Electrode Material Tradeoffs For Biodegradable Supercapacitors
SEP 1, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Biodegradable Supercapacitor Background and Objectives
Biodegradable supercapacitors represent a significant advancement in sustainable energy storage technology, emerging from the growing need for environmentally friendly power solutions. These devices combine the high power density and rapid charge-discharge capabilities of conventional supercapacitors with materials that can naturally decompose at their end-of-life, addressing critical environmental concerns associated with electronic waste accumulation.
The evolution of supercapacitor technology dates back to the 1950s, with commercial development beginning in the 1970s. However, the specific focus on biodegradability is relatively recent, gaining momentum in the past decade as sustainability has become a central concern in technological development. This shift has been driven by increasing electronic waste problems and stricter environmental regulations worldwide.
Biodegradable supercapacitors aim to maintain performance metrics comparable to conventional devices while introducing end-of-life degradability. Key technical objectives include achieving energy densities of 5-15 Wh/kg, power densities exceeding 10 kW/kg, cycle stability of at least 10,000 cycles, and complete biodegradation within 6-24 months under appropriate environmental conditions.
Electrode materials represent the most critical component in biodegradable supercapacitor design, directly influencing device performance, lifespan, and environmental impact. Traditional supercapacitors rely heavily on carbon-based materials like activated carbon, graphene, and carbon nanotubes, often combined with metal oxides that pose environmental concerns. The challenge lies in finding biodegradable alternatives that can deliver comparable electrical performance.
Current research focuses on several promising biodegradable electrode material categories: carbon-based materials derived from biomass (including activated carbon from agricultural waste, cellulose-derived carbons, and lignin-based materials), conducting polymers (particularly polypyrrole, polyaniline, and PEDOT:PSS modified for biodegradability), and natural polymers with conductive properties (such as polysaccharides and protein-based materials).
The technical trajectory aims to develop electrode materials that balance four critical factors: electrical performance (conductivity and surface area), mechanical stability during operation, controlled biodegradation after disposal, and cost-effectiveness for commercial viability. This balance represents the central challenge in the field.
Recent breakthroughs include the development of cellulose-derived carbon electrodes with specific capacitances approaching 200 F/g, biodegradable conducting polymer composites with enhanced stability, and novel electrolyte systems that facilitate both device performance and eventual decomposition. These advances suggest that commercially viable biodegradable supercapacitors could reach the market within the next 3-5 years, particularly for applications in wearable electronics, environmental sensors, and medical implants.
The evolution of supercapacitor technology dates back to the 1950s, with commercial development beginning in the 1970s. However, the specific focus on biodegradability is relatively recent, gaining momentum in the past decade as sustainability has become a central concern in technological development. This shift has been driven by increasing electronic waste problems and stricter environmental regulations worldwide.
Biodegradable supercapacitors aim to maintain performance metrics comparable to conventional devices while introducing end-of-life degradability. Key technical objectives include achieving energy densities of 5-15 Wh/kg, power densities exceeding 10 kW/kg, cycle stability of at least 10,000 cycles, and complete biodegradation within 6-24 months under appropriate environmental conditions.
Electrode materials represent the most critical component in biodegradable supercapacitor design, directly influencing device performance, lifespan, and environmental impact. Traditional supercapacitors rely heavily on carbon-based materials like activated carbon, graphene, and carbon nanotubes, often combined with metal oxides that pose environmental concerns. The challenge lies in finding biodegradable alternatives that can deliver comparable electrical performance.
Current research focuses on several promising biodegradable electrode material categories: carbon-based materials derived from biomass (including activated carbon from agricultural waste, cellulose-derived carbons, and lignin-based materials), conducting polymers (particularly polypyrrole, polyaniline, and PEDOT:PSS modified for biodegradability), and natural polymers with conductive properties (such as polysaccharides and protein-based materials).
The technical trajectory aims to develop electrode materials that balance four critical factors: electrical performance (conductivity and surface area), mechanical stability during operation, controlled biodegradation after disposal, and cost-effectiveness for commercial viability. This balance represents the central challenge in the field.
