratory equipment and instruments to create a cohesive workflow. These systems enable seamless data transfer between different devices, allowing for more efficient laboratory operations. The integration includes hardware interfaces, communication protocols, and software platforms that facilitate the coordination of different laboratory components, resulting in improved productivity and reduced manual intervention.- Automated laboratory systems integration: Integration of automated laboratory systems involves connecting various laboratory equipment and instruments to create a cohesive workflow. These systems enable seamless data transfer between different devices, allowing for remote monitoring and control of laboratory processes. The integration facilitates efficient sample handling, analysis, and data management, reducing manual intervention and increasing throughput in research and development environments.
- Cloud-based laboratory management: Cloud-based platforms for laboratory management enable real-time data sharing, remote access to laboratory resources, and collaborative research across distributed teams. These systems provide centralized storage for experimental data, facilitate workflow orchestration, and support decision-making through advanced analytics. The cloud infrastructure ensures scalability, reliability, and security for autonomous laboratory operations while enabling integration with existing laboratory information management systems.
- AI-driven experimental design and analysis: Artificial intelligence technologies are applied to optimize experimental design, predict outcomes, and analyze results in autonomous laboratories. Machine learning algorithms can identify patterns in experimental data, suggest modifications to protocols, and accelerate discovery processes. These AI systems continuously learn from experimental results, improving their predictive capabilities over time and enabling more efficient use of laboratory resources for complex research challenges.
- Robotic systems for laboratory automation: Robotic systems automate physical tasks in laboratories, including sample preparation, handling, and analysis. These systems incorporate precision robotics, computer vision, and sensor technologies to perform complex manipulations with high accuracy and repeatability. The integration of robotics reduces human error, increases throughput, and enables continuous operation of laboratory processes, particularly beneficial for hazardous or repetitive tasks in research environments.
- Modular laboratory infrastructure: Modular laboratory designs provide flexible infrastructure that can be reconfigured to accommodate changing research needs. These systems feature standardized interfaces for equipment integration, plug-and-play connectivity, and adaptable physical layouts. The modular approach facilitates rapid deployment of new technologies, efficient space utilization, and scalable laboratory operations, supporting the evolution of autonomous research capabilities over time.
02 Cloud-based laboratory management
Cloud-based laboratory management systems provide remote access and control of laboratory operations. These systems enable researchers to monitor experiments, analyze data, and collaborate with team members from anywhere. The cloud infrastructure supports real-time data sharing, storage, and processing, facilitating seamless integration between different laboratory locations. This approach enhances flexibility, scalability, and accessibility of laboratory resources while maintaining data security and integrity.Expand Specific Solutions03 AI-driven laboratory automation
Artificial intelligence technologies are being integrated into laboratory environments to enhance decision-making and process optimization. AI algorithms can analyze experimental data, predict outcomes, and suggest adjustments to protocols. These systems learn from previous experiments to improve future results, reducing the need for human intervention. AI-driven automation enables more complex experimental designs, faster iteration cycles, and more reliable results through intelligent data analysis and process control.Expand Specific Solutions04 Modular laboratory integration platforms
Modular integration platforms provide flexible frameworks for connecting various laboratory components. These platforms feature standardized interfaces and protocols that allow for plug-and-play functionality between different instruments and systems. The modular approach enables laboratories to customize their automation setup according to specific needs and gradually expand capabilities over time. This flexibility facilitates easier upgrades, maintenance, and reconfiguration of laboratory systems without disrupting ongoing operations.Expand Specific Solutions05 IoT-enabled laboratory devices
Internet of Things (IoT) technology is being applied to laboratory equipment to create networks of connected devices that can communicate and coordinate with each other. These smart laboratory devices can transmit data, receive instructions, and adjust operations based on real-time information. IoT-enabled laboratories feature sensors that monitor environmental conditions, equipment status, and experimental progress. This connectivity enhances laboratory efficiency, enables predictive maintenance, and provides comprehensive data collection for improved experimental reproducibility.Expand Specific Solutions
Leading Players in Autonomous Biotechnology Solutions
The autonomous lab integration in biotechnology is currently in an early growth phase, characterized by rapid technological advancements and increasing market adoption. The global market is expanding significantly, projected to reach substantial value as pharmaceutical and biotech companies seek efficiency improvements. Technologically, the field shows varying maturity levels across players: established companies like Roche Molecular Systems, Vertex Pharmaceuticals, and Regeneron demonstrate advanced capabilities in automated workflows, while newer entrants such as Mekonos and Quris Technologies are pioneering innovative approaches with AI-driven platforms and chip-based testing. Academic institutions including MIT and Zhejiang University are contributing fundamental research, creating a dynamic ecosystem where collaboration between industry leaders and research institutions is accelerating development of integrated autonomous laboratory solutions.
