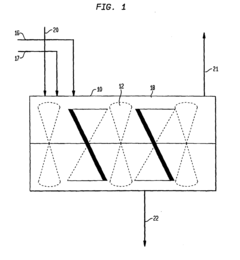
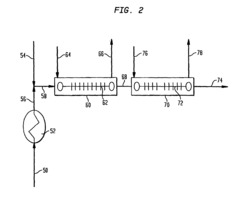
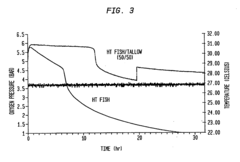
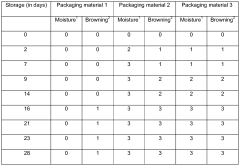
Long-Term Stability Before Degradation: Storage And Shelf-Life Controls
SEP 1, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Storage Stability Background and Objectives
Storage stability represents a critical aspect of product development across numerous industries, particularly in pharmaceuticals, food technology, electronics, and advanced materials. The concept encompasses the ability of a product to maintain its essential characteristics, functionality, and safety throughout its intended shelf life under specified storage conditions. Historically, stability concerns have evolved from simple empirical observations to sophisticated predictive modeling approaches that incorporate molecular-level understanding of degradation mechanisms.
The evolution of storage stability science has been marked by significant milestones, including the development of accelerated aging protocols in the 1950s, the establishment of ICH guidelines for pharmaceutical stability in the 1990s, and the recent integration of computational modeling with experimental data to predict long-term behavior. Current technological trends indicate a shift toward real-time monitoring systems, predictive analytics, and the application of artificial intelligence to anticipate stability issues before they manifest.
The primary objective of storage stability research is to develop robust methodologies for predicting and extending product shelf life while maintaining quality attributes throughout the supply chain. This involves identifying critical degradation pathways, establishing appropriate storage conditions, and designing effective containment systems that mitigate environmental stressors such as temperature fluctuations, humidity, light exposure, and oxidative processes.
Secondary objectives include the development of accelerated testing protocols that accurately reflect real-world aging, the establishment of meaningful stability indicators that serve as early warning systems, and the creation of mathematical models that can extrapolate short-term data to predict long-term performance with statistical confidence. These objectives align with broader industry goals of reducing waste, improving sustainability, and enhancing product reliability.
From a technological perspective, current challenges include the accurate prediction of complex degradation kinetics, particularly for multi-component systems where interactions between ingredients can accelerate or inhibit deterioration. The variability of environmental conditions during transportation and storage presents additional complications, as does the need to balance extended shelf life with consumer expectations for reduced preservatives and more natural formulations.
The strategic importance of storage stability extends beyond quality control to encompass supply chain optimization, market expansion opportunities, regulatory compliance, and ultimately consumer trust. As global distribution networks become more complex and consumer expectations for product longevity increase, the ability to ensure consistent performance throughout a product's shelf life has become a competitive differentiator and a cornerstone of brand reputation.
The evolution of storage stability science has been marked by significant milestones, including the development of accelerated aging protocols in the 1950s, the establishment of ICH guidelines for pharmaceutical stability in the 1990s, and the recent integration of computational modeling with experimental data to predict long-term behavior. Current technological trends indicate a shift toward real-time monitoring systems, predictive analytics, and the application of artificial intelligence to anticipate stability issues before they manifest.
The primary objective of storage stability research is to develop robust methodologies for predicting and extending product shelf life while maintaining quality attributes throughout the supply chain. This involves identifying critical degradation pathways, establishing appropriate storage conditions, and designing effective containment systems that mitigate environmental stressors such as temperature fluctuations, humidity, light exposure, and oxidative processes.
Secondary objectives include the development of accelerated testing protocols that accurately reflect real-world aging, the establishment of meaningful stability indicators that serve as early warning systems, and the creation of mathematical models that can extrapolate short-term data to predict long-term performance with statistical confidence. These objectives align with broader industry goals of reducing waste, improving sustainability, and enhancing product reliability.
