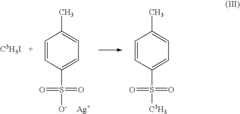
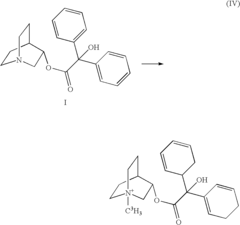
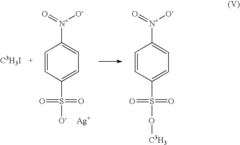
Breaking Down Alkyl Halides: Key Reactions and Uses
JUL 15, 20258 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Alkyl Halide Fundamentals
Alkyl halides, also known as haloalkanes, are a fundamental class of organic compounds characterized by the presence of a halogen atom (fluorine, chlorine, bromine, or iodine) bonded to an alkyl group. These compounds play a crucial role in organic synthesis and have diverse applications in industry and everyday life.
The structure of alkyl halides consists of a carbon-halogen bond, where the halogen atom replaces a hydrogen atom in the parent alkane. This substitution results in unique chemical and physical properties that distinguish alkyl halides from their hydrocarbon counterparts. The nature of the carbon-halogen bond is primarily covalent but exhibits some ionic character due to the electronegativity difference between carbon and the halogen atom.
Alkyl halides are classified based on the number of alkyl groups attached to the carbon bearing the halogen atom. Primary alkyl halides have one alkyl group, secondary have two, and tertiary have three. This classification is essential as it influences the compound's reactivity and behavior in various chemical reactions.
The physical properties of alkyl halides are largely determined by the size and electronegativity of the halogen atom. As the atomic number of the halogen increases, the boiling point and density of the alkyl halide generally increase. This trend is attributed to the increasing strength of intermolecular forces, particularly van der Waals interactions, as the halogen atom becomes larger.
The polarity of the carbon-halogen bond is a key factor in the chemical behavior of alkyl halides. The electronegativity difference between carbon and the halogen creates a dipole moment, with the halogen bearing a partial negative charge and the carbon a partial positive charge. This polarity influences the compound's solubility, reactivity, and its ability to participate in various organic reactions.
Alkyl halides serve as versatile precursors in organic synthesis due to their ability to undergo nucleophilic substitution and elimination reactions. These reactions form the basis for the conversion of alkyl halides into a wide range of other functional groups, making them invaluable in the synthesis of complex organic molecules.
The reactivity of alkyl halides is influenced by several factors, including the nature of the halogen, the structure of the alkyl group, and the reaction conditions. Generally, the reactivity of alkyl halides in nucleophilic substitution reactions follows the order: methyl > primary > secondary > tertiary. This trend is explained by steric hindrance and the stability of carbocation intermediates in SN1 reactions.
The structure of alkyl halides consists of a carbon-halogen bond, where the halogen atom replaces a hydrogen atom in the parent alkane. This substitution results in unique chemical and physical properties that distinguish alkyl halides from their hydrocarbon counterparts. The nature of the carbon-halogen bond is primarily covalent but exhibits some ionic character due to the electronegativity difference between carbon and the halogen atom.
Alkyl halides are classified based on the number of alkyl groups attached to the carbon bearing the halogen atom. Primary alkyl halides have one alkyl group, secondary have two, and tertiary have three. This classification is essential as it influences the compound's reactivity and behavior in various chemical reactions.
The physical properties of alkyl halides are largely determined by the size and electronegativity of the halogen atom. As the atomic number of the halogen increases, the boiling point and density of the alkyl halide generally increase. This trend is attributed to the increasing strength of intermolecular forces, particularly van der Waals interactions, as the halogen atom becomes larger.
The polarity of the carbon-halogen bond is a key factor in the chemical behavior of alkyl halides. The electronegativity difference between carbon and the halogen creates a dipole moment, with the halogen bearing a partial negative charge and the carbon a partial positive charge. This polarity influences the compound's solubility, reactivity, and its ability to participate in various organic reactions.
Alkyl halides serve as versatile precursors in organic synthesis due to their ability to undergo nucleophilic substitution and elimination reactions. These reactions form the basis for the conversion of alkyl halides into a wide range of other functional groups, making them invaluable in the synthesis of complex organic molecules.
The reactivity of alkyl halides is influenced by several factors, including the nature of the halogen, the structure of the alkyl group, and the reaction conditions. Generally, the reactivity of alkyl halides in nucleophilic substitution reactions follows the order: methyl > primary > secondary > tertiary. This trend is explained by steric hindrance and the stability of carbocation intermediates in SN1 reactions.