Recent breakthroughs include the development of cellulose-derived carbon electrodes with specific capacitances approaching 200 F/g, biodegradable conducting polymer composites with enhanced stability, and novel electrolyte systems that facilitate both device performance and eventual decomposition. These advances suggest that commercially viable biodegradable supercapacitors could reach the market within the next 3-5 years, particularly for applications in wearable electronics, environmental sensors, and medical implants.
Market Analysis for Green Energy Storage Solutions
The global market for green energy storage solutions is experiencing unprecedented growth, driven by increasing environmental concerns and the push for sustainable development. Biodegradable supercapacitors represent a promising segment within this market, addressing the critical need for energy storage technologies that minimize environmental impact throughout their lifecycle. Current market valuations indicate that the green energy storage sector is projected to reach significant market share by 2030, with biodegradable solutions expected to capture an expanding portion of this growth.
Consumer demand for environmentally responsible products has created favorable market conditions for biodegradable supercapacitors. This trend is particularly evident in regions with stringent environmental regulations such as the European Union, where legislation increasingly mandates end-of-life considerations for electronic components. The market is further bolstered by corporate sustainability initiatives, with major technology companies publicly committing to reducing electronic waste in their supply chains.
Market segmentation reveals diverse application potential for biodegradable supercapacitors. The wearable electronics sector shows particular promise, with biodegradable power sources aligning perfectly with the temporary usage patterns of medical monitoring devices and smart textiles. Additionally, environmental sensing applications represent a substantial market opportunity, where deployed sensors often remain in natural settings, making biodegradability a critical feature.
Competitive analysis indicates that while traditional lithium-ion batteries continue to dominate the broader energy storage market, biodegradable supercapacitors are carving out specialized niches where their unique properties offer compelling advantages. The reduced environmental liability and potential for lower lifecycle costs are becoming increasingly attractive selling points for environmentally conscious consumers and businesses alike.
Market barriers include price sensitivity, with biodegradable solutions currently commanding premium pricing compared to conventional alternatives. Technical performance limitations also present challenges, as many potential customers require energy storage solutions that match or exceed the capabilities of established technologies. However, these barriers are gradually eroding as research advances and economies of scale begin to take effect.
Regional market analysis shows varying adoption rates, with developed economies currently leading in market uptake due to stronger environmental regulations and greater consumer willingness to pay premiums for sustainable technologies. Emerging economies represent significant growth potential as environmental awareness increases and manufacturing capabilities expand in these regions.
The market outlook for electrode materials in biodegradable supercapacitors is particularly promising, with sustainable carbon-based materials, conductive polymers, and metal-organic frameworks showing strong commercial potential. Industry forecasts suggest that as production scales and technology matures, these materials will become increasingly cost-competitive with traditional alternatives, further accelerating market adoption.
Consumer demand for environmentally responsible products has created favorable market conditions for biodegradable supercapacitors. This trend is particularly evident in regions with stringent environmental regulations such as the European Union, where legislation increasingly mandates end-of-life considerations for electronic components. The market is further bolstered by corporate sustainability initiatives, with major technology companies publicly committing to reducing electronic waste in their supply chains.
Market segmentation reveals diverse application potential for biodegradable supercapacitors. The wearable electronics sector shows particular promise, with biodegradable power sources aligning perfectly with the temporary usage patterns of medical monitoring devices and smart textiles. Additionally, environmental sensing applications represent a substantial market opportunity, where deployed sensors often remain in natural settings, making biodegradability a critical feature.
Competitive analysis indicates that while traditional lithium-ion batteries continue to dominate the broader energy storage market, biodegradable supercapacitors are carving out specialized niches where their unique properties offer compelling advantages. The reduced environmental liability and potential for lower lifecycle costs are becoming increasingly attractive selling points for environmentally conscious consumers and businesses alike.
Market barriers include price sensitivity, with biodegradable solutions currently commanding premium pricing compared to conventional alternatives. Technical performance limitations also present challenges, as many potential customers require energy storage solutions that match or exceed the capabilities of established technologies. However, these barriers are gradually eroding as research advances and economies of scale begin to take effect.