From a technological perspective, current challenges include the accurate prediction of complex degradation kinetics, particularly for multi-component systems where interactions between ingredients can accelerate or inhibit deterioration. The variability of environmental conditions during transportation and storage presents additional complications, as does the need to balance extended shelf life with consumer expectations for reduced preservatives and more natural formulations.
The strategic importance of storage stability extends beyond quality control to encompass supply chain optimization, market expansion opportunities, regulatory compliance, and ultimately consumer trust. As global distribution networks become more complex and consumer expectations for product longevity increase, the ability to ensure consistent performance throughout a product's shelf life has become a competitive differentiator and a cornerstone of brand reputation.
Market Demand for Extended Shelf-Life Products
The global market for extended shelf-life products has witnessed substantial growth over the past decade, driven primarily by changing consumer lifestyles, increasing urbanization, and the growing demand for convenience foods. According to recent market research, the global extended shelf-life food market was valued at approximately $891 billion in 2022 and is projected to reach $1.3 trillion by 2028, growing at a CAGR of 6.5% during the forecast period.
Consumer preferences have significantly shifted towards products that maintain quality and freshness for longer periods while minimizing preservative content. This trend is particularly evident in developed markets where busy lifestyles necessitate less frequent shopping trips and greater reliance on stored products. A 2023 consumer survey revealed that 78% of respondents consider shelf-life as a critical factor influencing their purchasing decisions, with 65% willing to pay premium prices for products with extended stability.
The pharmaceutical industry represents another significant market segment demanding advanced shelf-life solutions. With biologics and complex formulations gaining prominence, maintaining product stability throughout the supply chain has become increasingly challenging. The global pharmaceutical stability testing market reached $1.7 billion in 2022 and is expected to grow at 8.2% annually through 2027, highlighting the critical importance of long-term stability controls.
Food waste reduction initiatives have further accelerated market demand for extended shelf-life technologies. With approximately one-third of global food production lost or wasted annually, solutions that prolong product viability directly address sustainability concerns. Regulatory bodies worldwide have implemented stricter guidelines for shelf-life claims, creating additional market pressure for scientifically validated stability control methods.
Emerging markets present substantial growth opportunities, with rising middle-class populations demanding higher quality packaged goods with longer usability periods. The Asia-Pacific region is expected to witness the fastest growth rate in extended shelf-life product adoption, with China and India leading regional demand. Market penetration in these regions is projected to increase by 12% annually over the next five years.
E-commerce expansion has created additional market pressure for products with enhanced stability profiles. Online grocery sales have grown by 25% annually since 2020, necessitating products that can withstand varied storage conditions and extended delivery timeframes. This channel-specific demand has prompted manufacturers to invest heavily in innovative packaging and preservation technologies that maintain product integrity throughout complex distribution networks.
Consumer preferences have significantly shifted towards products that maintain quality and freshness for longer periods while minimizing preservative content. This trend is particularly evident in developed markets where busy lifestyles necessitate less frequent shopping trips and greater reliance on stored products. A 2023 consumer survey revealed that 78% of respondents consider shelf-life as a critical factor influencing their purchasing decisions, with 65% willing to pay premium prices for products with extended stability.
The pharmaceutical industry represents another significant market segment demanding advanced shelf-life solutions. With biologics and complex formulations gaining prominence, maintaining product stability throughout the supply chain has become increasingly challenging. The global pharmaceutical stability testing market reached $1.7 billion in 2022 and is expected to grow at 8.2% annually through 2027, highlighting the critical importance of long-term stability controls.
Food waste reduction initiatives have further accelerated market demand for extended shelf-life technologies. With approximately one-third of global food production lost or wasted annually, solutions that prolong product viability directly address sustainability concerns. Regulatory bodies worldwide have implemented stricter guidelines for shelf-life claims, creating additional market pressure for scientifically validated stability control methods.
Emerging markets present substantial growth opportunities, with rising middle-class populations demanding higher quality packaged goods with longer usability periods. The Asia-Pacific region is expected to witness the fastest growth rate in extended shelf-life product adoption, with China and India leading regional demand. Market penetration in these regions is projected to increase by 12% annually over the next five years.
