Standards And Certification Pathways For Transient Electronic Devices
SEP 1, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Transient Electronics Background and Objectives
Transient electronics represents a revolutionary paradigm shift in electronic device design and functionality, characterized by the ability to physically disappear or degrade in a controlled manner after serving their intended purpose. This emerging field has evolved from the convergence of materials science, electronic engineering, and environmental sustainability concerns over the past decade. The evolution trajectory began with biodegradable polymers as substrates, progressing to fully dissolvable electronic components including conductors, semiconductors, and dielectrics.
The development of transient electronics addresses critical challenges in multiple sectors, particularly in medical implants, environmental monitoring, and secure hardware systems. Unlike conventional electronics designed for durability and longevity, transient devices are engineered with predetermined lifespans, after which they harmlessly decompose in biological environments or natural settings without requiring retrieval or generating electronic waste.
Current technical objectives in this field focus on establishing reliable degradation mechanisms, predictable dissolution rates, and functional performance comparable to conventional electronics during their operational lifetime. Researchers aim to develop materials and fabrication processes that enable precise control over device lifetime while maintaining electrical performance integrity until the designated dissolution point.
A significant challenge lies in standardizing the performance metrics and dissolution characteristics across different transient electronic platforms. The absence of universally accepted testing protocols and certification pathways has hindered widespread commercial adoption despite promising laboratory demonstrations. This technical gap necessitates comprehensive standards that can validate both functionality and transience claims.
The environmental impact potential of transient electronics represents a paradigm shift in sustainable technology development. By designing electronics that naturally degrade after use, the industry could significantly reduce electronic waste accumulation, which currently exceeds 50 million metric tons annually worldwide. This aligns with global sustainability initiatives and circular economy principles gaining momentum across technology sectors.
Medical applications represent a primary target for transient electronics development, with objectives centered on creating implantable diagnostic and therapeutic devices that eliminate secondary surgical removal procedures. These include dissolvable sensors for post-operative monitoring, temporary neural interfaces, and drug delivery systems with programmable degradation timelines.
The technical evolution of this field aims to establish fundamental design principles, material selection criteria, and manufacturing processes that can be standardized across the industry. This standardization would facilitate regulatory approval pathways, particularly for medical and environmental applications where safety and reliability are paramount concerns.
The development of transient electronics addresses critical challenges in multiple sectors, particularly in medical implants, environmental monitoring, and secure hardware systems. Unlike conventional electronics designed for durability and longevity, transient devices are engineered with predetermined lifespans, after which they harmlessly decompose in biological environments or natural settings without requiring retrieval or generating electronic waste.
Current technical objectives in this field focus on establishing reliable degradation mechanisms, predictable dissolution rates, and functional performance comparable to conventional electronics during their operational lifetime. Researchers aim to develop materials and fabrication processes that enable precise control over device lifetime while maintaining electrical performance integrity until the designated dissolution point.
A significant challenge lies in standardizing the performance metrics and dissolution characteristics across different transient electronic platforms. The absence of universally accepted testing protocols and certification pathways has hindered widespread commercial adoption despite promising laboratory demonstrations. This technical gap necessitates comprehensive standards that can validate both functionality and transience claims.
The environmental impact potential of transient electronics represents a paradigm shift in sustainable technology development. By designing electronics that naturally degrade after use, the industry could significantly reduce electronic waste accumulation, which currently exceeds 50 million metric tons annually worldwide. This aligns with global sustainability initiatives and circular economy principles gaining momentum across technology sectors.
Medical applications represent a primary target for transient electronics development, with objectives centered on creating implantable diagnostic and therapeutic devices that eliminate secondary surgical removal procedures. These include dissolvable sensors for post-operative monitoring, temporary neural interfaces, and drug delivery systems with programmable degradation timelines.
The technical evolution of this field aims to establish fundamental design principles, material selection criteria, and manufacturing processes that can be standardized across the industry. This standardization would facilitate regulatory approval pathways, particularly for medical and environmental applications where safety and reliability are paramount concerns.