Roche Molecular Systems, Inc.
Technical Solution: Roche has pioneered an autonomous laboratory platform specifically designed for molecular diagnostics and biotechnology research. Their solution integrates high-throughput screening capabilities with automated sample preparation and analysis workflows. The system employs a sophisticated scheduling algorithm that dynamically allocates resources based on priority, urgency, and available equipment capacity. Roche's platform features a proprietary liquid handling system with advanced error detection and correction mechanisms, achieving sub-microliter precision across diverse biological samples[2]. The company has implemented a comprehensive data management infrastructure that enables real-time monitoring of experiments, automated quality control checks, and seamless integration with laboratory information management systems (LIMS). Their autonomous lab solution incorporates predictive maintenance capabilities that minimize downtime by anticipating equipment failures before they occur[4].
Strengths: Roche's deep expertise in molecular diagnostics provides highly specialized automation solutions tailored to biotechnology applications. Their systems offer exceptional precision and reproducibility for critical assays. Weaknesses: The platform may have limited flexibility for novel experimental protocols outside established workflows, and integration with third-party instruments can be challenging.
Massachusetts Institute of Technology
Technical Solution: MIT has developed a groundbreaking autonomous laboratory platform that leverages advanced robotics and machine learning to accelerate biotechnology research. Their system implements a closed-loop experimental design approach where AI algorithms continuously analyze results and automatically generate optimized follow-up experiments without human intervention. MIT's solution features a flexible robotic architecture that can be rapidly reconfigured for different experimental protocols, from cell culture maintenance to complex genetic engineering tasks. The platform incorporates computer vision systems that monitor experimental progress in real-time, detecting anomalies and making adjustments to ensure experimental success[5]. MIT researchers have implemented reinforcement learning algorithms that progressively improve experimental efficiency by learning from past successes and failures. Their autonomous lab solution also includes a collaborative interface that allows remote researchers to monitor experiments, provide input on decision points, and access results in real-time regardless of physical location[7].
Strengths: MIT's cutting-edge AI algorithms provide superior experimental optimization and novel discovery capabilities. Their open architecture approach facilitates adaptation to emerging biotechnology techniques. Weaknesses: As a research-focused platform, it may lack the industrial robustness and regulatory compliance features needed for commercial deployment in regulated environments.
Key Technical Innovations in Lab Automation
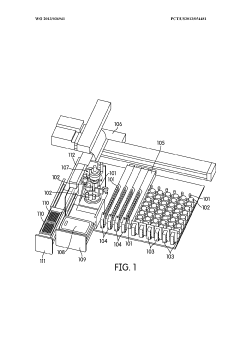
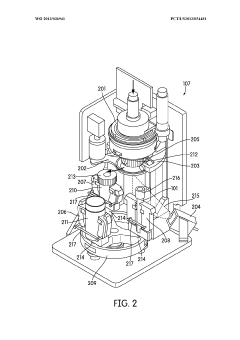
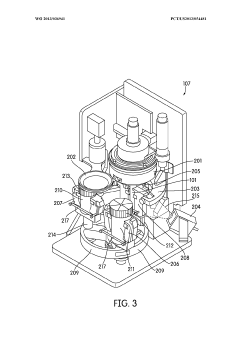
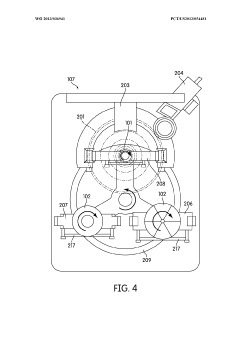
Automated sample handling instrumentation, systems, processes, and methods
PatentWO2013036941A2
Innovation
- A processing station with a rotatable platform, capping/decapping mechanism, data scanning, and mucoid detection, integrated into an automated instrument for handling various container sizes and shapes, incorporating machine vision and real-time inventory management to ensure accurate and efficient sample handling.