Industrial Applications
Alkyl halides play a crucial role in various industrial applications, serving as versatile intermediates and reagents in numerous chemical processes. Their widespread use stems from their reactivity and the ease with which they can be transformed into other valuable compounds. In the pharmaceutical industry, alkyl halides are essential building blocks for the synthesis of many drugs and active pharmaceutical ingredients (APIs). They serve as key intermediates in the production of antibiotics, anti-inflammatory drugs, and other therapeutic agents. The ability to manipulate alkyl halides through nucleophilic substitution and elimination reactions allows for the creation of complex molecular structures necessary for drug development.
The polymer industry heavily relies on alkyl halides for the production of various plastics and synthetic materials. Polyvinyl chloride (PVC), one of the world's most widely used plastics, is manufactured using vinyl chloride, an alkyl halide. PVC finds applications in construction, automotive, and consumer goods sectors. Additionally, alkyl halides are used in the synthesis of other important polymers, such as polyvinylidene chloride (PVDC) and fluoropolymers, which are valued for their unique properties like chemical resistance and low friction.
In the agrochemical sector, alkyl halides are utilized in the production of pesticides, herbicides, and fungicides. These compounds often serve as precursors or active ingredients in crop protection products, helping to improve agricultural yields and food security. The ability to fine-tune the properties of alkyl halides allows for the development of targeted and environmentally friendly agrochemicals.
The electronics industry benefits from alkyl halides in the production of semiconductors and electronic components. Chlorinated and fluorinated alkyl compounds are used as cleaning agents and etchants in semiconductor manufacturing processes. They play a crucial role in creating the intricate patterns and structures required for modern electronic devices.
Alkyl halides also find applications in the production of dyes and pigments. Their reactivity allows for the introduction of various functional groups, enabling the synthesis of a wide range of colorants used in textiles, paints, and printing inks. The versatility of alkyl halides in color chemistry contributes to the development of vibrant and durable products across multiple industries.
In the field of surface treatment and coatings, alkyl halides are employed in the production of water-repellent and stain-resistant finishes. Fluorinated alkyl compounds, in particular, are used to create durable and non-stick coatings for cookware, textiles, and industrial equipment. These applications leverage the unique properties of alkyl halides to enhance the performance and longevity of various consumer and industrial products.
The polymer industry heavily relies on alkyl halides for the production of various plastics and synthetic materials. Polyvinyl chloride (PVC), one of the world's most widely used plastics, is manufactured using vinyl chloride, an alkyl halide. PVC finds applications in construction, automotive, and consumer goods sectors. Additionally, alkyl halides are used in the synthesis of other important polymers, such as polyvinylidene chloride (PVDC) and fluoropolymers, which are valued for their unique properties like chemical resistance and low friction.
In the agrochemical sector, alkyl halides are utilized in the production of pesticides, herbicides, and fungicides. These compounds often serve as precursors or active ingredients in crop protection products, helping to improve agricultural yields and food security. The ability to fine-tune the properties of alkyl halides allows for the development of targeted and environmentally friendly agrochemicals.
The electronics industry benefits from alkyl halides in the production of semiconductors and electronic components. Chlorinated and fluorinated alkyl compounds are used as cleaning agents and etchants in semiconductor manufacturing processes. They play a crucial role in creating the intricate patterns and structures required for modern electronic devices.
Alkyl halides also find applications in the production of dyes and pigments. Their reactivity allows for the introduction of various functional groups, enabling the synthesis of a wide range of colorants used in textiles, paints, and printing inks. The versatility of alkyl halides in color chemistry contributes to the development of vibrant and durable products across multiple industries.
In the field of surface treatment and coatings, alkyl halides are employed in the production of water-repellent and stain-resistant finishes. Fluorinated alkyl compounds, in particular, are used to create durable and non-stick coatings for cookware, textiles, and industrial equipment. These applications leverage the unique properties of alkyl halides to enhance the performance and longevity of various consumer and industrial products.
Reaction Mechanisms
The reaction mechanisms of alkyl halides are fundamental to understanding their key reactions and uses. These mechanisms primarily involve nucleophilic substitution and elimination reactions, which are influenced by factors such as the structure of the alkyl halide, the nature of the nucleophile, and the reaction conditions.