Market Analysis for Degradable Electronic Solutions
The global market for degradable electronic solutions is experiencing significant growth, driven by increasing environmental concerns and regulatory pressures. Current market valuations indicate that the transient electronics sector is projected to reach approximately 3.5 billion USD by 2028, with a compound annual growth rate exceeding 20% between 2023 and 2028. This remarkable growth trajectory is primarily fueled by heightened awareness of electronic waste management challenges and the push toward sustainable technology solutions.
Consumer electronics represents the largest application segment for degradable electronic solutions, accounting for nearly 40% of the market share. This dominance stems from the short lifecycle of consumer devices and growing consumer preference for environmentally responsible products. Healthcare applications follow closely, with biodegradable medical implants and transient diagnostic devices gaining substantial traction due to their ability to eliminate secondary surgical procedures for device removal.
Regional analysis reveals that North America currently leads the market with approximately 35% share, attributed to advanced research infrastructure and favorable regulatory frameworks supporting innovative electronic materials. However, the Asia-Pacific region is expected to witness the fastest growth rate, driven by rapid industrialization, expanding electronics manufacturing capabilities, and increasing adoption of green technologies in countries like China, Japan, and South Korea.
Key market drivers include stringent environmental regulations limiting electronic waste disposal, growing corporate sustainability initiatives, and increasing consumer awareness about environmental impacts of traditional electronics. The European Union's Waste Electrical and Electronic Equipment (WEEE) Directive and similar regulations worldwide are creating strong market pull for degradable alternatives to conventional electronic components.
Market challenges primarily revolve around cost considerations, as degradable electronic solutions currently command a premium of 30-50% over traditional counterparts. Performance limitations and reliability concerns also present significant barriers to widespread adoption, particularly in applications requiring extended operational lifespans or harsh environmental conditions.
Emerging market opportunities exist in temporary electronics for specialized applications such as environmental monitoring, agricultural sensing, and military surveillance. The integration of degradable electronics with Internet of Things (IoT) technologies represents another promising growth avenue, potentially creating a specialized market segment valued at over 1 billion USD by 2030.
Customer segmentation analysis indicates that early adopters primarily include research institutions, healthcare providers, and environmentally conscious technology companies. Mass market penetration remains limited but is expected to accelerate as production scales drive costs down and performance characteristics improve through continued research and development efforts.
Consumer electronics represents the largest application segment for degradable electronic solutions, accounting for nearly 40% of the market share. This dominance stems from the short lifecycle of consumer devices and growing consumer preference for environmentally responsible products. Healthcare applications follow closely, with biodegradable medical implants and transient diagnostic devices gaining substantial traction due to their ability to eliminate secondary surgical procedures for device removal.
Regional analysis reveals that North America currently leads the market with approximately 35% share, attributed to advanced research infrastructure and favorable regulatory frameworks supporting innovative electronic materials. However, the Asia-Pacific region is expected to witness the fastest growth rate, driven by rapid industrialization, expanding electronics manufacturing capabilities, and increasing adoption of green technologies in countries like China, Japan, and South Korea.
Key market drivers include stringent environmental regulations limiting electronic waste disposal, growing corporate sustainability initiatives, and increasing consumer awareness about environmental impacts of traditional electronics. The European Union's Waste Electrical and Electronic Equipment (WEEE) Directive and similar regulations worldwide are creating strong market pull for degradable alternatives to conventional electronic components.
Market challenges primarily revolve around cost considerations, as degradable electronic solutions currently command a premium of 30-50% over traditional counterparts. Performance limitations and reliability concerns also present significant barriers to widespread adoption, particularly in applications requiring extended operational lifespans or harsh environmental conditions.
Emerging market opportunities exist in temporary electronics for specialized applications such as environmental monitoring, agricultural sensing, and military surveillance. The integration of degradable electronics with Internet of Things (IoT) technologies represents another promising growth avenue, potentially creating a specialized market segment valued at over 1 billion USD by 2030.
Customer segmentation analysis indicates that early adopters primarily include research institutions, healthcare providers, and environmentally conscious technology companies. Mass market penetration remains limited but is expected to accelerate as production scales drive costs down and performance characteristics improve through continued research and development efforts.