Regional market analysis shows varying adoption rates, with developed economies currently leading in market uptake due to stronger environmental regulations and greater consumer willingness to pay premiums for sustainable technologies. Emerging economies represent significant growth potential as environmental awareness increases and manufacturing capabilities expand in these regions.
The market outlook for electrode materials in biodegradable supercapacitors is particularly promising, with sustainable carbon-based materials, conductive polymers, and metal-organic frameworks showing strong commercial potential. Industry forecasts suggest that as production scales and technology matures, these materials will become increasingly cost-competitive with traditional alternatives, further accelerating market adoption.
Current Electrode Materials and Technical Challenges
Biodegradable supercapacitors represent a promising solution for sustainable energy storage, with electrode materials playing a crucial role in their performance. Currently, the most widely used electrode materials include carbon-based materials, conducting polymers, and metal-based compounds, each offering distinct advantages and limitations.
Carbon-based materials such as activated carbon, carbon nanotubes (CNTs), and graphene have gained significant attention due to their high surface area, excellent electrical conductivity, and relatively good stability. Activated carbon derived from biomass sources like cellulose, lignin, and chitin demonstrates promising biodegradability while maintaining acceptable capacitive performance. However, these materials often suffer from limited energy density and variable degradation rates depending on environmental conditions.
Conducting polymers, including polyaniline (PANI), polypyrrole (PPy), and poly(3,4-ethylenedioxythiophene) (PEDOT), offer pseudocapacitive properties that enhance energy density. Their organic nature makes them potentially biodegradable, particularly when synthesized from natural monomers. Nevertheless, these materials face challenges related to cycling stability, with performance degradation occurring after repeated charge-discharge cycles.
Metal-based compounds, especially transition metal oxides and hydroxides (MnO2, Fe2O3, Ni(OH)2), provide high theoretical capacitance but present significant biodegradability challenges. Recent research has focused on incorporating these materials into biodegradable matrices or developing metal-organic frameworks with controlled degradation pathways.
A major technical challenge in this field involves balancing the trade-off between electrical performance and biodegradability. Materials with excellent capacitive properties often exhibit poor degradation characteristics, while highly biodegradable materials typically deliver suboptimal electrical performance. This fundamental tension necessitates innovative composite approaches and novel material design strategies.
Another significant challenge is controlling degradation kinetics. Ideal biodegradable supercapacitors should maintain stable performance during their operational lifetime and then degrade rapidly when disposed of. Achieving this precise temporal control remains difficult, as environmental factors like temperature, humidity, and microbial activity significantly influence degradation rates.
Fabrication techniques present additional hurdles. Traditional electrode manufacturing often involves non-biodegradable binders and current collectors, compromising the overall biodegradability of the device. Developing fully biodegradable assembly methods requires rethinking conventional fabrication approaches.
Standardization of testing protocols represents another obstacle. Currently, there is no universally accepted methodology for evaluating both the electrochemical performance and biodegradation characteristics of electrode materials, making direct comparisons between different research efforts challenging and potentially misleading.
Addressing these challenges requires interdisciplinary collaboration between materials scientists, electrochemists, and environmental engineers to develop next-generation electrode materials that effectively balance performance requirements with environmental considerations.
Carbon-based materials such as activated carbon, carbon nanotubes (CNTs), and graphene have gained significant attention due to their high surface area, excellent electrical conductivity, and relatively good stability. Activated carbon derived from biomass sources like cellulose, lignin, and chitin demonstrates promising biodegradability while maintaining acceptable capacitive performance. However, these materials often suffer from limited energy density and variable degradation rates depending on environmental conditions.
Conducting polymers, including polyaniline (PANI), polypyrrole (PPy), and poly(3,4-ethylenedioxythiophene) (PEDOT), offer pseudocapacitive properties that enhance energy density. Their organic nature makes them potentially biodegradable, particularly when synthesized from natural monomers. Nevertheless, these materials face challenges related to cycling stability, with performance degradation occurring after repeated charge-discharge cycles.
Metal-based compounds, especially transition metal oxides and hydroxides (MnO2, Fe2O3, Ni(OH)2), provide high theoretical capacitance but present significant biodegradability challenges. Recent research has focused on incorporating these materials into biodegradable matrices or developing metal-organic frameworks with controlled degradation pathways.
