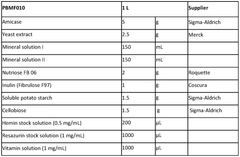
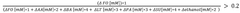Butyrate's Dualistic Effects on Human Health: Therapeutic Potential and Associated Risks
Butyrate Research Background and Objectives
Butyrate, a short-chain fatty acid produced by gut microbiota through fermentation of dietary fibers, has emerged as a significant molecule in human health research. The exploration of butyrate's effects on human health has a rich history dating back to the early 20th century, with initial studies focusing on its role in colonic health. Over the decades, research has expanded to encompass a wide range of potential therapeutic applications, from gastrointestinal disorders to metabolic syndromes and even neurological conditions.
The evolution of butyrate research has been marked by significant milestones, including the discovery of its histone deacetylase (HDAC) inhibitory properties in the 1970s, which opened new avenues for understanding its molecular mechanisms. Recent advancements in microbiome research have further propelled the interest in butyrate, highlighting its role as a key mediator in the gut-brain axis and its potential impact on systemic health.
Current research trends indicate a growing interest in elucidating the dual nature of butyrate's effects on human health. While numerous studies have demonstrated its beneficial properties, including anti-inflammatory, anti-carcinogenic, and metabolic regulatory effects, emerging evidence suggests potential risks associated with excessive butyrate levels or exposure in certain contexts. This duality presents both opportunities and challenges for therapeutic applications.
The primary objectives of contemporary butyrate research are multifaceted. Firstly, there is a pressing need to comprehensively map the molecular pathways through which butyrate exerts its diverse effects on different organ systems. This includes investigating its interactions with various cellular receptors and its influence on gene expression patterns. Secondly, researchers aim to delineate the optimal therapeutic windows for butyrate supplementation, considering factors such as dosage, duration, and delivery methods.
Another critical goal is to elucidate the complex interplay between butyrate, the gut microbiome, and host physiology. This involves exploring how dietary interventions and probiotic supplementations can modulate butyrate production and its subsequent health impacts. Additionally, there is a growing focus on developing targeted delivery systems to enhance butyrate's therapeutic efficacy while minimizing potential adverse effects.
The field also seeks to address the paradoxical effects of butyrate observed in different physiological states and disease conditions. Understanding these context-dependent outcomes is crucial for developing safe and effective butyrate-based therapies. Lastly, long-term studies are being prioritized to assess the sustained benefits and potential risks of chronic butyrate exposure, particularly in the context of preventive medicine and management of chronic diseases.
Market Analysis of Butyrate-Based Therapeutics
The market for butyrate-based therapeutics is experiencing significant growth, driven by increasing awareness of the compound's potential health benefits and its dual role in human physiology. The global market for butyrate-related products is expanding across various sectors, including pharmaceuticals, nutraceuticals, and functional foods.
In the pharmaceutical sector, butyrate-based drugs are being developed for a range of gastrointestinal disorders, including inflammatory bowel diseases (IBD) such as Crohn's disease and ulcerative colitis. The market for IBD treatments alone is projected to grow substantially in the coming years, with butyrate-based therapies poised to capture a significant share.
The nutraceutical industry is also capitalizing on butyrate's potential, with an increasing number of dietary supplements containing butyrate or its precursors entering the market. These products are marketed for gut health, immune support, and potential metabolic benefits, appealing to health-conscious consumers seeking natural solutions for wellness.
Functional foods fortified with butyrate or designed to promote its production in the gut represent another growing market segment. Prebiotic fibers that support butyrate-producing bacteria in the colon are gaining popularity among food manufacturers looking to enhance the health profile of their products.
However, the market for butyrate-based therapeutics faces challenges. The compound's dual nature, with both beneficial and potentially harmful effects, necessitates careful product development and marketing strategies. Regulatory scrutiny is likely to increase as more products enter the market, potentially impacting approval processes and time-to-market for new therapies.
Despite these challenges, the overall market outlook remains positive. The aging population in many developed countries, coupled with rising rates of gastrointestinal disorders and metabolic diseases, is expected to drive demand for butyrate-based interventions. Additionally, ongoing research into butyrate's effects on various aspects of human health, including potential applications in neurological and cardiovascular disorders, may open new market opportunities.
Geographically, North America and Europe currently lead the market for butyrate-based therapeutics, owing to advanced healthcare infrastructure and higher consumer awareness. However, emerging markets in Asia-Pacific and Latin America are expected to show rapid growth as healthcare spending increases and awareness of gut health improves.
Current Challenges in Butyrate Research
Despite the promising therapeutic potential of butyrate, researchers face several significant challenges in advancing its clinical applications. One of the primary obstacles is the limited bioavailability of butyrate due to its rapid absorption and metabolism in the upper gastrointestinal tract. This necessitates the development of novel delivery systems to ensure sufficient concentrations reach the target tissues, particularly the colon.