E-commerce expansion has created additional market pressure for products with enhanced stability profiles. Online grocery sales have grown by 25% annually since 2020, necessitating products that can withstand varied storage conditions and extended delivery timeframes. This channel-specific demand has prompted manufacturers to invest heavily in innovative packaging and preservation technologies that maintain product integrity throughout complex distribution networks.
Current Challenges in Long-Term Storage Stability
Despite significant advancements in storage technologies, maintaining long-term stability of products remains a formidable challenge across multiple industries. The primary obstacle lies in the inherent chemical and physical degradation processes that occur over time, even under controlled conditions. These processes are often accelerated by environmental factors such as temperature fluctuations, humidity, light exposure, and oxygen presence, creating a complex matrix of variables that must be simultaneously controlled.
Material compatibility issues represent another significant challenge, particularly in pharmaceutical and food industries. Container-content interactions can lead to leaching of compounds, adsorption of active ingredients onto packaging surfaces, or catalytic degradation reactions that compromise product integrity. These interactions are often difficult to predict during initial stability testing and may only become apparent after extended storage periods.
The variability in global storage conditions presents a universal challenge for multinational companies. Products must maintain stability across diverse climatic zones, from tropical humidity to arid desert conditions, often requiring different formulation strategies or packaging solutions for different markets. This geographical diversity complicates standardization efforts and increases development costs.
Regulatory frameworks for stability testing and shelf-life determination vary significantly across regions, creating compliance challenges for global products. While ICH guidelines provide some harmonization for pharmaceuticals, many industries face inconsistent requirements that necessitate multiple stability programs to satisfy different authorities, adding complexity and cost to product development.
Accelerated stability testing methodologies, while valuable for expediting development, often fail to accurately predict real-time degradation pathways. The correlation between accelerated and real-time stability data remains imperfect, with some degradation mechanisms only manifesting under specific long-term conditions that cannot be reliably simulated through acceleration factors.
The emergence of novel materials and complex formulations, including biologics, nanomaterials, and combination products, has introduced unprecedented stability challenges. These advanced products often exhibit unique degradation profiles that traditional stability models fail to capture, requiring innovative analytical methods and predictive tools.
Economic pressures to extend shelf-life while reducing packaging costs create conflicting objectives that technical teams must balance. The drive for sustainability further complicates this equation, as environmentally friendly packaging solutions may not always provide optimal barrier properties for long-term stability.
Material compatibility issues represent another significant challenge, particularly in pharmaceutical and food industries. Container-content interactions can lead to leaching of compounds, adsorption of active ingredients onto packaging surfaces, or catalytic degradation reactions that compromise product integrity. These interactions are often difficult to predict during initial stability testing and may only become apparent after extended storage periods.
The variability in global storage conditions presents a universal challenge for multinational companies. Products must maintain stability across diverse climatic zones, from tropical humidity to arid desert conditions, often requiring different formulation strategies or packaging solutions for different markets. This geographical diversity complicates standardization efforts and increases development costs.
Regulatory frameworks for stability testing and shelf-life determination vary significantly across regions, creating compliance challenges for global products. While ICH guidelines provide some harmonization for pharmaceuticals, many industries face inconsistent requirements that necessitate multiple stability programs to satisfy different authorities, adding complexity and cost to product development.
Accelerated stability testing methodologies, while valuable for expediting development, often fail to accurately predict real-time degradation pathways. The correlation between accelerated and real-time stability data remains imperfect, with some degradation mechanisms only manifesting under specific long-term conditions that cannot be reliably simulated through acceleration factors.
The emergence of novel materials and complex formulations, including biologics, nanomaterials, and combination products, has introduced unprecedented stability challenges. These advanced products often exhibit unique degradation profiles that traditional stability models fail to capture, requiring innovative analytical methods and predictive tools.
Economic pressures to extend shelf-life while reducing packaging costs create conflicting objectives that technical teams must balance. The drive for sustainability further complicates this equation, as environmentally friendly packaging solutions may not always provide optimal barrier properties for long-term stability.