A major technical challenge in this field involves balancing the trade-off between electrical performance and biodegradability. Materials with excellent capacitive properties often exhibit poor degradation characteristics, while highly biodegradable materials typically deliver suboptimal electrical performance. This fundamental tension necessitates innovative composite approaches and novel material design strategies.
Another significant challenge is controlling degradation kinetics. Ideal biodegradable supercapacitors should maintain stable performance during their operational lifetime and then degrade rapidly when disposed of. Achieving this precise temporal control remains difficult, as environmental factors like temperature, humidity, and microbial activity significantly influence degradation rates.
Fabrication techniques present additional hurdles. Traditional electrode manufacturing often involves non-biodegradable binders and current collectors, compromising the overall biodegradability of the device. Developing fully biodegradable assembly methods requires rethinking conventional fabrication approaches.
Standardization of testing protocols represents another obstacle. Currently, there is no universally accepted methodology for evaluating both the electrochemical performance and biodegradation characteristics of electrode materials, making direct comparisons between different research efforts challenging and potentially misleading.
Addressing these challenges requires interdisciplinary collaboration between materials scientists, electrochemists, and environmental engineers to develop next-generation electrode materials that effectively balance performance requirements with environmental considerations.
Current Electrode Material Solutions and Tradeoffs
01 Carbon-based biodegradable electrode materials
Carbon-based materials derived from sustainable sources are widely used in biodegradable supercapacitor electrodes. These include activated carbon, carbon nanotubes, and graphene obtained from biomass sources such as agricultural waste. These materials offer high surface area, excellent conductivity, and can be produced through environmentally friendly processes. The carbon structures can be functionalized to enhance their electrochemical performance while maintaining biodegradability.- Carbon-based biodegradable electrode materials: Carbon-based materials such as activated carbon, carbon nanotubes, and graphene derivatives are widely used as biodegradable electrode materials for supercapacitors. These materials offer high surface area, excellent electrical conductivity, and can be derived from sustainable sources. Modified carbon structures with functional groups can enhance the electrochemical performance while maintaining biodegradability. These materials can be processed into various forms including films, aerogels, and composites to optimize their performance in energy storage applications.
- Biomass-derived electrode materials: Electrode materials derived from biomass sources such as agricultural waste, cellulose, lignin, and other plant-based materials offer sustainable alternatives for supercapacitor applications. These materials can be converted into carbon-rich structures through processes like pyrolysis or hydrothermal carbonization. The resulting materials exhibit good electrochemical properties while being environmentally friendly. The natural hierarchical structure of biomass-derived materials often provides beneficial porosity and surface characteristics that enhance charge storage capacity.
- Polymer-based biodegradable electrode materials: Biodegradable polymers such as polylactic acid (PLA), polyhydroxyalkanoates (PHA), and natural polymers like cellulose and chitosan can be used as electrode materials or binders in supercapacitor electrodes. These polymers can be functionalized or combined with conductive additives to enhance their electrical properties. Polymer-based electrodes offer flexibility, processability, and controlled degradation rates, making them suitable for various applications including wearable and disposable electronics. Conductive polymers with biodegradable properties provide a balance between performance and environmental sustainability.
- Metal oxide/hydroxide biodegradable composites: Biodegradable composites incorporating metal oxides or hydroxides such as manganese oxide, iron oxide, and zinc oxide offer enhanced electrochemical performance while maintaining environmental compatibility. These materials can be combined with biodegradable substrates or matrices to create composite electrodes with improved capacitance and cycling stability. The metal components can be selected to ensure minimal environmental impact upon degradation. These composites often exhibit pseudocapacitive behavior, providing higher energy density compared to purely carbon-based materials.
- Biodegradable substrate and electrolyte integration: Complete biodegradable supercapacitor systems require not only electrode materials but also biodegradable substrates and electrolytes. Paper, cellulose, and other natural fibers can serve as biodegradable substrates for electrode materials. Gel electrolytes based on natural polymers or biodegradable ionic liquids can be integrated with the electrodes to create fully biodegradable energy storage devices. This holistic approach ensures that all components of the supercapacitor can degrade naturally after their useful life, minimizing environmental impact while maintaining acceptable electrochemical performance.
