Biocompatible Materials For Implantable Transient Sensors: Safety Criteria
SEP 1, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Implantable Transient Sensors Background and Objectives
Implantable transient sensors represent a revolutionary advancement in medical monitoring technology, designed to dissolve or degrade within the body after fulfilling their diagnostic or therapeutic functions. The evolution of these sensors began in the early 2000s with rudimentary biodegradable electronics, progressing significantly over the past decade with the integration of advanced biocompatible materials and miniaturized sensing components.
The technological trajectory has been driven by increasing demands for less invasive medical monitoring solutions that eliminate the need for secondary removal surgeries. Initial developments focused primarily on simple circuit elements using magnesium and silicon, while recent innovations have expanded to include complex functional systems capable of monitoring various physiological parameters such as temperature, pressure, pH levels, and specific biomarkers.
Current research emphasizes the development of materials that maintain functional integrity during the required operational period while ensuring complete dissolution afterward without producing toxic byproducts. This balance between operational stability and controlled degradation represents one of the field's central technical challenges.
The primary objective of implantable transient sensor technology is to create fully functional diagnostic devices that can be safely introduced into the human body, perform reliable measurements for a predetermined period, and subsequently disappear through natural metabolic processes without requiring surgical extraction or leaving permanent foreign materials.
Safety criteria for biocompatible materials in these sensors focus on several critical parameters: biocompatibility during functional lifetime, controlled degradation kinetics, non-toxic degradation products, minimal inflammatory response, and compatibility with sterilization processes. These criteria must be met while maintaining sensor performance specifications including sensitivity, accuracy, and signal stability.
The field is experiencing accelerated growth due to convergence with other technological trends including wireless power transfer, flexible electronics, and advanced biomaterials engineering. Market projections indicate substantial expansion potential in applications ranging from post-surgical monitoring to chronic disease management and therapeutic drug monitoring.
Technical objectives for the next generation of implantable transient sensors include extending operational lifetimes from days to months, enhancing wireless communication capabilities, improving power efficiency, and developing materials with more precisely controlled degradation profiles tailored to specific clinical applications.
Regulatory pathways for these novel devices are still evolving, with significant attention being paid to establishing standardized testing protocols for safety assessment and performance validation. The FDA and equivalent international regulatory bodies are actively developing frameworks specifically addressing the unique characteristics and risk profiles of transient implantable technologies.
The technological trajectory has been driven by increasing demands for less invasive medical monitoring solutions that eliminate the need for secondary removal surgeries. Initial developments focused primarily on simple circuit elements using magnesium and silicon, while recent innovations have expanded to include complex functional systems capable of monitoring various physiological parameters such as temperature, pressure, pH levels, and specific biomarkers.
Current research emphasizes the development of materials that maintain functional integrity during the required operational period while ensuring complete dissolution afterward without producing toxic byproducts. This balance between operational stability and controlled degradation represents one of the field's central technical challenges.
The primary objective of implantable transient sensor technology is to create fully functional diagnostic devices that can be safely introduced into the human body, perform reliable measurements for a predetermined period, and subsequently disappear through natural metabolic processes without requiring surgical extraction or leaving permanent foreign materials.
Safety criteria for biocompatible materials in these sensors focus on several critical parameters: biocompatibility during functional lifetime, controlled degradation kinetics, non-toxic degradation products, minimal inflammatory response, and compatibility with sterilization processes. These criteria must be met while maintaining sensor performance specifications including sensitivity, accuracy, and signal stability.
The field is experiencing accelerated growth due to convergence with other technological trends including wireless power transfer, flexible electronics, and advanced biomaterials engineering. Market projections indicate substantial expansion potential in applications ranging from post-surgical monitoring to chronic disease management and therapeutic drug monitoring.
Technical objectives for the next generation of implantable transient sensors include extending operational lifetimes from days to months, enhancing wireless communication capabilities, improving power efficiency, and developing materials with more precisely controlled degradation profiles tailored to specific clinical applications.
Regulatory pathways for these novel devices are still evolving, with significant attention being paid to establishing standardized testing protocols for safety assessment and performance validation. The FDA and equivalent international regulatory bodies are actively developing frameworks specifically addressing the unique characteristics and risk profiles of transient implantable technologies.