Current Degradation Prevention Methodologies
01 Temperature-controlled storage systems
Temperature-controlled storage systems are essential for maintaining product stability over extended periods. These systems monitor and regulate storage temperatures to prevent degradation of sensitive ingredients. Advanced temperature control mechanisms can include automated alerts for temperature excursions, continuous monitoring capabilities, and specialized cooling technologies that ensure consistent conditions throughout the storage area, thereby extending product shelf-life and maintaining efficacy.- Temperature-controlled storage systems: Temperature-controlled storage systems are essential for maintaining product stability over extended periods. These systems monitor and regulate storage temperatures to prevent degradation of sensitive ingredients. Advanced systems may include automated temperature monitoring, alerts for deviations, and backup systems to ensure continuous temperature control even during power outages. Proper temperature control is particularly critical for pharmaceutical products, biologics, and certain cosmetic formulations where active ingredients may degrade under temperature fluctuations.
- Packaging innovations for extended shelf-life: Specialized packaging materials and designs can significantly extend product shelf-life by providing barriers against moisture, oxygen, light, and microbial contamination. These innovations include multi-layer packaging, modified atmosphere packaging, oxygen scavengers, and UV-blocking containers. Smart packaging technologies may incorporate freshness indicators or time-temperature indicators that provide visual cues about product quality and remaining shelf-life. Proper packaging selection based on product characteristics is crucial for maintaining stability throughout the intended storage period.
- Stability testing protocols and predictive models: Comprehensive stability testing protocols are implemented to evaluate product performance under various environmental conditions over time. These include accelerated aging tests, real-time stability studies, and stress testing to identify potential degradation pathways. Advanced predictive models and algorithms can forecast long-term stability based on short-term data, helping manufacturers establish appropriate expiration dates and storage recommendations. Regular stability monitoring throughout a product's lifecycle ensures continued compliance with quality specifications.
- Preservative systems and antioxidant strategies: Effective preservative systems and antioxidant strategies are crucial for preventing microbial growth and oxidative degradation during storage. These may include traditional preservatives, natural antimicrobial compounds, chelating agents, and free radical scavengers. Synergistic combinations of preservatives can provide broad-spectrum protection while minimizing the concentration of individual components. The selection of appropriate preservative systems must balance efficacy, safety, regulatory compliance, and consumer preferences for product formulations with extended shelf-life.
- Digital monitoring and inventory management systems: Digital monitoring and inventory management systems leverage technology to track product conditions throughout storage and distribution. These systems may incorporate IoT sensors, blockchain technology, and cloud-based platforms to provide real-time visibility of storage conditions and product quality. Automated inventory management ensures proper stock rotation following first-expired-first-out principles. Advanced analytics can identify patterns affecting stability and shelf-life, enabling proactive interventions before quality issues arise. These digital solutions enhance compliance with regulatory requirements while optimizing inventory management.