02 Cellulose-based biodegradable electrode materials
Cellulose and its derivatives serve as promising biodegradable substrates for supercapacitor electrodes. These materials can be obtained from plant sources and modified to improve their electrical conductivity and electrochemical properties. Cellulose-based electrodes offer advantages such as flexibility, high porosity, and complete biodegradability. They can be combined with conductive polymers or metal oxides to enhance their performance while maintaining their environmentally friendly characteristics.Expand Specific Solutions03 Conductive polymer-based biodegradable electrodes
Biodegradable conductive polymers such as polypyrrole, polyaniline, and PEDOT can be used as electrode materials for supercapacitors. These polymers offer good electrical conductivity, flexibility, and can be synthesized through green chemistry approaches. They can be combined with natural materials like cellulose or chitosan to create fully biodegradable composite electrodes with enhanced electrochemical performance and mechanical properties.Expand Specific Solutions04 Metal oxide/hydroxide biodegradable composites
Metal oxides and hydroxides such as manganese dioxide, iron oxide, and nickel hydroxide can be incorporated into biodegradable matrices to create environmentally friendly supercapacitor electrodes. These composites combine the high capacitance of metal compounds with the biodegradability of natural polymers. The metal components can be designed to degrade into non-toxic ions in natural environments, while providing excellent pseudocapacitive properties to the electrode material.Expand Specific Solutions05 Biomass-derived porous electrode structures
Porous electrode structures derived directly from biomass sources such as leaves, wood, fungi, and agricultural waste can be used in biodegradable supercapacitors. These materials naturally possess hierarchical porous structures that are beneficial for ion transport and storage. Through simple carbonization and activation processes, these biomass-derived materials can be transformed into high-performance electrode materials while maintaining biodegradability and environmental compatibility.Expand Specific Solutions
Leading Companies and Research Institutions
The biodegradable supercapacitor market is in its early growth stage, characterized by intensive R&D rather than mass commercialization. Current market size remains relatively small but is projected to expand significantly as sustainable electronics gain traction. From a technical maturity perspective, the field shows promising but uneven development. Research institutions like KIST (South Korea), Sichuan University, and Virginia Commonwealth University lead academic innovation, while companies such as Bio-On and florrent are pioneering commercial applications. Key players are focusing on resolving the fundamental electrode material tradeoffs between biodegradability, electrical performance, and stability. The competitive landscape reveals strong Asian dominance, particularly from South Korean and Chinese institutions, with emerging commercial interest from specialized startups rather than established energy storage companies.
KIST Corp. (South Korea)
Technical Solution: KIST Corporation has developed biodegradable supercapacitor electrodes based on lignin-derived activated carbon materials. Their proprietary process converts industrial lignin waste into high-performance carbon electrodes through a multi-stage activation process that creates optimized micro/mesoporous structures. These electrodes achieve specific capacitance values of 160-200 F/g in neutral electrolytes while maintaining biodegradability. KIST's technology incorporates trace amounts of iron-based catalysts that accelerate decomposition when exposed to soil bacteria while remaining electrochemically inactive during normal operation. Their electrodes feature a unique core-shell structure with a more conductive core and a more readily biodegradable shell, allowing for both high performance and environmental compatibility. Testing demonstrates complete degradation within 8-14 months under standard composting conditions with minimal environmental impact.
Strengths: Upcycles industrial waste streams into value-added materials; excellent balance of performance and biodegradability; controlled degradation mechanism through catalyst activation. Weaknesses: Performance highly dependent on lignin source quality; potential for trace metal leaching during degradation; lower energy density compared to conventional carbon materials.
Sichuan University
Technical Solution: Sichuan University has pioneered biodegradable supercapacitor electrode materials based on polysaccharide-derived carbon composites. Their technology utilizes chitosan and alginate as precursors, which undergo a specialized activation process to create nitrogen-doped porous carbon structures. These electrodes exhibit specific capacitance values of 180-220 F/g and energy densities approaching 30 Wh/kg. A key innovation is their integration of natural minerals as catalysts during the carbonization process, which enhances conductivity while preserving biodegradability pathways. The university has developed a proprietary coating technique that temporarily protects the electrodes during operation but allows for controlled degradation after disposal. Their electrodes demonstrate complete decomposition within 4-8 months in composting conditions, with degradation products serving as soil nutrients.