Current Certification Challenges for Transient Devices
Transient electronic devices face significant certification challenges due to their unique nature and intended functionality. Unlike conventional electronics designed for durability, these devices are purposefully created to degrade or dissolve under specific conditions. This fundamental difference creates a regulatory gap as current certification frameworks were established for permanent electronics with stable performance characteristics over time.
The primary challenge lies in the absence of standardized testing methodologies specifically designed for transient electronics. Existing protocols for electronic device certification typically evaluate long-term reliability, whereas transient devices require assessment of controlled degradation patterns and end-of-life behavior. Regulatory bodies such as the FDA, FCC, and ISO have yet to establish clear guidelines addressing the unique performance metrics relevant to transient electronics.
For medical applications, which represent a significant potential market for transient electronics, the certification pathway is particularly complex. Current medical device regulations require extensive stability testing and shelf-life validation, concepts that are fundamentally at odds with devices designed to disappear. The FDA's premarket approval process lacks specific provisions for evaluating the safety of degradation byproducts or the predictability of dissolution timelines.
Environmental certification presents another significant hurdle. While transient electronics offer potential environmental benefits through reduced e-waste, paradoxically, they face challenges in obtaining eco-certifications. Current environmental standards often evaluate recyclability rather than biodegradability or benign dissolution, creating a misalignment with the core value proposition of transient technologies.
Material safety certification represents a critical gap in the regulatory landscape. As transient devices degrade, they release various compounds into their surrounding environment. Current toxicity assessment frameworks are not optimized to evaluate the dynamic release profiles characteristic of dissolving electronics, particularly in biological contexts where these devices may be implanted or ingested.
Electromagnetic compliance testing for transient devices introduces additional complexity. As these devices degrade, their electromagnetic properties may change, potentially affecting their compliance with FCC or CE standards over their functional lifetime. Current certification processes typically evaluate devices at a single point in time rather than across a degradation continuum.
Performance reliability standards present perhaps the most fundamental contradiction. Traditional certification approaches emphasize consistent performance throughout a device's lifetime, while transient electronics must balance reliable functionality during their operational period with predictable degradation thereafter. This dual requirement has no precedent in existing certification frameworks, leaving manufacturers without clear guidance on validation requirements.
The primary challenge lies in the absence of standardized testing methodologies specifically designed for transient electronics. Existing protocols for electronic device certification typically evaluate long-term reliability, whereas transient devices require assessment of controlled degradation patterns and end-of-life behavior. Regulatory bodies such as the FDA, FCC, and ISO have yet to establish clear guidelines addressing the unique performance metrics relevant to transient electronics.
For medical applications, which represent a significant potential market for transient electronics, the certification pathway is particularly complex. Current medical device regulations require extensive stability testing and shelf-life validation, concepts that are fundamentally at odds with devices designed to disappear. The FDA's premarket approval process lacks specific provisions for evaluating the safety of degradation byproducts or the predictability of dissolution timelines.
Environmental certification presents another significant hurdle. While transient electronics offer potential environmental benefits through reduced e-waste, paradoxically, they face challenges in obtaining eco-certifications. Current environmental standards often evaluate recyclability rather than biodegradability or benign dissolution, creating a misalignment with the core value proposition of transient technologies.
Material safety certification represents a critical gap in the regulatory landscape. As transient devices degrade, they release various compounds into their surrounding environment. Current toxicity assessment frameworks are not optimized to evaluate the dynamic release profiles characteristic of dissolving electronics, particularly in biological contexts where these devices may be implanted or ingested.
Electromagnetic compliance testing for transient devices introduces additional complexity. As these devices degrade, their electromagnetic properties may change, potentially affecting their compliance with FCC or CE standards over their functional lifetime. Current certification processes typically evaluate devices at a single point in time rather than across a degradation continuum.
Performance reliability standards present perhaps the most fundamental contradiction. Traditional certification approaches emphasize consistent performance throughout a device's lifetime, while transient electronics must balance reliable functionality during their operational period with predictable degradation thereafter. This dual requirement has no precedent in existing certification frameworks, leaving manufacturers without clear guidance on validation requirements.