Market Analysis for Biodegradable Medical Implants
The global market for biodegradable medical implants is experiencing significant growth, driven by increasing demand for advanced medical technologies that reduce long-term complications and eliminate secondary removal surgeries. Currently valued at approximately $5.7 billion in 2023, this market is projected to reach $9.8 billion by 2028, representing a compound annual growth rate (CAGR) of 11.4% during the forecast period.
North America dominates the market with a share of 42%, followed by Europe at 31% and Asia-Pacific at 21%. The remaining 6% is distributed across other regions. This regional distribution reflects the concentration of advanced healthcare infrastructure, research capabilities, and regulatory frameworks that support innovation in biodegradable implant technologies.
Within the biodegradable implants sector, transient sensors represent an emerging segment with particularly strong growth potential. These sensors, which can monitor physiological parameters and then safely degrade in the body, are expected to grow at a CAGR of 14.2% through 2028, outpacing the overall market growth rate.
Key market drivers include the aging global population, rising prevalence of chronic diseases requiring implantable monitoring solutions, and increasing healthcare expenditure. Additionally, growing patient preference for minimally invasive procedures and the push toward personalized medicine are creating favorable conditions for market expansion.
Reimbursement policies are evolving to support these technologies, with several major insurance providers now covering biodegradable implants. This trend is expected to continue as clinical evidence demonstrates the cost-effectiveness of these solutions compared to traditional permanent implants that may require removal procedures.
Regulatory pathways for biodegradable implants are becoming more streamlined in major markets, with the FDA and EMA establishing specific guidance for these technologies. This regulatory clarity is accelerating time-to-market for new products and encouraging further investment in the sector.
Venture capital funding for startups developing biodegradable medical implants has increased by 27% over the past three years, with particular interest in companies focusing on biocompatible materials for transient sensing applications. This investment trend underscores the perceived market potential and technological promise of this field.
Customer segmentation reveals that academic medical centers and large hospital systems are early adopters of these technologies, while community hospitals follow as costs decrease and clinical protocols become standardized. Patient demographics show strongest adoption among those aged 45-70 who require long-term physiological monitoring but wish to avoid permanent implants.
North America dominates the market with a share of 42%, followed by Europe at 31% and Asia-Pacific at 21%. The remaining 6% is distributed across other regions. This regional distribution reflects the concentration of advanced healthcare infrastructure, research capabilities, and regulatory frameworks that support innovation in biodegradable implant technologies.
Within the biodegradable implants sector, transient sensors represent an emerging segment with particularly strong growth potential. These sensors, which can monitor physiological parameters and then safely degrade in the body, are expected to grow at a CAGR of 14.2% through 2028, outpacing the overall market growth rate.
Key market drivers include the aging global population, rising prevalence of chronic diseases requiring implantable monitoring solutions, and increasing healthcare expenditure. Additionally, growing patient preference for minimally invasive procedures and the push toward personalized medicine are creating favorable conditions for market expansion.
Reimbursement policies are evolving to support these technologies, with several major insurance providers now covering biodegradable implants. This trend is expected to continue as clinical evidence demonstrates the cost-effectiveness of these solutions compared to traditional permanent implants that may require removal procedures.
Regulatory pathways for biodegradable implants are becoming more streamlined in major markets, with the FDA and EMA establishing specific guidance for these technologies. This regulatory clarity is accelerating time-to-market for new products and encouraging further investment in the sector.
Venture capital funding for startups developing biodegradable medical implants has increased by 27% over the past three years, with particular interest in companies focusing on biocompatible materials for transient sensing applications. This investment trend underscores the perceived market potential and technological promise of this field.
Customer segmentation reveals that academic medical centers and large hospital systems are early adopters of these technologies, while community hospitals follow as costs decrease and clinical protocols become standardized. Patient demographics show strongest adoption among those aged 45-70 who require long-term physiological monitoring but wish to avoid permanent implants.
Biocompatible Materials: Current Status and Challenges
The global landscape of biocompatible materials for implantable transient sensors has evolved significantly over the past decade. Currently, the field is dominated by three primary material categories: biodegradable polymers, water-soluble metals, and silicon-based semiconductors. Each category presents unique advantages and limitations when applied to transient sensing applications.
Biodegradable polymers such as poly(lactic-co-glycolic acid) (PLGA) and polycaprolactone (PCL) have demonstrated excellent biocompatibility profiles in clinical settings. However, these materials face challenges related to controlled degradation rates and mechanical stability during the functional lifetime of sensors. Recent advancements have improved degradation predictability, but precise temporal control remains elusive.