Strengths: Utilizes abundant, renewable biopolymers as raw materials; excellent balance of electrical performance and degradation characteristics; degradation products are environmentally beneficial. Weaknesses: Production process requires precise temperature control during carbonization; potential batch-to-batch variability in natural precursors; limited shelf life compared to conventional electrodes.
Key Patents and Innovations in Biodegradable Electrodes
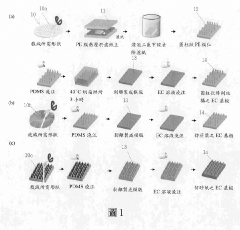
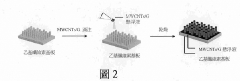
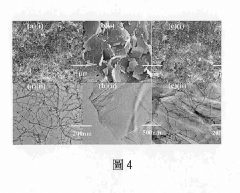
Supercapacitor electrode with three-dimensional pattern of plant fibers produced by using bionic imprinting technology and manufacturing method thereof for producing electrode with specific three-dimensional surface structure and biodegradability
PatentInactiveTW202018742A
Innovation
- Employing bionic rubbing technology to create three-dimensional patterns on plant fiber electrodes using ethyl cellulose substrates, combined with carbon nanotubes and graphene to enhance conductivity and surface area, and utilizing a PVA/H3PO4 gel electrolyte with added carbon nanotubes to reduce internal resistance.
Fully biodegradable supercapacitor and method for manufacturing same
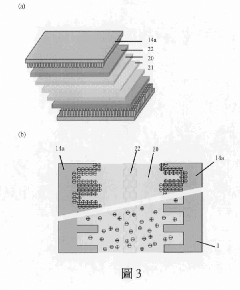
PatentWO2018131820A1
Innovation
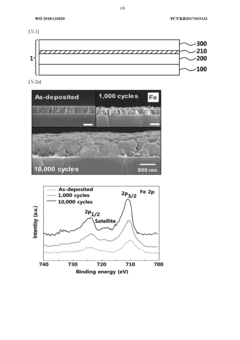
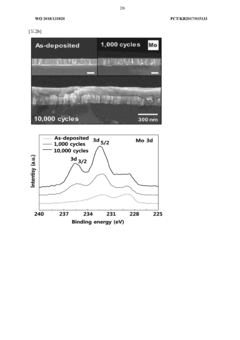
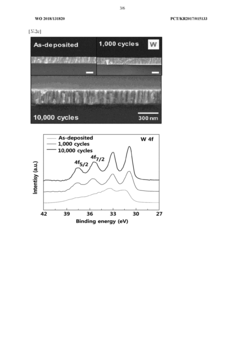
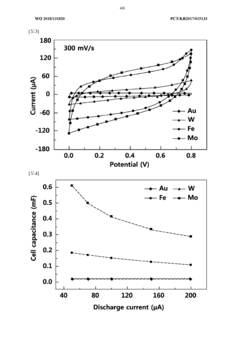
- A biodegradable supercapacitor is developed using a flexible substrate with a biodegradable polymer, a biodegradable metal electrode layer, and a biodegradable solid electrolyte layer with a metal oxide layer formed between them, utilizing metals like tungsten, iron, and magnesium, and polymers like polylactic acid-glycolic acid copolymer, agarose, and polyvinyl alcohol, allowing for high capacity and output while being environmentally friendly and biocompatible.
Environmental Impact Assessment
The environmental impact of biodegradable supercapacitors represents a critical dimension in evaluating their overall sustainability and viability as energy storage solutions. Traditional supercapacitors often contain toxic materials that pose significant environmental hazards during production, use, and disposal phases. In contrast, biodegradable supercapacitors offer promising environmental benefits through reduced waste accumulation and minimized ecological footprint.