Nucleophilic substitution reactions of alkyl halides can proceed via two distinct mechanisms: SN1 (unimolecular nucleophilic substitution) and SN2 (bimolecular nucleophilic substitution). The SN1 mechanism occurs in two steps, beginning with the slow, rate-determining dissociation of the alkyl halide to form a carbocation intermediate. This is followed by the rapid attack of the nucleophile on the carbocation. SN1 reactions are favored by tertiary alkyl halides and polar protic solvents.
In contrast, the SN2 mechanism is a concerted, one-step process where the nucleophile attacks the alkyl halide from the backside, opposite the leaving group. This results in an inversion of stereochemistry at the reaction center. SN2 reactions are favored by primary and secondary alkyl halides, strong nucleophiles, and polar aprotic solvents.
Elimination reactions of alkyl halides can also occur via two mechanisms: E1 (unimolecular elimination) and E2 (bimolecular elimination). The E1 mechanism, like SN1, involves the formation of a carbocation intermediate, followed by the removal of a proton to form an alkene. E1 reactions are favored by tertiary alkyl halides and conditions similar to those that promote SN1 reactions.
The E2 mechanism is a concerted process where the base removes a proton while the leaving group departs, resulting in the formation of an alkene. E2 reactions are favored by secondary and tertiary alkyl halides, strong bases, and typically occur under conditions similar to those that promote SN2 reactions.
The competition between substitution and elimination reactions is influenced by various factors, including the structure of the alkyl halide, the strength and steric bulk of the nucleophile or base, the solvent, and the temperature. Understanding these mechanistic pathways is crucial for predicting and controlling the outcomes of reactions involving alkyl halides.
Recent advancements in computational chemistry and experimental techniques have provided deeper insights into these reaction mechanisms, allowing for more precise control and prediction of reaction outcomes. This knowledge has led to the development of new synthetic methodologies and improved understanding of the reactivity of alkyl halides in various chemical and biological systems.
Nucleophilic substitution reactions of alkyl halides can proceed via two distinct mechanisms: SN1 (unimolecular nucleophilic substitution) and SN2 (bimolecular nucleophilic substitution). The SN1 mechanism occurs in two steps, beginning with the slow, rate-determining dissociation of the alkyl halide to form a carbocation intermediate. This is followed by the rapid attack of the nucleophile on the carbocation. SN1 reactions are favored by tertiary alkyl halides and polar protic solvents.
In contrast, the SN2 mechanism is a concerted, one-step process where the nucleophile attacks the alkyl halide from the backside, opposite the leaving group. This results in an inversion of stereochemistry at the reaction center. SN2 reactions are favored by primary and secondary alkyl halides, strong nucleophiles, and polar aprotic solvents.
Elimination reactions of alkyl halides can also occur via two mechanisms: E1 (unimolecular elimination) and E2 (bimolecular elimination). The E1 mechanism, like SN1, involves the formation of a carbocation intermediate, followed by the removal of a proton to form an alkene. E1 reactions are favored by tertiary alkyl halides and conditions similar to those that promote SN1 reactions.
The E2 mechanism is a concerted process where the base removes a proton while the leaving group departs, resulting in the formation of an alkene. E2 reactions are favored by secondary and tertiary alkyl halides, strong bases, and typically occur under conditions similar to those that promote SN2 reactions.
The competition between substitution and elimination reactions is influenced by various factors, including the structure of the alkyl halide, the strength and steric bulk of the nucleophile or base, the solvent, and the temperature. Understanding these mechanistic pathways is crucial for predicting and controlling the outcomes of reactions involving alkyl halides.
Recent advancements in computational chemistry and experimental techniques have provided deeper insights into these reaction mechanisms, allowing for more precise control and prediction of reaction outcomes. This knowledge has led to the development of new synthetic methodologies and improved understanding of the reactivity of alkyl halides in various chemical and biological systems.
Current Synthetic Methods
01 Synthesis of alkyl halides
Various methods for synthesizing alkyl halides are described, including halogenation of alkanes, addition of hydrogen halides to alkenes, and substitution reactions of alcohols with halogenating agents. These processes often involve specific catalysts, reaction conditions, and purification techniques to obtain the desired alkyl halide products.- Synthesis of alkyl halides: Various methods for synthesizing alkyl halides are described, including halogenation of alkanes, addition of hydrogen halides to alkenes, and substitution reactions of alcohols with halogenating agents. These processes often involve specific catalysts, reaction conditions, and purification techniques to obtain high-quality alkyl halide products.
- Applications of alkyl halides in organic synthesis: Alkyl halides serve as versatile intermediates in organic synthesis, participating in numerous reactions such as nucleophilic substitution, elimination, and coupling reactions. They are used to introduce alkyl groups into various organic compounds and play a crucial role in the synthesis of pharmaceuticals, agrochemicals, and other fine chemicals.