Water-soluble metals, particularly magnesium and zinc alloys, have gained significant attention due to their electrical conductivity and natural presence in physiological systems. The primary challenge with these materials lies in controlling corrosion rates in vivo, as premature degradation can lead to sensor failure and potentially harmful local pH changes. Current research focuses on surface modifications and alloying strategies to optimize dissolution kinetics.
Silicon-based materials, including monocrystalline silicon and silicon dioxide, offer superior electronic properties but face biocompatibility concerns related to inflammatory responses and potential toxicity of degradation byproducts. Recent innovations in ultrathin silicon membranes have shown promising results in minimizing foreign body reactions.
A significant challenge across all material platforms is achieving the delicate balance between functional lifetime and complete dissolution. Most current systems exhibit either premature degradation or persistent residual fragments that may trigger chronic inflammation. This dichotomy represents a fundamental technical barrier requiring innovative materials engineering approaches.
Geographically, research leadership in this field shows distinct patterns. North American institutions lead in silicon-based transient electronics, while European research centers have made significant advances in biodegradable polymers. Asian research groups, particularly in Japan and South Korea, have contributed substantially to water-soluble metal technologies.
Regulatory frameworks present another critical challenge, as safety standards for transient implantable materials remain inconsistent globally. The FDA has established preliminary guidance for biodegradable implants, but specific criteria for transient electronic materials are still evolving. This regulatory uncertainty has slowed clinical translation despite promising preclinical results.
Emerging concerns include the potential for nanoscale degradation products to cross biological barriers and the long-term effects of repeated exposure to degradation byproducts. These safety considerations have prompted increased focus on comprehensive toxicological profiling beyond standard biocompatibility testing.
Biodegradable polymers such as poly(lactic-co-glycolic acid) (PLGA) and polycaprolactone (PCL) have demonstrated excellent biocompatibility profiles in clinical settings. However, these materials face challenges related to controlled degradation rates and mechanical stability during the functional lifetime of sensors. Recent advancements have improved degradation predictability, but precise temporal control remains elusive.
Water-soluble metals, particularly magnesium and zinc alloys, have gained significant attention due to their electrical conductivity and natural presence in physiological systems. The primary challenge with these materials lies in controlling corrosion rates in vivo, as premature degradation can lead to sensor failure and potentially harmful local pH changes. Current research focuses on surface modifications and alloying strategies to optimize dissolution kinetics.
Silicon-based materials, including monocrystalline silicon and silicon dioxide, offer superior electronic properties but face biocompatibility concerns related to inflammatory responses and potential toxicity of degradation byproducts. Recent innovations in ultrathin silicon membranes have shown promising results in minimizing foreign body reactions.
A significant challenge across all material platforms is achieving the delicate balance between functional lifetime and complete dissolution. Most current systems exhibit either premature degradation or persistent residual fragments that may trigger chronic inflammation. This dichotomy represents a fundamental technical barrier requiring innovative materials engineering approaches.
Geographically, research leadership in this field shows distinct patterns. North American institutions lead in silicon-based transient electronics, while European research centers have made significant advances in biodegradable polymers. Asian research groups, particularly in Japan and South Korea, have contributed substantially to water-soluble metal technologies.
Regulatory frameworks present another critical challenge, as safety standards for transient implantable materials remain inconsistent globally. The FDA has established preliminary guidance for biodegradable implants, but specific criteria for transient electronic materials are still evolving. This regulatory uncertainty has slowed clinical translation despite promising preclinical results.
Emerging concerns include the potential for nanoscale degradation products to cross biological barriers and the long-term effects of repeated exposure to degradation byproducts. These safety considerations have prompted increased focus on comprehensive toxicological profiling beyond standard biocompatibility testing.
Current Biocompatible Material Solutions and Safety Profiles
01 Biodegradable materials for transient sensors
Biodegradable materials are essential for implantable transient sensors as they eliminate the need for surgical removal after the sensor's functional lifetime. These materials naturally break down in the body over time, reducing long-term biocompatibility concerns. Common biodegradable materials include certain polymers, magnesium alloys, and silk fibroin that can be engineered to dissolve at controlled rates matching the required monitoring period. The degradation products must be non-toxic and easily metabolized or excreted by the body.- Biodegradable materials for transient sensors: Biodegradable materials are essential for implantable transient sensors as they eliminate the need for surgical removal after the sensor's functional lifetime. These materials naturally break down into non-toxic byproducts that can be safely absorbed or excreted by the body. Common biodegradable materials include certain polymers, magnesium alloys, and silk fibroin that provide temporary structural support while maintaining biocompatibility. The degradation rate can be engineered to match the required functional lifetime of the sensor.