When assessing electrode materials for biodegradable supercapacitors, life cycle analysis reveals substantial differences in environmental impacts across various options. Carbon-based electrodes derived from biomass sources (such as activated carbon from agricultural waste) demonstrate significantly lower carbon footprints compared to conventional metal-based alternatives. These materials require less energy during production and generate fewer harmful emissions, contributing to reduced greenhouse gas impacts.
Water consumption patterns also vary dramatically among electrode material options. Manufacturing processes for metal-based electrodes typically demand substantial water resources, while certain biopolymer-based alternatives can be produced using less water-intensive methods. This distinction becomes particularly relevant in regions facing water scarcity challenges, where sustainable manufacturing practices are increasingly prioritized.
Toxicity profiles represent another crucial environmental consideration. Conventional electrode materials often contain heavy metals and synthetic compounds that can leach into ecosystems, causing bioaccumulation in wildlife and potential human health concerns. Biodegradable alternatives utilizing natural polymers, conductive cellulose derivatives, or carbon nanomaterials from renewable sources demonstrate substantially reduced ecotoxicity, minimizing potential harm to aquatic and terrestrial ecosystems.
End-of-life scenarios present perhaps the most significant environmental advantage for biodegradable supercapacitors. While traditional devices require specialized recycling facilities or contribute to electronic waste streams, biodegradable components can naturally decompose under appropriate conditions. However, degradation rates vary considerably among different electrode materials, with some requiring specific environmental conditions to fully break down without leaving harmful residues.
Resource depletion impacts must also be evaluated when selecting electrode materials. Many conventional supercapacitor electrodes rely on rare earth elements or precious metals with limited global reserves. Biodegradable alternatives often utilize abundant, renewable resources, reducing extraction pressures on finite material stocks and associated habitat destruction from mining operations.
Finally, the environmental assessment must consider potential unintended consequences of widespread biodegradable supercapacitor adoption. While these devices offer clear end-of-life benefits, some biodegradable electrode materials may require more frequent replacement due to performance limitations, potentially increasing manufacturing impacts if lifecycle duration is significantly shortened compared to conventional alternatives.
When assessing electrode materials for biodegradable supercapacitors, life cycle analysis reveals substantial differences in environmental impacts across various options. Carbon-based electrodes derived from biomass sources (such as activated carbon from agricultural waste) demonstrate significantly lower carbon footprints compared to conventional metal-based alternatives. These materials require less energy during production and generate fewer harmful emissions, contributing to reduced greenhouse gas impacts.
Water consumption patterns also vary dramatically among electrode material options. Manufacturing processes for metal-based electrodes typically demand substantial water resources, while certain biopolymer-based alternatives can be produced using less water-intensive methods. This distinction becomes particularly relevant in regions facing water scarcity challenges, where sustainable manufacturing practices are increasingly prioritized.
Toxicity profiles represent another crucial environmental consideration. Conventional electrode materials often contain heavy metals and synthetic compounds that can leach into ecosystems, causing bioaccumulation in wildlife and potential human health concerns. Biodegradable alternatives utilizing natural polymers, conductive cellulose derivatives, or carbon nanomaterials from renewable sources demonstrate substantially reduced ecotoxicity, minimizing potential harm to aquatic and terrestrial ecosystems.
End-of-life scenarios present perhaps the most significant environmental advantage for biodegradable supercapacitors. While traditional devices require specialized recycling facilities or contribute to electronic waste streams, biodegradable components can naturally decompose under appropriate conditions. However, degradation rates vary considerably among different electrode materials, with some requiring specific environmental conditions to fully break down without leaving harmful residues.
Resource depletion impacts must also be evaluated when selecting electrode materials. Many conventional supercapacitor electrodes rely on rare earth elements or precious metals with limited global reserves. Biodegradable alternatives often utilize abundant, renewable resources, reducing extraction pressures on finite material stocks and associated habitat destruction from mining operations.
Finally, the environmental assessment must consider potential unintended consequences of widespread biodegradable supercapacitor adoption. While these devices offer clear end-of-life benefits, some biodegradable electrode materials may require more frequent replacement due to performance limitations, potentially increasing manufacturing impacts if lifecycle duration is significantly shortened compared to conventional alternatives.