Another challenge lies in the complex and sometimes contradictory effects of butyrate on different cell types and tissues. While butyrate has shown anti-inflammatory and anti-carcinogenic properties in some contexts, it may also promote inflammation and tumor growth under certain conditions. This dualistic nature complicates the development of targeted therapies and requires a more nuanced understanding of butyrate's mechanisms of action in various physiological states.
The dosage and timing of butyrate administration present additional hurdles. Determining the optimal therapeutic window for different conditions, such as inflammatory bowel diseases or colorectal cancer, remains a critical area of investigation. Moreover, the potential long-term effects of sustained butyrate supplementation on gut microbiota composition and overall health are not yet fully understood, necessitating extensive longitudinal studies.
Researchers also grapple with the challenge of standardizing butyrate production and delivery methods. The variability in butyrate-producing bacteria strains and dietary fiber sources can lead to inconsistent results across studies, making it difficult to draw definitive conclusions about butyrate's efficacy in different therapeutic applications.
Furthermore, the interaction between butyrate and other short-chain fatty acids, as well as its effects on various signaling pathways, adds another layer of complexity to research efforts. Elucidating these intricate relationships is crucial for developing targeted interventions that maximize therapeutic benefits while minimizing potential risks.
Lastly, translating promising in vitro and animal model findings to human clinical trials poses significant challenges. The differences in gut physiology and microbiome composition between species necessitate careful consideration when extrapolating results. Additionally, designing and conducting well-controlled human studies that account for the myriad factors influencing butyrate production and metabolism in the gut remains a formidable task for researchers in the field.
Existing Butyrate Delivery Methods
01 Gut health and microbiome modulation
Butyrate plays a crucial role in maintaining gut health and modulating the microbiome. It serves as an energy source for colonocytes, strengthens the intestinal barrier, and promotes the growth of beneficial bacteria. This can lead to improved digestion, reduced inflammation, and enhanced overall gut function.- Gut health and microbiome modulation: Butyrate plays a crucial role in maintaining gut health and modulating the microbiome. It serves as a primary energy source for colonocytes and has anti-inflammatory properties in the intestine. Butyrate supplementation can improve gut barrier function, reduce intestinal permeability, and promote the growth of beneficial bacteria.
- Metabolic health and weight management: Butyrate has been shown to have positive effects on metabolic health and weight management. It can improve insulin sensitivity, regulate glucose metabolism, and reduce fat accumulation. Studies suggest that butyrate supplementation may help in the prevention and management of obesity and related metabolic disorders.
- Neuroprotective and cognitive effects: Emerging research indicates that butyrate may have neuroprotective properties and positive effects on cognitive function. It has been associated with improved memory, reduced neuroinflammation, and potential benefits in neurodegenerative disorders. Butyrate's ability to cross the blood-brain barrier makes it a promising candidate for brain health applications.
- Anti-inflammatory and immune-modulating properties: Butyrate exhibits potent anti-inflammatory effects and can modulate the immune system. It has been shown to suppress pro-inflammatory cytokines, enhance the production of regulatory T cells, and improve overall immune function. These properties make butyrate potentially beneficial in managing inflammatory conditions and autoimmune disorders.
- Cancer prevention and treatment potential: Studies have suggested that butyrate may have anticancer properties, particularly in colorectal cancer. It has been shown to induce apoptosis in cancer cells, inhibit tumor growth, and enhance the effectiveness of certain chemotherapeutic agents. Butyrate's potential in cancer prevention and as an adjunct therapy is an area of ongoing research.
02 Anti-inflammatory and immune-modulating effects
Butyrate exhibits potent anti-inflammatory properties and helps modulate the immune system. It can suppress pro-inflammatory cytokines, enhance regulatory T-cell function, and promote a balanced immune response. These effects may be beneficial in managing various inflammatory conditions and autoimmune disorders.Expand Specific Solutions03 Metabolic health and weight management
Butyrate has been shown to positively impact metabolic health and weight management. It can improve insulin sensitivity, regulate glucose metabolism, and enhance energy expenditure. These effects may contribute to better weight control and reduced risk of metabolic disorders such as type 2 diabetes and obesity.Expand Specific Solutions04 Neuroprotective and cognitive benefits
Emerging research suggests that butyrate may have neuroprotective properties and cognitive benefits. It can cross the blood-brain barrier, promote neuroplasticity, and support the production of neurotransmitters. These effects may have potential applications in managing neurodegenerative disorders and improving cognitive function.Expand Specific Solutions05 Cancer prevention and treatment potential
Butyrate has shown promise in cancer prevention and as a potential adjunct therapy in cancer treatment. It can induce apoptosis in cancer cells, inhibit tumor growth, and enhance the effectiveness of certain chemotherapeutic agents. These properties make butyrate an interesting target for further research in oncology.Expand Specific Solutions
Key Players in Butyrate Research and Development
The research on butyrate's dual effects on human health is in a dynamic phase, with the market showing significant growth potential. The field is characterized by a mix of established pharmaceutical companies, academic institutions, and emerging biotech firms, indicating a diverse competitive landscape. Companies like Société des Produits Nestlé SA, Astellas Pharma, Inc., and DSM IP Assets BV are leveraging their resources to explore butyrate's therapeutic applications. Academic institutions such as The University of Chicago and Boston University are contributing to foundational research. The technology's maturity varies across different applications, with some areas more advanced than others. Emerging players like Axcess Global Sciences LLC and Evopoint Biosciences Co., Ltd. are focusing on innovative approaches, potentially disrupting the market. Overall, the field is marked by ongoing research and development, with a trend towards personalized therapeutic solutions.