- Industrial production of alkyl halides: Large-scale production methods for alkyl halides are discussed, focusing on optimizing yield, purity, and cost-effectiveness. These processes often involve continuous flow reactors, specialized catalysts, and efficient separation techniques to meet industrial demands for high-volume production of various alkyl halides.
- Environmental and safety considerations in alkyl halide handling: Due to the potential environmental impact and health hazards associated with some alkyl halides, various safety measures and handling protocols are described. This includes the development of less toxic alternatives, improved containment systems, and waste treatment methods to minimize environmental contamination and ensure worker safety in industrial settings.
- Novel applications of alkyl halides: Emerging applications of alkyl halides in various fields are explored, including their use in advanced materials, nanotechnology, and energy storage systems. These novel applications leverage the unique properties of alkyl halides to develop innovative products and processes with potential impacts across multiple industries.
02 Applications of alkyl halides in organic synthesis
Alkyl halides serve as versatile intermediates in organic synthesis, participating in numerous reactions such as nucleophilic substitution, elimination, and coupling reactions. They are used to introduce alkyl groups into molecules and can be transformed into other functional groups, making them valuable building blocks in the synthesis of complex organic compounds.Expand Specific Solutions03 Industrial uses of alkyl halides
Alkyl halides find applications in various industrial processes, including the production of polymers, solvents, and refrigerants. They are also used as intermediates in the manufacture of pharmaceuticals, agrochemicals, and other specialty chemicals. Some alkyl halides serve as flame retardants or as components in cleaning agents and aerosol propellants.Expand Specific Solutions04 Environmental and safety considerations
The use and production of alkyl halides raise environmental and safety concerns due to their potential toxicity and ozone-depleting properties. Research focuses on developing safer alternatives, improving handling and disposal methods, and implementing regulations to minimize their environmental impact. Efforts are also made to develop more environmentally friendly synthesis routes for alkyl halides.Expand Specific Solutions05 Analytical methods for alkyl halides
Various analytical techniques are employed to characterize and quantify alkyl halides in different matrices. These include gas chromatography, mass spectrometry, and spectroscopic methods such as NMR and IR. Advanced separation and detection methods are developed to improve the sensitivity and selectivity of alkyl halide analysis in environmental samples, industrial products, and biological systems.Expand Specific Solutions
Key Industry Players
The market for alkyl halide reactions and applications is in a mature stage, with established players across the petrochemical, pharmaceutical, and specialty chemical industries. Key companies like China Petroleum & Chemical Corp., Pfizer Inc., and Eastman Chemical Co. have significant market presence. The global market size for alkyl halide-related products is substantial, driven by diverse applications in organic synthesis, pharmaceuticals, and materials science. Technologically, the field is well-developed, with ongoing research focused on improving efficiency and sustainability. Companies like Sinopec Research Institute and AbbVie are actively engaged in R&D to enhance existing processes and develop novel applications, indicating a competitive landscape with opportunities for innovation and market expansion.
China Petroleum & Chemical Corp.
Technical Solution: China Petroleum & Chemical Corp. (Sinopec) has developed advanced catalytic processes for alkyl halide conversion in petroleum refining. Their technology focuses on hydrodechlorination reactions to remove chlorine from alkyl halides, producing valuable hydrocarbons. Sinopec's approach utilizes noble metal catalysts supported on high-surface-area materials to achieve high conversion rates and selectivity[1]. The process operates under moderate hydrogen pressure and temperature conditions, typically 200-300°C and 2-5 MPa, to maximize efficiency while minimizing energy consumption[3]. Sinopec has also implemented continuous flow reactors for large-scale industrial applications, allowing for improved process control and product quality[5].
Strengths: Extensive experience in large-scale chemical processes, strong R&D capabilities, and established industrial infrastructure. Weaknesses: Potential environmental concerns related to chlorinated byproducts and catalyst disposal.
Eastman Chemical Co.
Technical Solution: Eastman Chemical Co. has developed innovative approaches for alkyl halide transformations, particularly in the production of specialty chemicals and advanced materials. Their technology focuses on nucleophilic substitution reactions of alkyl halides to introduce various functional groups. Eastman's process utilizes phase-transfer catalysis to enhance reaction rates and selectivity in biphasic systems[2]. They have also implemented continuous flow microreactor technology for precise control of reaction conditions and improved safety[4]. Eastman's approach allows for the synthesis of complex molecules from alkyl halide precursors, including pharmaceuticals and high-performance polymers. The company has recently explored green chemistry alternatives, such as using ionic liquids as solvents to reduce environmental impact[6].