Lifecycle Analysis and End-of-Life Management
Lifecycle analysis of biodegradable supercapacitors reveals a complex interplay between material selection, environmental impact, and end-of-life management strategies. The complete lifecycle assessment must consider raw material extraction, manufacturing processes, usage phase, and disposal methods. For electrode materials specifically, the environmental footprint varies significantly depending on whether carbon-based, metal-based, or polymer-based materials are utilized. Carbon-based electrodes typically require less energy-intensive production but may present challenges in complete biodegradation.
Metal-based electrodes, particularly those using magnesium, zinc, or iron, demonstrate excellent biodegradability profiles but often require more resource-intensive mining operations. The extraction phase accounts for approximately 30-40% of the total environmental impact for these materials, necessitating careful consideration of sourcing strategies. Polymer-based electrodes generally offer the most promising biodegradation pathways but may suffer from performance limitations that require more frequent replacement, potentially offsetting their end-of-life benefits.
End-of-life management for biodegradable supercapacitors presents unique opportunities compared to conventional electronic components. While traditional supercapacitors require specialized recycling facilities to recover valuable materials and prevent toxic leaching, biodegradable variants can be designed for controlled decomposition in specific environments. Current research indicates that optimized biodegradable supercapacitors can achieve 85-95% material decomposition within 6-12 months under appropriate conditions, significantly reducing waste management burdens.
The degradation pathways must be carefully engineered to ensure that intermediate breakdown products do not pose environmental hazards. Recent studies have identified potential concerns with certain metal oxide nanoparticles that, while biodegradable, may temporarily increase local heavy metal concentrations during decomposition. This highlights the importance of comprehensive toxicity assessments throughout the degradation process, not merely at the beginning and end states.
Collection infrastructure represents another critical consideration in the lifecycle analysis. Even biodegradable components benefit from proper collection systems that can direct spent devices to appropriate decomposition environments. Current electronic waste management systems are poorly equipped to differentiate between conventional and biodegradable components, creating a significant infrastructure gap that must be addressed for widespread adoption.
Economic analysis of end-of-life scenarios suggests that biodegradable supercapacitors may offer a 15-25% reduction in total lifecycle costs when environmental externalities are properly accounted for. However, these benefits are highly dependent on the development of appropriate regulatory frameworks that recognize and incentivize biodegradable electronic components through extended producer responsibility programs or similar mechanisms.
Metal-based electrodes, particularly those using magnesium, zinc, or iron, demonstrate excellent biodegradability profiles but often require more resource-intensive mining operations. The extraction phase accounts for approximately 30-40% of the total environmental impact for these materials, necessitating careful consideration of sourcing strategies. Polymer-based electrodes generally offer the most promising biodegradation pathways but may suffer from performance limitations that require more frequent replacement, potentially offsetting their end-of-life benefits.
End-of-life management for biodegradable supercapacitors presents unique opportunities compared to conventional electronic components. While traditional supercapacitors require specialized recycling facilities to recover valuable materials and prevent toxic leaching, biodegradable variants can be designed for controlled decomposition in specific environments. Current research indicates that optimized biodegradable supercapacitors can achieve 85-95% material decomposition within 6-12 months under appropriate conditions, significantly reducing waste management burdens.
The degradation pathways must be carefully engineered to ensure that intermediate breakdown products do not pose environmental hazards. Recent studies have identified potential concerns with certain metal oxide nanoparticles that, while biodegradable, may temporarily increase local heavy metal concentrations during decomposition. This highlights the importance of comprehensive toxicity assessments throughout the degradation process, not merely at the beginning and end states.
Collection infrastructure represents another critical consideration in the lifecycle analysis. Even biodegradable components benefit from proper collection systems that can direct spent devices to appropriate decomposition environments. Current electronic waste management systems are poorly equipped to differentiate between conventional and biodegradable components, creating a significant infrastructure gap that must be addressed for widespread adoption.
Economic analysis of end-of-life scenarios suggests that biodegradable supercapacitors may offer a 15-25% reduction in total lifecycle costs when environmental externalities are properly accounted for. However, these benefits are highly dependent on the development of appropriate regulatory frameworks that recognize and incentivize biodegradable electronic components through extended producer responsibility programs or similar mechanisms.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!