02 Stability testing protocols
Comprehensive stability testing protocols are crucial for predicting and ensuring long-term product viability. These protocols typically involve accelerated aging tests under various environmental conditions, real-time stability monitoring, and periodic quality assessments. By subjecting products to controlled stress conditions such as varying temperatures, humidity levels, and light exposure, manufacturers can accurately determine shelf-life expectations and establish appropriate storage recommendations for maintaining product integrity.Expand Specific Solutions03 Packaging innovations for extended shelf-life
Advanced packaging solutions play a significant role in extending product shelf-life. Innovations include barrier materials that prevent moisture ingress, oxygen scavengers that reduce oxidative degradation, UV-protective containers that shield light-sensitive components, and modified atmosphere packaging that creates optimal internal environments. These packaging technologies work together to maintain product stability by protecting against environmental factors that accelerate degradation, thereby ensuring longer-term efficacy and safety.Expand Specific Solutions04 Digital monitoring and tracking systems
Digital monitoring and tracking systems enable real-time oversight of storage conditions and product stability. These systems utilize sensors, IoT technology, and cloud-based platforms to continuously monitor critical parameters such as temperature, humidity, and light exposure. Advanced analytics can predict potential stability issues before they occur, while blockchain or similar technologies ensure data integrity throughout the product lifecycle, allowing for comprehensive stability documentation and regulatory compliance.Expand Specific Solutions05 Stabilizing additives and formulation techniques
Specialized stabilizing additives and formulation techniques can significantly enhance product shelf-life. These include antioxidants that prevent oxidative degradation, pH buffers that maintain optimal acidity levels, chelating agents that bind destabilizing metal ions, and specialized excipients that improve overall formulation stability. Advanced processing methods such as microencapsulation can also protect sensitive ingredients from environmental factors, ensuring consistent product performance throughout its intended shelf-life.Expand Specific Solutions
Key Industry Players in Stability Control Solutions
The long-term stability and shelf-life control market is currently in a growth phase, with an estimated global value exceeding $5 billion annually. The technology maturity varies across sectors, with pharmaceutical applications leading the advancement curve. Novo Nordisk A/S and Biogen MA have established strong positions in biopharmaceutical stability, while Novozymes and Chr. Hansen dominate in enzyme and microbial preservation technologies. In the food sector, companies like Arla Foods, The Clorox Co., and Apeel Technology are developing innovative preservation solutions. Tata Consultancy Services and Canon are emerging players in digital monitoring systems for stability tracking. The competitive landscape is characterized by increasing cross-industry collaboration, with research institutions like University of Strathclyde and Norwegian University of Science & Technology partnering with industry to advance fundamental stability science and practical applications.
Novo Nordisk A/S
Technical Solution: Novo Nordisk has developed comprehensive stability control systems for their pharmaceutical products, particularly insulin and GLP-1 receptor agonists. Their approach includes advanced protein stabilization technologies that prevent degradation during long-term storage. The company employs proprietary formulation techniques incorporating specific buffer systems, stabilizing excipients, and optimized pH environments to maintain molecular integrity. Their SmartCare™ technology platform integrates temperature-controlled supply chain management with real-time monitoring systems that track environmental conditions throughout product lifecycle. Novo Nordisk has pioneered the use of specialized container closure systems with minimal extractables and leachables profiles, significantly extending shelf-life of sensitive biopharmaceuticals. Their stability testing protocols exceed regulatory requirements, implementing accelerated and real-time testing under various stress conditions to predict long-term stability profiles accurately.
Strengths: Industry-leading expertise in protein stabilization for injectable products; comprehensive end-to-end stability management from manufacturing through distribution. Weaknesses: Solutions primarily optimized for cold-chain products; relatively high implementation costs for their advanced monitoring systems.
Novozymes A/S
Technical Solution: Novozymes has developed the "EnzStable" platform specifically addressing enzyme stability during long-term storage across various industrial applications. Their approach combines protein engineering with advanced formulation science to create enzymes with enhanced thermostability and pH tolerance. The company employs proprietary computational modeling to predict protein unfolding pathways and design targeted modifications that block degradation mechanisms. Their stabilization technology includes specialized carrier materials and encapsulation systems that create protective microenvironments around enzyme molecules. Novozymes' liquid enzyme formulations incorporate synergistic stabilizer packages containing polyols, salts, and proprietary compounds that prevent aggregation and denaturation during storage. For powdered enzyme products, they've developed moisture-resistant granulation technologies that maintain activity even under challenging humidity conditions. Their stability testing protocols simulate real-world storage scenarios across temperature ranges from -20°C to 40°C with controlled humidity cycling.
Strengths: Unparalleled expertise in enzyme stabilization across diverse applications; customizable solutions for specific industry requirements; comprehensive stability prediction models. Weaknesses: Some advanced stabilization technologies carry premium pricing; certain solutions require specialized handling equipment for implementation.
Critical Stability Control Patents and Research
Polyunsaturated fatty acid monovalent and divalent metal salt synthesis
PatentInactiveUS20110039932A1
Innovation
- The process involves saponifying unsaturated fatty acid glycerides in a reduced oxygen atmosphere and blending with an antioxidant-effective stabilizing oil to enhance storage stability, allowing for the production of free-flowing, storage-stable fatty acid metal salts without diluting high glyceride content oils.