Strengths: Diverse product portfolio, strong intellectual property position, and expertise in specialty chemical manufacturing. Weaknesses: Higher production costs compared to bulk chemical processes and potential scalability challenges for some specialty products.
Innovative Reaction Pathways
Isotopically labeled chemically stable reagents and process for the synthesis thereof
PatentActiveUS8058464B2
Innovation
- A radioisotope labeled reagent with the formula L-(aCbH2)naCbH3, where L is a chemically stable leaving group, is synthesized by reacting an isotope-enriched methyl halide with a metal or onium ion under anhydrous conditions in an aprotic solvent, providing enhanced stability and allowing for extended storage and remote isotopic labeling reactions.
Synthesis of primary alkyl halides
PatentInactiveUS3799999A
Innovation
- A new synthesis method involving an interchange reaction between a primary alkyl halide and an ethyl halide in the presence of alkali and alkaline earth metal halide salts as catalysts in an aprotic solvent, allowing for the efficient production of primary alkyl halides like chlorides, bromides, and iodides with minimal material loss.
Environmental Considerations
The environmental impact of alkyl halide reactions and uses is a critical consideration in modern chemistry. These compounds, while valuable in many industrial and research applications, pose significant environmental risks if not properly managed.
Alkyl halides are known to contribute to ozone depletion when released into the atmosphere. Chlorofluorocarbons (CFCs), a subset of alkyl halides, have been phased out globally due to their destructive effect on the ozone layer. This has led to the development of alternative compounds and processes that minimize environmental harm.
Water and soil contamination are also major concerns associated with alkyl halides. These compounds can persist in the environment, potentially entering groundwater systems and food chains. Their bioaccumulation in aquatic organisms can lead to long-term ecological damage and pose risks to human health through contaminated water sources and seafood consumption.
The disposal of alkyl halide waste presents another environmental challenge. Improper disposal can result in soil and water pollution, necessitating strict regulations and guidelines for waste management in laboratories and industrial settings. Many countries have implemented stringent protocols for the handling, storage, and disposal of these compounds to mitigate environmental risks.
In response to these environmental concerns, there has been a shift towards green chemistry principles in alkyl halide reactions. Researchers are developing alternative synthetic routes that reduce or eliminate the use of harmful alkyl halides. This includes exploring bio-based alternatives, using ionic liquids as solvents, and implementing catalytic processes that minimize waste production.
The concept of atom economy has gained prominence in alkyl halide chemistry, focusing on maximizing the incorporation of reactants into the final product to reduce waste. This approach not only improves environmental sustainability but also enhances economic efficiency in chemical processes.
Efforts are also being made to improve the recyclability and biodegradability of alkyl halide-derived products. This includes developing new polymer materials that can be more easily broken down at the end of their lifecycle, reducing long-term environmental impact.
Monitoring and remediation technologies for alkyl halide contamination have advanced significantly. Improved detection methods allow for early identification of environmental contamination, while innovative remediation techniques, such as bioremediation and advanced oxidation processes, offer more effective and environmentally friendly cleanup solutions.
Alkyl halides are known to contribute to ozone depletion when released into the atmosphere. Chlorofluorocarbons (CFCs), a subset of alkyl halides, have been phased out globally due to their destructive effect on the ozone layer. This has led to the development of alternative compounds and processes that minimize environmental harm.
Water and soil contamination are also major concerns associated with alkyl halides. These compounds can persist in the environment, potentially entering groundwater systems and food chains. Their bioaccumulation in aquatic organisms can lead to long-term ecological damage and pose risks to human health through contaminated water sources and seafood consumption.
The disposal of alkyl halide waste presents another environmental challenge. Improper disposal can result in soil and water pollution, necessitating strict regulations and guidelines for waste management in laboratories and industrial settings. Many countries have implemented stringent protocols for the handling, storage, and disposal of these compounds to mitigate environmental risks.
In response to these environmental concerns, there has been a shift towards green chemistry principles in alkyl halide reactions. Researchers are developing alternative synthetic routes that reduce or eliminate the use of harmful alkyl halides. This includes exploring bio-based alternatives, using ionic liquids as solvents, and implementing catalytic processes that minimize waste production.
The concept of atom economy has gained prominence in alkyl halide chemistry, focusing on maximizing the incorporation of reactants into the final product to reduce waste. This approach not only improves environmental sustainability but also enhances economic efficiency in chemical processes.