- Biocompatible encapsulation materials: Encapsulation materials serve as protective barriers between the electronic components of transient sensors and the surrounding biological environment. These materials must prevent immune responses while allowing the sensor to function properly. Biocompatible encapsulation materials include medical-grade silicones, parylene, and certain hydrogels that provide hermetic sealing while remaining non-toxic. The encapsulation must maintain integrity during the sensor's operational lifetime while eventually allowing controlled degradation or dissolution.
- Bioresorbable electronic components: Bioresorbable electronic components are designed to dissolve or degrade safely within the body after fulfilling their sensing function. These components include conductive materials like magnesium, zinc, or silicon nanomembranes that can transmit signals while eventually breaking down into biocompatible byproducts. The dissolution rates of these materials can be tailored through composition and structure to ensure proper functionality during the required monitoring period before safe elimination from the body.
- Biocompatibility testing protocols: Comprehensive testing protocols are essential to evaluate the safety of materials used in implantable transient sensors. These protocols include cytotoxicity assessments, genotoxicity testing, sensitization studies, and long-term implantation tests. Advanced in vitro and in vivo models help predict potential adverse reactions and ensure materials meet regulatory standards. Biocompatibility testing must evaluate both the intact sensor materials and their degradation products to confirm safety throughout the entire lifecycle of the implant.
- Controlled degradation mechanisms: Controlled degradation mechanisms ensure that implantable transient sensors break down predictably and safely after completing their function. These mechanisms include hydrolytic degradation, enzymatic breakdown, or dissolution triggered by specific physiological conditions. The degradation process must be carefully engineered to prevent sudden release of potentially harmful components and to maintain sensor functionality until the intended end of its operational lifetime. Advanced designs incorporate gradual dissolution pathways that minimize local pH changes and inflammatory responses.
02 Biocompatible coatings and encapsulation techniques
Protective coatings and encapsulation methods are crucial for implantable sensors to prevent adverse immune responses and ensure device functionality. These coatings create barriers between the electronic components and bodily fluids, preventing corrosion while maintaining sensor performance. Materials such as parylene, biocompatible hydrogels, and certain oxide layers can effectively encapsulate sensors while allowing necessary analyte diffusion. Advanced techniques include layer-by-layer deposition and conformal coating processes that ensure complete coverage of complex sensor geometries.Expand Specific Solutions03 Inflammation and foreign body response mitigation
Controlling inflammation and foreign body responses is essential for implantable sensor safety and longevity. Strategies include surface modifications to reduce protein adsorption, incorporation of anti-inflammatory agents, and designs that minimize mechanical irritation to surrounding tissues. Materials with specific surface topographies or chemical modifications can reduce fibrotic encapsulation that would otherwise compromise sensor function. Some approaches utilize controlled release of anti-inflammatory compounds or incorporate materials that actively promote tissue integration rather than rejection.Expand Specific Solutions04 Wireless and passive sensing technologies
Wireless and passive sensing technologies eliminate the need for batteries or wired connections in implantable sensors, enhancing safety by reducing foreign materials and potential failure points. These systems often utilize resonant circuits, RFID technology, or other passive components that can be interrogated externally. The elimination of batteries removes risks associated with battery leakage or depletion. Materials used in these systems must be carefully selected to maintain electromagnetic properties while meeting biocompatibility requirements. These technologies enable long-term monitoring without the complications of power sources.Expand Specific Solutions05 Regulatory compliance and safety testing protocols
Comprehensive safety testing protocols and regulatory compliance are mandatory for implantable transient sensors. These include biocompatibility testing according to ISO 10993 standards, leachable/extractable testing, sterilization validation, and long-term implantation studies. Safety criteria encompass cytotoxicity, sensitization, irritation, systemic toxicity, and genotoxicity assessments. Regulatory frameworks require demonstration of both material safety and functional reliability under physiological conditions. Testing must account for degradation products and their potential effects on surrounding tissues and overall patient health.Expand Specific Solutions
Leading Organizations in Transient Electronics Development
The biocompatible materials for implantable transient sensors market is currently in an early growth phase, characterized by intensive research and development activities. The market size is expanding steadily, driven by increasing applications in medical monitoring and diagnostics, with projections suggesting significant growth over the next decade. Regarding technological maturity, academic institutions like University of Illinois, Purdue Research Foundation, and University of Southern California are leading fundamental research, while established medical device companies such as Medtronic, Biotronik, and F. Hoffmann-La Roche are advancing commercial applications. Research organizations like IMEC are bridging the gap between academic innovation and industry implementation. The competitive landscape shows a collaborative ecosystem where universities develop novel materials and safety protocols, while commercial entities focus on clinical validation and regulatory approval pathways for these advanced biocompatible sensing technologies.