Existing Certification Frameworks and Methodologies
01 Certification standards for transient electronic devices
Transient electronic devices require specific certification standards to ensure their safety, reliability, and proper functionality. These standards define the requirements for testing, validation, and certification of transient electronic devices before they can be marketed or used in various applications. The certification process typically involves evaluating the device's performance, durability, and compliance with industry regulations.- Certification standards for transient electronic devices: Transient electronic devices require specific certification standards to ensure their safety, reliability, and proper functionality. These standards define the requirements for testing, validation, and certification of transient electronic devices before they can be marketed or used in various applications. The certification process typically involves assessment of the device's performance, durability, and compliance with relevant regulations.
- Security protocols for transient electronic systems: Security protocols are essential for transient electronic devices to protect sensitive data and prevent unauthorized access. These protocols include encryption methods, authentication mechanisms, and secure communication channels. Implementing robust security measures ensures that transient electronic devices can safely handle confidential information while maintaining their temporary nature, which is particularly important in applications involving personal or financial data.
- Environmental compliance and sustainability standards: Environmental standards for transient electronic devices focus on their biodegradability, recyclability, and overall environmental impact. These standards ensure that transient devices decompose safely after their intended use period without releasing harmful substances into the environment. Compliance with these standards involves using eco-friendly materials, designing for minimal waste generation, and implementing proper disposal protocols to minimize the ecological footprint of these temporary electronic systems.
- Performance validation and testing methodologies: Specific testing methodologies are required to validate the performance of transient electronic devices, particularly regarding their controlled degradation characteristics. These methodologies include accelerated aging tests, environmental stress testing, and functionality assessments under various conditions. The testing protocols ensure that transient devices perform reliably during their intended operational lifetime and degrade predictably afterward, which is crucial for applications in medical implants, environmental monitoring, and temporary security systems.
- Regulatory frameworks for commercial deployment: Regulatory frameworks govern the commercial deployment of transient electronic devices across different industries and regions. These frameworks include licensing requirements, market authorization procedures, and compliance with industry-specific regulations. Navigating these regulatory landscapes is essential for manufacturers and developers to legally market and distribute transient electronic products, particularly in highly regulated sectors such as healthcare, defense, and consumer electronics.
02 Security protocols for transient electronic systems
Security protocols are essential for transient electronic devices to protect sensitive data and prevent unauthorized access. These protocols include encryption methods, authentication mechanisms, and secure communication channels. Implementing robust security measures ensures that transient electronic devices can operate safely in various environments while maintaining data integrity and confidentiality throughout their operational lifecycle.Expand Specific Solutions03 Verification and validation methodologies
Verification and validation methodologies are crucial for ensuring that transient electronic devices meet their intended specifications and performance requirements. These methodologies involve systematic testing procedures, quality assurance protocols, and compliance checks against established standards. By implementing comprehensive verification and validation processes, manufacturers can ensure the reliability and functionality of transient electronic devices before deployment.Expand Specific Solutions04 Electronic transaction certification for temporary devices
Electronic transaction certification systems are designed to authenticate and secure transactions conducted through transient electronic devices. These systems implement digital signatures, secure payment protocols, and transaction verification mechanisms to ensure the integrity and legitimacy of electronic transactions. Certification standards for electronic transactions help prevent fraud and establish trust in transient electronic payment systems.Expand Specific Solutions05 Biomedical standards for transient electronics
Biomedical applications of transient electronic devices require specialized standards and certification processes to ensure patient safety and device efficacy. These standards address biocompatibility, sterility, degradation behavior, and performance requirements specific to medical applications. Certification for biomedical transient electronics involves rigorous testing protocols and compliance with healthcare regulations to validate their safety for use in or on the human body.Expand Specific Solutions
Leading Organizations in Transient Electronics Standardization
The transient electronics standards and certification landscape is currently in an early development stage, characterized by emerging regulatory frameworks and growing market interest. The global market for transient electronic devices is expanding rapidly, projected to reach significant scale as applications in medical, environmental, and security sectors gain traction. From a technical maturity perspective, the field shows varying degrees of advancement among key players. Academic institutions like the University of Illinois and Tufts College are pioneering fundamental research, while major technology corporations including IBM, Microsoft, Samsung, and Huawei are developing commercial applications. State Grid Corporation of China and telecommunications companies such as ZTE and British Telecommunications are exploring infrastructure implementations. The ecosystem also includes specialized manufacturers like FlexEnable and STMicroelectronics advancing material science and fabrication techniques necessary for standardization pathways.