System and method for biopsy processing
PatentWO2024197223A2
Innovation
- An automated system for biopsy processing that includes a closed fluid transfer system for washing, digesting, and transferring cells to a culture flask, utilizing a programmable logic controller for precise fluid management and temperature control, and an interactive user interface for guiding the cell harvesting process, ensuring sterility and minimizing contamination risks.
Regulatory Compliance for Automated Biotechnology Systems
The integration of autonomous laboratories in biotechnology faces significant regulatory challenges that must be addressed to ensure compliance with established standards. Regulatory frameworks for automated biotechnology systems vary globally, with the FDA, EMA, and NMPA imposing different requirements for validation, verification, and documentation. These agencies have established Good Laboratory Practices (GLP), Good Manufacturing Practices (GMP), and Good Automated Manufacturing Practice (GAMP) guidelines that directly impact autonomous lab implementations.
Automated systems must demonstrate consistent adherence to data integrity principles, particularly the ALCOA+ framework (Attributable, Legible, Contemporaneous, Original, Accurate, plus Complete, Consistent, Enduring, and Available). This presents unique challenges for AI-driven autonomous labs where decision-making processes may lack transparency. Regulatory bodies increasingly require explainable AI models and comprehensive audit trails for all automated processes.
Validation protocols for autonomous systems require more rigorous testing than traditional laboratory equipment. This includes Installation Qualification (IQ), Operational Qualification (OQ), and Performance Qualification (PQ) with additional emphasis on algorithm validation and continuous monitoring systems. The dynamic nature of machine learning models in autonomous labs necessitates novel approaches to change management and revalidation procedures.
Risk management frameworks such as ICH Q9 must be adapted specifically for autonomous systems, incorporating failure mode and effects analysis (FMEA) for both hardware components and software algorithms. Regulatory agencies now expect comprehensive risk assessments that address potential algorithm drift, data security vulnerabilities, and system reliability under various operating conditions.
Compliance with data privacy regulations presents another layer of complexity, particularly when handling sensitive genetic information or patient data. GDPR in Europe, HIPAA in the US, and equivalent regulations in other regions impose strict requirements on data handling, storage, and transfer that must be engineered into autonomous lab systems from the ground up.
Solutions to these regulatory challenges include implementing modular validation approaches that separate hardware, software, and algorithmic components for more efficient compliance management. Leading organizations are developing regulatory-specific AI documentation templates and continuous compliance monitoring systems that track regulatory changes across jurisdictions and automatically flag potential compliance gaps in autonomous lab operations.
Collaborative initiatives between industry stakeholders and regulatory bodies are emerging to establish standardized frameworks specifically for autonomous laboratories. These include the development of technical standards for algorithm validation, data integrity in automated systems, and interoperability requirements that facilitate regulatory acceptance while maintaining innovation pace.
Automated systems must demonstrate consistent adherence to data integrity principles, particularly the ALCOA+ framework (Attributable, Legible, Contemporaneous, Original, Accurate, plus Complete, Consistent, Enduring, and Available). This presents unique challenges for AI-driven autonomous labs where decision-making processes may lack transparency. Regulatory bodies increasingly require explainable AI models and comprehensive audit trails for all automated processes.
Validation protocols for autonomous systems require more rigorous testing than traditional laboratory equipment. This includes Installation Qualification (IQ), Operational Qualification (OQ), and Performance Qualification (PQ) with additional emphasis on algorithm validation and continuous monitoring systems. The dynamic nature of machine learning models in autonomous labs necessitates novel approaches to change management and revalidation procedures.
Risk management frameworks such as ICH Q9 must be adapted specifically for autonomous systems, incorporating failure mode and effects analysis (FMEA) for both hardware components and software algorithms. Regulatory agencies now expect comprehensive risk assessments that address potential algorithm drift, data security vulnerabilities, and system reliability under various operating conditions.
Compliance with data privacy regulations presents another layer of complexity, particularly when handling sensitive genetic information or patient data. GDPR in Europe, HIPAA in the US, and equivalent regulations in other regions impose strict requirements on data handling, storage, and transfer that must be engineered into autonomous lab systems from the ground up.
Solutions to these regulatory challenges include implementing modular validation approaches that separate hardware, software, and algorithmic components for more efficient compliance management. Leading organizations are developing regulatory-specific AI documentation templates and continuous compliance monitoring systems that track regulatory changes across jurisdictions and automatically flag potential compliance gaps in autonomous lab operations.