The Board of Trustees of the University of Illinois
Technical Solution: The University of Illinois has developed a comprehensive transient electronics platform based on silicon nanomembranes and biodegradable polymers specifically engineered for implantable sensing applications. Their technology utilizes ultrathin silicon (50-100 nm) that dissolves into non-toxic silicic acid at controlled rates, combined with magnesium conductors and molybdenum electrodes that form biocompatible ionic species upon dissolution. A key innovation is their multilayer encapsulation approach using precisely engineered silk fibroin and poly(lactic-co-glycolic acid) (PLGA) with tailored molecular weights to achieve programmed degradation timelines ranging from days to months. Their materials undergo rigorous biocompatibility testing including cytotoxicity, genotoxicity, and systemic toxicity evaluations according to ISO 10993 standards. The university has demonstrated successful in vivo applications for intracranial pressure monitoring and temperature sensing with complete resorption and minimal inflammatory response in animal models.
Strengths: Pioneering research in transient electronics; extensive fundamental understanding of dissolution mechanisms; strong publication record establishing safety criteria. Weaknesses: Challenges in scaling manufacturing processes; limited clinical validation in human subjects; potential variability in degradation rates under different physiological conditions.
Purdue Research Foundation
Technical Solution: Purdue Research Foundation has developed an innovative platform for implantable transient sensors based on naturally derived biopolymers and biocompatible metals. Their technology centers on cellulose-derived nanomaterials combined with zinc and iron-based conductors that safely degrade into non-toxic components. A distinguishing feature is their "triggered transience" approach, where sensors remain stable until exposed to specific biochemical triggers or controlled electrical stimulation, allowing precise control over functional lifetime. Their materials incorporate specialized anti-inflammatory compounds embedded within the polymer matrix that are gradually released during degradation to actively mitigate foreign body responses. Purdue has established comprehensive safety criteria including degradation product characterization, local tissue response evaluation, and systemic distribution studies of dissolution byproducts. Their sensors have demonstrated successful application in post-surgical monitoring with programmable dissolution timelines ranging from weeks to months depending on the specific clinical need.
Strengths: Innovative triggered degradation mechanisms; strong focus on naturally derived materials; excellent control over dissolution kinetics. Weaknesses: Less established clinical validation pathway; potential challenges with sensor stability before triggered degradation; limited commercial manufacturing experience compared to industry leaders.
Key Patents and Research in Implantable Transient Materials
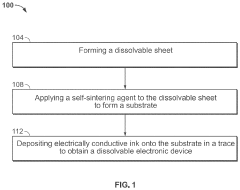
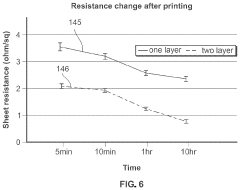
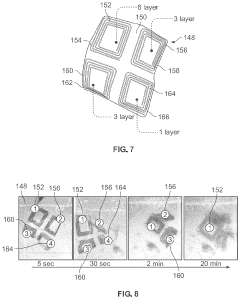
Method of manufacturing transient electronics
PatentPendingUS20240080991A1
Innovation
- The development of transient electronic devices that can chemically or physically dissolve, disintegrate, or degrade over time, using methods such as additive manufacturing to create dissolvable sheets with self-sintering agents and electrically conductive ink, or by integrating conductive materials into meltable or edible mediums, allowing for controlled end-of-life processes.