The Board of Trustees of the University of Illinois
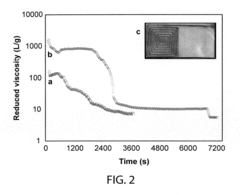
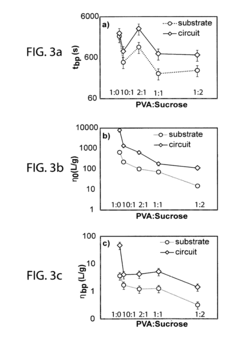
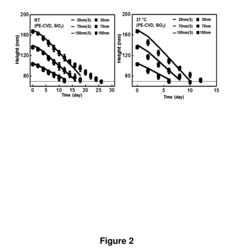
Technical Solution: The University of Illinois has pioneered significant advancements in transient electronics through their development of water-soluble electronic components that can safely dissolve in biological environments. Their technology utilizes biocompatible materials such as silicon nanomembranes, magnesium conductors, and silk protein encapsulation to create devices with controlled dissolution rates. The university's research team has established comprehensive testing protocols for verifying dissolution characteristics and biocompatibility, working closely with the FDA to develop certification pathways for implantable transient medical devices. Their standardization efforts focus on establishing metrics for dissolution time, degradation byproducts safety, and functional performance during the operational lifetime, creating a framework that balances innovation with patient safety requirements.
Strengths: Strong academic research foundation with extensive laboratory testing capabilities and established relationships with regulatory bodies. Weaknesses: May face challenges in scaling laboratory innovations to commercial manufacturing standards and navigating the complex regulatory landscape without industrial partners.
International Business Machines Corp.
Technical Solution: IBM has developed a comprehensive approach to transient electronics certification focusing on environmentally triggered self-destruction mechanisms for secure data applications. Their "trigger-and-forget" technology platform incorporates specialized silicon-based substrates with engineered stress points that can be activated remotely, causing complete device disintegration within seconds. IBM's certification pathway emphasizes security standards, establishing verification protocols for complete data elimination during the transience process. The company has created a multi-tiered testing framework that evaluates transient performance across various environmental conditions, ensuring reliability in field deployments. IBM collaborates with the National Institute of Standards and Technology (NIST) to develop standardized testing methodologies for transient electronic security features, particularly for government and defense applications where data security is paramount.
Strengths: Robust security-focused approach with established government partnerships and comprehensive testing infrastructure. Weaknesses: Solutions may be overly specialized for high-security applications, potentially limiting broader commercial adoption in consumer markets.
Key Technical Standards for Transient Electronic Materials
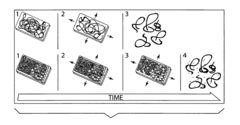
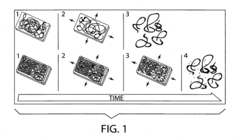
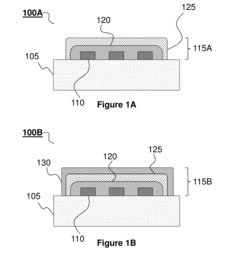
Transient electronic device
PatentInactiveUS20180053964A1
Innovation
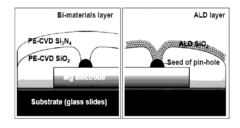
- The use of colloidal metallic particles deposited as conductive thin films on soluble polymer substrates, which are designed to re-disperse in solvent-containing media, allowing for concurrent deconstruction of both the substrate and electronic components, thereby controlling the transiency rate through the swelling force and dissolution characteristics.
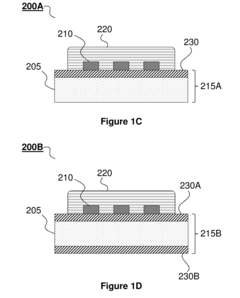
Transient Electronic Devices Comprising Inorganic or Hybrid Inorganic and Organic Substrates and Encapsulates
PatentActiveUS20170164482A1
Innovation
- The development of transient electronic devices incorporating inorganic materials as substrates and encapsulants, which can be selectively removed in response to internal or external stimuli, allowing for programmable transformation and precise control over the device's transience properties, such as temporal and physical changes.