Collaborative initiatives between industry stakeholders and regulatory bodies are emerging to establish standardized frameworks specifically for autonomous laboratories. These include the development of technical standards for algorithm validation, data integrity in automated systems, and interoperability requirements that facilitate regulatory acceptance while maintaining innovation pace.
Data Security and IP Protection in Autonomous Labs
The integration of autonomous laboratories in biotechnology introduces significant data security and intellectual property (IP) protection challenges. As these labs generate vast amounts of sensitive biological data, proprietary methodologies, and potentially patentable discoveries, implementing robust security frameworks becomes paramount. Traditional security measures often prove inadequate due to the unique requirements of autonomous systems that operate with minimal human intervention.
Data encryption represents the first line of defense in autonomous labs, with advanced encryption protocols necessary for both data at rest and in transit. Implementing multi-layered encryption strategies ensures that even if one security layer is compromised, the underlying data remains protected. However, encryption must be balanced with accessibility to avoid hindering the real-time data processing capabilities essential for autonomous operations.
Access control mechanisms require particular attention in autonomous lab environments. Biometric authentication, multi-factor verification, and role-based access systems help ensure that only authorized personnel can interact with sensitive data and equipment. These systems must be designed to accommodate the distributed nature of autonomous operations while maintaining comprehensive security logs for audit purposes.
Blockchain technology offers promising solutions for maintaining data integrity in autonomous labs. By creating immutable records of all experimental procedures, results, and modifications, blockchain provides transparent verification of research provenance while protecting intellectual property. Several biotechnology firms have already implemented blockchain-based systems to secure their autonomous research workflows and establish clear ownership of discoveries.
IP protection in autonomous labs presents unique challenges as machine learning algorithms increasingly generate novel compounds and methodologies. Current legal frameworks struggle to address inventions created by AI systems with minimal human guidance. Some jurisdictions have begun developing specialized regulations for AI-generated IP, while others maintain that human involvement in system design constitutes sufficient basis for traditional patent protection.
Cloud security vulnerabilities represent another significant concern, as many autonomous labs leverage cloud infrastructure for data storage and computational resources. Implementing secure API gateways, regular penetration testing, and comprehensive threat monitoring systems helps mitigate these risks. Additionally, data residency considerations must be addressed to comply with varying international regulations regarding biological data storage and transfer.
Collaborative research environments further complicate security requirements, as autonomous labs often operate within networks of partner organizations. Establishing clear data sharing agreements, implementing secure collaboration platforms, and utilizing federated learning approaches allows organizations to maintain control over proprietary information while still benefiting from collaborative innovation.
Data encryption represents the first line of defense in autonomous labs, with advanced encryption protocols necessary for both data at rest and in transit. Implementing multi-layered encryption strategies ensures that even if one security layer is compromised, the underlying data remains protected. However, encryption must be balanced with accessibility to avoid hindering the real-time data processing capabilities essential for autonomous operations.
Access control mechanisms require particular attention in autonomous lab environments. Biometric authentication, multi-factor verification, and role-based access systems help ensure that only authorized personnel can interact with sensitive data and equipment. These systems must be designed to accommodate the distributed nature of autonomous operations while maintaining comprehensive security logs for audit purposes.
Blockchain technology offers promising solutions for maintaining data integrity in autonomous labs. By creating immutable records of all experimental procedures, results, and modifications, blockchain provides transparent verification of research provenance while protecting intellectual property. Several biotechnology firms have already implemented blockchain-based systems to secure their autonomous research workflows and establish clear ownership of discoveries.
IP protection in autonomous labs presents unique challenges as machine learning algorithms increasingly generate novel compounds and methodologies. Current legal frameworks struggle to address inventions created by AI systems with minimal human guidance. Some jurisdictions have begun developing specialized regulations for AI-generated IP, while others maintain that human involvement in system design constitutes sufficient basis for traditional patent protection.
Cloud security vulnerabilities represent another significant concern, as many autonomous labs leverage cloud infrastructure for data storage and computational resources. Implementing secure API gateways, regular penetration testing, and comprehensive threat monitoring systems helps mitigate these risks. Additionally, data residency considerations must be addressed to comply with varying international regulations regarding biological data storage and transfer.
Collaborative research environments further complicate security requirements, as autonomous labs often operate within networks of partner organizations. Establishing clear data sharing agreements, implementing secure collaboration platforms, and utilizing federated learning approaches allows organizations to maintain control over proprietary information while still benefiting from collaborative innovation.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!