Process for increasing the shelf life of a food or agricultural product
PatentWO2013034568A1
Innovation
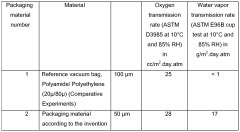
- A packaging system utilizing a thermoplastic, monolithic film with specific water vapor transmission and oxygen permeability rates, allowing a controlled mix of oxygen, carbon dioxide, and water vapor to maintain product freshness, which can be applied as a patch, label, or used in various packaging formats.
Regulatory Framework for Shelf-Life Claims
The regulatory landscape governing shelf-life claims for products spans multiple jurisdictions and agencies, each with specific requirements that manufacturers must adhere to. In the United States, the Food and Drug Administration (FDA) establishes comprehensive guidelines for pharmaceutical products, requiring stability testing under various environmental conditions to substantiate shelf-life claims. These regulations are codified in 21 CFR Parts 210 and 211, with additional guidance provided in ICH Q1A(R2) for stability testing protocols.
The European Medicines Agency (EMA) implements parallel but distinct regulatory frameworks through EU Directive 2001/83/EC and the Guidelines on Stability Testing of Existing Active Substances and Related Finished Products. These frameworks mandate accelerated and long-term stability studies, with specific requirements for temperature, humidity, and light exposure parameters during testing.
For consumer products, regulatory oversight varies significantly by product category. Cosmetics in the EU fall under Regulation (EC) No 1223/2009, which requires a Cosmetic Product Safety Report including stability assessment data. Food products are governed by Regulation (EU) No 1169/2011, which distinguishes between "use-by" dates for perishable items and "best-before" dates for quality considerations.
International harmonization efforts are led by organizations such as the International Conference on Harmonisation (ICH) and the International Organization for Standardization (ISO), which have developed standards like ISO 16128 for cosmetics and ISO 22000 for food safety management systems that include stability considerations.
Regulatory compliance necessitates robust documentation practices, including detailed stability protocols, validation of analytical methods, and comprehensive data management systems. Manufacturers must maintain records of all stability studies, including out-of-specification results and investigations, typically for at least one year beyond the expiration date of the batch.
Recent regulatory trends indicate increasing scrutiny of stability claims, with authorities demanding more rigorous scientific evidence. The FDA's 2019 guidance on Expiration Dating and Stability Testing for Human Drug Products emphasizes the need for statistical approaches to stability data analysis and the importance of considering variability in manufacturing processes when establishing shelf-life claims.
Penalties for non-compliance with shelf-life regulations can be severe, ranging from product recalls and import restrictions to significant financial penalties and potential criminal charges for willful violations that endanger public health.
The European Medicines Agency (EMA) implements parallel but distinct regulatory frameworks through EU Directive 2001/83/EC and the Guidelines on Stability Testing of Existing Active Substances and Related Finished Products. These frameworks mandate accelerated and long-term stability studies, with specific requirements for temperature, humidity, and light exposure parameters during testing.
For consumer products, regulatory oversight varies significantly by product category. Cosmetics in the EU fall under Regulation (EC) No 1223/2009, which requires a Cosmetic Product Safety Report including stability assessment data. Food products are governed by Regulation (EU) No 1169/2011, which distinguishes between "use-by" dates for perishable items and "best-before" dates for quality considerations.
International harmonization efforts are led by organizations such as the International Conference on Harmonisation (ICH) and the International Organization for Standardization (ISO), which have developed standards like ISO 16128 for cosmetics and ISO 22000 for food safety management systems that include stability considerations.
Regulatory compliance necessitates robust documentation practices, including detailed stability protocols, validation of analytical methods, and comprehensive data management systems. Manufacturers must maintain records of all stability studies, including out-of-specification results and investigations, typically for at least one year beyond the expiration date of the batch.
Recent regulatory trends indicate increasing scrutiny of stability claims, with authorities demanding more rigorous scientific evidence. The FDA's 2019 guidance on Expiration Dating and Stability Testing for Human Drug Products emphasizes the need for statistical approaches to stability data analysis and the importance of considering variability in manufacturing processes when establishing shelf-life claims.