Environmental Impact Assessment Protocols
The assessment of environmental impacts for transient electronic devices requires specialized protocols that differ significantly from those used for conventional electronics. These devices, designed to degrade or dissolve after a predetermined period, present unique challenges for environmental impact evaluation. Current assessment methodologies must be adapted to account for the temporary nature of these technologies and their intended degradation pathways.
Traditional life cycle assessment (LCA) frameworks prove inadequate when applied to transient electronics, as they typically assume product permanence rather than intentional degradation. A modified LCA approach must incorporate degradation timelines, intermediate breakdown products, and final residual components. This requires the development of new metrics that can quantify environmental benefits from reduced e-waste alongside potential risks from degradation byproducts.
Ecotoxicological testing protocols for transient electronics must evaluate both intact devices and their degradation products. Standardized leaching tests need modification to simulate various environmental conditions that might accelerate or alter degradation pathways. Aquatic toxicity testing is particularly relevant, as many transient electronics are designed to dissolve in liquid environments, potentially releasing constituent materials into water systems.
Biodegradability assessment presents another critical challenge, requiring protocols that can verify manufacturer claims about degradation rates and completeness. Current standards like ASTM D5338 or ISO 14855 for biodegradable plastics provide starting points but must be adapted for the complex material compositions found in transient electronics. These protocols should establish clear thresholds for what constitutes "acceptable" biodegradation in terms of both time frame and residual materials.
Material recovery assessment protocols represent another essential component, evaluating the potential for reclaiming valuable materials from partially degraded devices. This includes quantifying recovery efficiency and energy requirements for various reclamation processes, which may differ significantly from conventional electronics recycling methods.
Carbon footprint analysis for transient electronics must account for both manufacturing impacts and end-of-life scenarios, including the energy and resources saved by avoiding conventional disposal methods. This requires developing comparative frameworks that can accurately represent the environmental trade-offs between conventional and transient electronic technologies.
Implementation of these specialized environmental assessment protocols will require collaboration between regulatory bodies, industry stakeholders, and research institutions. The development of standardized testing methodologies will be crucial for establishing certification pathways that can verify environmental claims and ensure consumer confidence in transient electronic technologies.
Traditional life cycle assessment (LCA) frameworks prove inadequate when applied to transient electronics, as they typically assume product permanence rather than intentional degradation. A modified LCA approach must incorporate degradation timelines, intermediate breakdown products, and final residual components. This requires the development of new metrics that can quantify environmental benefits from reduced e-waste alongside potential risks from degradation byproducts.
Ecotoxicological testing protocols for transient electronics must evaluate both intact devices and their degradation products. Standardized leaching tests need modification to simulate various environmental conditions that might accelerate or alter degradation pathways. Aquatic toxicity testing is particularly relevant, as many transient electronics are designed to dissolve in liquid environments, potentially releasing constituent materials into water systems.
Biodegradability assessment presents another critical challenge, requiring protocols that can verify manufacturer claims about degradation rates and completeness. Current standards like ASTM D5338 or ISO 14855 for biodegradable plastics provide starting points but must be adapted for the complex material compositions found in transient electronics. These protocols should establish clear thresholds for what constitutes "acceptable" biodegradation in terms of both time frame and residual materials.
Material recovery assessment protocols represent another essential component, evaluating the potential for reclaiming valuable materials from partially degraded devices. This includes quantifying recovery efficiency and energy requirements for various reclamation processes, which may differ significantly from conventional electronics recycling methods.
Carbon footprint analysis for transient electronics must account for both manufacturing impacts and end-of-life scenarios, including the energy and resources saved by avoiding conventional disposal methods. This requires developing comparative frameworks that can accurately represent the environmental trade-offs between conventional and transient electronic technologies.
Implementation of these specialized environmental assessment protocols will require collaboration between regulatory bodies, industry stakeholders, and research institutions. The development of standardized testing methodologies will be crucial for establishing certification pathways that can verify environmental claims and ensure consumer confidence in transient electronic technologies.