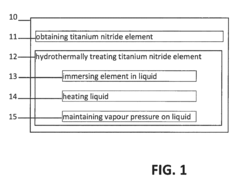
Method for decreasing the impedance of a titanium nitride electrode
PatentActiveEP2876435A1
Innovation
- Hydrothermal treatment of titanium nitride electrodes in a heated aqueous solution to increase surface roughness and capacitance, reducing impedance by a factor of 5 to 100, making them compatible with CMOS processing and cost-effective.
Regulatory Framework for Implantable Medical Devices
The regulatory landscape for implantable medical devices, particularly transient sensors utilizing biocompatible materials, is complex and multifaceted. In the United States, the Food and Drug Administration (FDA) classifies implantable sensors as Class III medical devices, requiring the most stringent regulatory controls due to their high-risk nature. Manufacturers must submit a Premarket Approval (PMA) application, which necessitates comprehensive clinical trials demonstrating both safety and efficacy.
The European Union operates under the Medical Device Regulation (MDR 2017/745), which replaced the previous Medical Device Directive in 2021. This framework introduced more rigorous requirements for clinical evaluation, post-market surveillance, and unique device identification. Implantable sensors are typically classified as Class III devices, requiring conformity assessment by a Notified Body and CE marking before market access.
International standards play a crucial role in establishing uniform safety criteria. ISO 10993 series specifically addresses the biological evaluation of medical devices, with ISO 10993-1 providing a framework for risk assessment. For implantable transient sensors, ISO 14708 (Implantable Medical Devices) offers additional guidance on particular requirements for active implantable medical devices.
Regulatory bodies increasingly focus on the degradation products of transient sensors. The FDA's guidance on biocompatibility testing emphasizes the need for comprehensive toxicological risk assessments of all materials and their degradation byproducts. This includes evaluation of local tissue responses, systemic toxicity, and potential immunological reactions over the entire lifecycle of the device.
Japan's Pharmaceuticals and Medical Devices Agency (PMDA) and China's National Medical Products Administration (NMPA) have established their own regulatory frameworks, though they generally harmonize with international standards. Both require extensive preclinical and clinical data before approval, with particular emphasis on biocompatibility and safety monitoring.
Recent regulatory trends indicate a shift toward adaptive licensing pathways for innovative medical technologies. The FDA's Breakthrough Devices Program and the EU's MDCG guidance on clinical evaluation provide accelerated review processes for novel technologies that address unmet medical needs, potentially benefiting developers of transient sensor technologies.
Post-market surveillance requirements have become increasingly stringent across all major markets. Manufacturers must implement robust systems for tracking device performance, adverse events, and long-term safety outcomes. For transient sensors specifically, monitoring the complete degradation process and its physiological impact represents a unique regulatory challenge that must be addressed through comprehensive risk management strategies.
The European Union operates under the Medical Device Regulation (MDR 2017/745), which replaced the previous Medical Device Directive in 2021. This framework introduced more rigorous requirements for clinical evaluation, post-market surveillance, and unique device identification. Implantable sensors are typically classified as Class III devices, requiring conformity assessment by a Notified Body and CE marking before market access.
International standards play a crucial role in establishing uniform safety criteria. ISO 10993 series specifically addresses the biological evaluation of medical devices, with ISO 10993-1 providing a framework for risk assessment. For implantable transient sensors, ISO 14708 (Implantable Medical Devices) offers additional guidance on particular requirements for active implantable medical devices.
Regulatory bodies increasingly focus on the degradation products of transient sensors. The FDA's guidance on biocompatibility testing emphasizes the need for comprehensive toxicological risk assessments of all materials and their degradation byproducts. This includes evaluation of local tissue responses, systemic toxicity, and potential immunological reactions over the entire lifecycle of the device.
Japan's Pharmaceuticals and Medical Devices Agency (PMDA) and China's National Medical Products Administration (NMPA) have established their own regulatory frameworks, though they generally harmonize with international standards. Both require extensive preclinical and clinical data before approval, with particular emphasis on biocompatibility and safety monitoring.
Recent regulatory trends indicate a shift toward adaptive licensing pathways for innovative medical technologies. The FDA's Breakthrough Devices Program and the EU's MDCG guidance on clinical evaluation provide accelerated review processes for novel technologies that address unmet medical needs, potentially benefiting developers of transient sensor technologies.
Post-market surveillance requirements have become increasingly stringent across all major markets. Manufacturers must implement robust systems for tracking device performance, adverse events, and long-term safety outcomes. For transient sensors specifically, monitoring the complete degradation process and its physiological impact represents a unique regulatory challenge that must be addressed through comprehensive risk management strategies.