Efforts are also being made to improve the recyclability and biodegradability of alkyl halide-derived products. This includes developing new polymer materials that can be more easily broken down at the end of their lifecycle, reducing long-term environmental impact.
Monitoring and remediation technologies for alkyl halide contamination have advanced significantly. Improved detection methods allow for early identification of environmental contamination, while innovative remediation techniques, such as bioremediation and advanced oxidation processes, offer more effective and environmentally friendly cleanup solutions.
Safety and Handling Protocols
Alkyl halides are highly reactive compounds that require careful handling and storage to ensure safety in laboratory and industrial settings. Proper safety protocols are essential to mitigate risks associated with their use. Personal protective equipment (PPE) is crucial when working with alkyl halides. This includes wearing chemical-resistant gloves, safety goggles, and a lab coat. In cases where vapors may be present, a fume hood or respiratory protection should be utilized.
Storage of alkyl halides demands special attention. These compounds should be kept in tightly sealed containers made of materials resistant to halogenated hydrocarbons, such as glass or certain plastics. Storage areas must be well-ventilated and away from sources of heat or ignition. It is advisable to store alkyl halides separately from incompatible materials, particularly strong oxidizers and bases.
Handling procedures for alkyl halides should minimize the risk of spills or exposure. Transfer of these compounds should be performed in a fume hood using appropriate techniques to prevent splashing or aerosolization. When possible, use of sealed systems or transfer techniques that limit exposure, such as cannula transfer, is recommended.
In the event of a spill, prompt action is critical. Small spills can be contained and absorbed using inert materials like vermiculite or activated charcoal. Larger spills may require professional hazardous material handling teams. Proper disposal of alkyl halides and contaminated materials is essential, following local regulations for hazardous waste management.
Training and education are vital components of safety protocols. All personnel working with alkyl halides should receive comprehensive training on the hazards, proper handling techniques, and emergency procedures. Regular refresher courses and safety drills can help maintain a high level of preparedness.
Emergency response planning is crucial when working with alkyl halides. Laboratories and facilities should have clearly defined procedures for addressing accidents, including spills, fires, or personal exposure. This includes readily accessible safety showers, eyewash stations, and appropriate fire suppression equipment. Additionally, maintaining up-to-date safety data sheets (SDS) for all alkyl halides in use is essential for quick reference in emergency situations.
Implementing a robust monitoring system can further enhance safety. This may include regular inspections of storage areas, integrity checks on containers, and environmental monitoring for potential leaks or vapor accumulation. By adhering to these comprehensive safety and handling protocols, the risks associated with alkyl halides can be significantly reduced, ensuring a safer working environment for all involved.
Storage of alkyl halides demands special attention. These compounds should be kept in tightly sealed containers made of materials resistant to halogenated hydrocarbons, such as glass or certain plastics. Storage areas must be well-ventilated and away from sources of heat or ignition. It is advisable to store alkyl halides separately from incompatible materials, particularly strong oxidizers and bases.
Handling procedures for alkyl halides should minimize the risk of spills or exposure. Transfer of these compounds should be performed in a fume hood using appropriate techniques to prevent splashing or aerosolization. When possible, use of sealed systems or transfer techniques that limit exposure, such as cannula transfer, is recommended.
In the event of a spill, prompt action is critical. Small spills can be contained and absorbed using inert materials like vermiculite or activated charcoal. Larger spills may require professional hazardous material handling teams. Proper disposal of alkyl halides and contaminated materials is essential, following local regulations for hazardous waste management.
Training and education are vital components of safety protocols. All personnel working with alkyl halides should receive comprehensive training on the hazards, proper handling techniques, and emergency procedures. Regular refresher courses and safety drills can help maintain a high level of preparedness.
Emergency response planning is crucial when working with alkyl halides. Laboratories and facilities should have clearly defined procedures for addressing accidents, including spills, fires, or personal exposure. This includes readily accessible safety showers, eyewash stations, and appropriate fire suppression equipment. Additionally, maintaining up-to-date safety data sheets (SDS) for all alkyl halides in use is essential for quick reference in emergency situations.
Implementing a robust monitoring system can further enhance safety. This may include regular inspections of storage areas, integrity checks on containers, and environmental monitoring for potential leaks or vapor accumulation. By adhering to these comprehensive safety and handling protocols, the risks associated with alkyl halides can be significantly reduced, ensuring a safer working environment for all involved.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!