International Regulatory Harmonization Opportunities
The fragmented nature of global regulatory frameworks presents significant challenges for the commercialization of transient electronic devices. Currently, these innovative technologies face a complex landscape of disparate standards across different regions, creating barriers to market entry and increasing compliance costs. Harmonization efforts between major regulatory bodies such as the FDA, EMA, and NMPA could substantially accelerate the global adoption of transient electronics while ensuring consistent safety and performance standards.
Key opportunities for international regulatory harmonization exist in developing unified terminology and classification systems specifically for transient electronics. The establishment of standardized definitions for terms like "controlled degradation," "programmed dissolution," and "bioabsorption kinetics" would create a common language for regulators worldwide, facilitating more efficient approval processes and reducing redundant testing requirements.
Mutual recognition agreements (MRAs) represent another promising pathway for harmonization. Expanding existing MRAs between regulatory authorities to explicitly include transient electronic devices would allow manufacturers to leverage approvals from one jurisdiction to expedite clearance in others. This approach has proven successful for conventional medical devices and could be adapted to address the unique characteristics of transient electronics.
International standards organizations such as ISO and IEC are well-positioned to lead collaborative efforts in developing globally recognized testing protocols and performance benchmarks. Working groups specifically focused on transient electronics could coordinate input from diverse stakeholders including industry representatives, academic researchers, and regulatory experts to create consensus-based standards that balance innovation with safety considerations.
The creation of international data sharing platforms for post-market surveillance would significantly enhance the regulatory oversight of transient electronic devices. Such systems would enable rapid identification of safety signals across different markets and facilitate coordinated responses to emerging concerns, ultimately strengthening consumer protection while reducing regulatory burden through resource sharing.
Regional regulatory sandboxes offer another promising approach to harmonization. These controlled testing environments allow manufacturers to evaluate transient electronic products under multiple regulatory frameworks simultaneously, generating evidence that satisfies requirements across jurisdictions while identifying opportunities for alignment. Successful sandbox initiatives in financial technology regulation provide valuable models that could be adapted for transient electronics.
Ultimately, achieving meaningful international regulatory harmonization will require sustained diplomatic engagement and technical cooperation. Industry consortia, academic institutions, and public-private partnerships all have crucial roles to play in building consensus around appropriate regulatory pathways for these revolutionary technologies.
Key opportunities for international regulatory harmonization exist in developing unified terminology and classification systems specifically for transient electronics. The establishment of standardized definitions for terms like "controlled degradation," "programmed dissolution," and "bioabsorption kinetics" would create a common language for regulators worldwide, facilitating more efficient approval processes and reducing redundant testing requirements.
Mutual recognition agreements (MRAs) represent another promising pathway for harmonization. Expanding existing MRAs between regulatory authorities to explicitly include transient electronic devices would allow manufacturers to leverage approvals from one jurisdiction to expedite clearance in others. This approach has proven successful for conventional medical devices and could be adapted to address the unique characteristics of transient electronics.
International standards organizations such as ISO and IEC are well-positioned to lead collaborative efforts in developing globally recognized testing protocols and performance benchmarks. Working groups specifically focused on transient electronics could coordinate input from diverse stakeholders including industry representatives, academic researchers, and regulatory experts to create consensus-based standards that balance innovation with safety considerations.
The creation of international data sharing platforms for post-market surveillance would significantly enhance the regulatory oversight of transient electronic devices. Such systems would enable rapid identification of safety signals across different markets and facilitate coordinated responses to emerging concerns, ultimately strengthening consumer protection while reducing regulatory burden through resource sharing.
Regional regulatory sandboxes offer another promising approach to harmonization. These controlled testing environments allow manufacturers to evaluate transient electronic products under multiple regulatory frameworks simultaneously, generating evidence that satisfies requirements across jurisdictions while identifying opportunities for alignment. Successful sandbox initiatives in financial technology regulation provide valuable models that could be adapted for transient electronics.
Ultimately, achieving meaningful international regulatory harmonization will require sustained diplomatic engagement and technical cooperation. Industry consortia, academic institutions, and public-private partnerships all have crucial roles to play in building consensus around appropriate regulatory pathways for these revolutionary technologies.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!