Penalties for non-compliance with shelf-life regulations can be severe, ranging from product recalls and import restrictions to significant financial penalties and potential criminal charges for willful violations that endanger public health.
Economic Impact of Extended Product Stability
The economic implications of extending product stability are profound and multifaceted across various industries. Companies investing in advanced storage and shelf-life control technologies can realize significant cost reductions through decreased waste management expenses. When products maintain their integrity for longer periods, the frequency of disposal due to degradation diminishes substantially, resulting in annual savings that can range from 15-30% in waste-related costs for manufacturers in pharmaceutical and food sectors.
Extended stability directly impacts inventory management economics by allowing businesses to maintain larger stockpiles without increased spoilage risk. This optimization reduces the need for frequent reordering and enables more efficient bulk purchasing strategies, potentially lowering procurement costs by 8-12% according to recent supply chain analyses. Additionally, companies can strategically time their market entries based on favorable economic conditions rather than being constrained by imminent expiration dates.
The distribution economics also transform significantly with enhanced product stability. Transportation logistics become more flexible, enabling less frequent shipments and more consolidated delivery routes. Studies indicate that companies implementing advanced stability technologies have reduced their distribution costs by approximately 10-15% through optimized shipping schedules and reduced emergency expediting requirements. Furthermore, geographical market expansion becomes economically viable as products can withstand longer transit times to distant markets without quality compromise.
From a revenue perspective, extended product stability creates opportunities for premium pricing strategies. Products with demonstrably longer useful lives command price premiums of 5-20% in consumer markets, particularly in sectors where freshness and efficacy are paramount concerns. This translates to improved profit margins without proportional increases in production costs, enhancing overall business profitability.
The macroeconomic impact extends to global trade facilitation, where products with extended stability can navigate complex international supply chains more effectively. This has particular significance for developing markets where cold chain infrastructure may be inconsistent. Economic analyses suggest that stability-enhanced products can reduce total landed costs in such markets by up to 25%, making previously unprofitable market entries commercially viable and expanding global economic participation.
Insurance and risk management economics also benefit substantially, with companies reporting reduced premium costs for product liability coverage when implementing advanced stability controls. The quantifiable reduction in product failure risk translates to 7-12% savings in related insurance expenses, contributing to improved overall operational economics.
Extended stability directly impacts inventory management economics by allowing businesses to maintain larger stockpiles without increased spoilage risk. This optimization reduces the need for frequent reordering and enables more efficient bulk purchasing strategies, potentially lowering procurement costs by 8-12% according to recent supply chain analyses. Additionally, companies can strategically time their market entries based on favorable economic conditions rather than being constrained by imminent expiration dates.
The distribution economics also transform significantly with enhanced product stability. Transportation logistics become more flexible, enabling less frequent shipments and more consolidated delivery routes. Studies indicate that companies implementing advanced stability technologies have reduced their distribution costs by approximately 10-15% through optimized shipping schedules and reduced emergency expediting requirements. Furthermore, geographical market expansion becomes economically viable as products can withstand longer transit times to distant markets without quality compromise.
From a revenue perspective, extended product stability creates opportunities for premium pricing strategies. Products with demonstrably longer useful lives command price premiums of 5-20% in consumer markets, particularly in sectors where freshness and efficacy are paramount concerns. This translates to improved profit margins without proportional increases in production costs, enhancing overall business profitability.
The macroeconomic impact extends to global trade facilitation, where products with extended stability can navigate complex international supply chains more effectively. This has particular significance for developing markets where cold chain infrastructure may be inconsistent. Economic analyses suggest that stability-enhanced products can reduce total landed costs in such markets by up to 25%, making previously unprofitable market entries commercially viable and expanding global economic participation.
Insurance and risk management economics also benefit substantially, with companies reporting reduced premium costs for product liability coverage when implementing advanced stability controls. The quantifiable reduction in product failure risk translates to 7-12% savings in related insurance expenses, contributing to improved overall operational economics.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!