Bioethical Considerations and Patient Safety Standards
The ethical framework surrounding implantable transient sensors demands rigorous consideration of patient autonomy, informed consent, and the right to bodily integrity. These bioethical principles must be balanced with technological advancement to ensure that innovation does not compromise patient welfare. Current bioethical standards require transparent communication about the nature, purpose, and potential risks of implantable devices, particularly those designed to degrade within the body over time.
Patient safety standards for biocompatible materials in transient sensors have evolved significantly, with regulatory bodies including the FDA, EMA, and ISO establishing comprehensive guidelines. ISO 10993 series specifically addresses the biological evaluation of medical devices, with particular emphasis on implantable technologies. These standards mandate extensive testing protocols including cytotoxicity, sensitization, irritation, acute systemic toxicity, and genotoxicity assessments for all materials intended for implantation.
The concept of "do no harm" remains paramount in the development of transient sensor technologies. This necessitates not only initial safety testing but also long-term monitoring systems to track potential delayed adverse reactions. The degradation products of transient materials present unique challenges, requiring thorough characterization of all breakdown components and their potential biological interactions throughout the entire lifecycle of the device.
Privacy considerations have become increasingly significant as implantable sensors often collect sensitive health data. Regulatory frameworks such as GDPR in Europe and HIPAA in the United States establish requirements for data protection, while ethical guidelines emphasize the importance of maintaining patient confidentiality and preventing unauthorized access to sensor-collected information.
Risk-benefit analysis methodologies have been standardized for implantable devices, with particular attention to vulnerable populations. Special considerations apply to pediatric patients, elderly individuals, and those with compromised immune systems, where the safety threshold for biocompatible materials must be exceptionally high. These populations may experience different degradation kinetics or immune responses to transient materials.
The principle of justice in healthcare delivery necessitates equitable access to advanced medical technologies. This raises questions about the cost and accessibility of transient sensor technologies, particularly in resource-limited settings. Ethical frameworks increasingly emphasize the importance of developing technologies that can be widely implemented across diverse healthcare systems.
Emerging ethical considerations include the psychological impact of implantable devices on patient identity and bodily integrity, even when these devices are designed to be temporary. Patient advocacy groups have called for greater involvement in the development and testing phases of new implantable technologies, ensuring that end-user perspectives inform safety criteria and design parameters.
Patient safety standards for biocompatible materials in transient sensors have evolved significantly, with regulatory bodies including the FDA, EMA, and ISO establishing comprehensive guidelines. ISO 10993 series specifically addresses the biological evaluation of medical devices, with particular emphasis on implantable technologies. These standards mandate extensive testing protocols including cytotoxicity, sensitization, irritation, acute systemic toxicity, and genotoxicity assessments for all materials intended for implantation.
The concept of "do no harm" remains paramount in the development of transient sensor technologies. This necessitates not only initial safety testing but also long-term monitoring systems to track potential delayed adverse reactions. The degradation products of transient materials present unique challenges, requiring thorough characterization of all breakdown components and their potential biological interactions throughout the entire lifecycle of the device.
Privacy considerations have become increasingly significant as implantable sensors often collect sensitive health data. Regulatory frameworks such as GDPR in Europe and HIPAA in the United States establish requirements for data protection, while ethical guidelines emphasize the importance of maintaining patient confidentiality and preventing unauthorized access to sensor-collected information.
Risk-benefit analysis methodologies have been standardized for implantable devices, with particular attention to vulnerable populations. Special considerations apply to pediatric patients, elderly individuals, and those with compromised immune systems, where the safety threshold for biocompatible materials must be exceptionally high. These populations may experience different degradation kinetics or immune responses to transient materials.
The principle of justice in healthcare delivery necessitates equitable access to advanced medical technologies. This raises questions about the cost and accessibility of transient sensor technologies, particularly in resource-limited settings. Ethical frameworks increasingly emphasize the importance of developing technologies that can be widely implemented across diverse healthcare systems.
Emerging ethical considerations include the psychological impact of implantable devices on patient identity and bodily integrity, even when these devices are designed to be temporary. Patient advocacy groups have called for greater involvement in the development and testing phases of new implantable technologies, ensuring that end-user perspectives inform safety criteria and design parameters.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!