The University of Chicago
Astellas Pharma, Inc.
Breakthrough Studies on Butyrate Mechanisms
- Development of oral, systemically bioavailable butyrate conjugates with amino acids like serine, threonine, and tyrosine, which enhance bioavailability and allow butyrate to bypass initial metabolism, facilitating its transport into the bloodstream and across the blood-brain barrier.
- Development of defined consortia of anaerobic bacterial strains, specifically including Agathobacter rectalis, Anaerostipes caccae, and Anaerobutyricum hallii, which are selected to enhance butyrate production through continuous co-culture fermentations, achieving metabolic equilibrium and stability, and are designed to treat conditions related to intestinal dysbiosis.
Regulatory Framework for Butyrate-Based Therapies
The regulatory framework for butyrate-based therapies is a critical aspect of their development and implementation in clinical settings. As butyrate's therapeutic potential gains recognition, regulatory bodies worldwide are working to establish guidelines for its use in medical applications.
In the United States, the Food and Drug Administration (FDA) plays a pivotal role in overseeing butyrate-based therapies. The FDA categorizes these treatments based on their intended use and formulation. For instance, butyrate supplements may be regulated as dietary supplements under the Dietary Supplement Health and Education Act (DSHEA), while more targeted therapeutic applications might fall under the purview of drug regulations.
The European Medicines Agency (EMA) has also been actively involved in shaping the regulatory landscape for butyrate-based therapies in the European Union. The EMA's approach focuses on evaluating the safety and efficacy of these treatments through rigorous clinical trials and post-market surveillance.
Regulatory considerations for butyrate-based therapies extend beyond traditional pharmaceutical regulations. Given butyrate's role in gut health and its potential impact on the microbiome, regulatory bodies are developing new frameworks to address the unique challenges posed by microbiome-modulating therapies.
Safety monitoring is a crucial component of the regulatory framework. Long-term studies are required to assess the potential risks associated with prolonged butyrate supplementation or treatment. This includes monitoring for adverse effects on gut health, metabolic processes, and potential interactions with other medications.
The regulatory landscape also addresses the quality control and standardization of butyrate-based products. This includes establishing guidelines for manufacturing processes, dosage forms, and stability testing to ensure consistent product quality and efficacy.
As research continues to uncover new applications for butyrate, regulatory frameworks are evolving to accommodate emerging therapeutic approaches. This includes considerations for personalized medicine, where butyrate treatments may be tailored to individual patient needs based on their microbiome profile or genetic factors.
International harmonization efforts are underway to align regulatory approaches across different regions. This aims to facilitate global development and access to butyrate-based therapies while maintaining high standards of safety and efficacy.
Safety and Risk Assessment of Butyrate Interventions
The safety and risk assessment of butyrate interventions is a critical aspect of evaluating their therapeutic potential. While butyrate has shown promising effects in various health conditions, it is essential to consider potential risks and adverse effects associated with its use. One primary concern is the potential for gastrointestinal disturbances, as butyrate supplementation may lead to bloating, abdominal discomfort, and diarrhea in some individuals. These side effects are generally mild and transient but should be monitored closely during clinical trials and therapeutic applications.
Another important consideration is the potential impact of butyrate on the gut microbiome. While butyrate is generally considered beneficial for gut health, excessive supplementation may disrupt the delicate balance of the microbial ecosystem. This could potentially lead to unintended consequences, such as the overgrowth of certain bacterial species or alterations in metabolic processes within the gut.
The long-term effects of butyrate supplementation also require careful evaluation. Limited data are available on the safety of prolonged butyrate use, particularly in specific patient populations such as those with compromised immune systems or chronic diseases. Researchers must conduct longitudinal studies to assess any potential cumulative effects or delayed adverse reactions that may arise from extended butyrate interventions.
Dosage and administration routes are crucial factors in the safety profile of butyrate interventions. Oral supplementation, enemas, and intravenous administration each present unique challenges and potential risks. For instance, oral butyrate supplements may have limited bioavailability due to rapid absorption in the upper gastrointestinal tract, while enemas and intravenous administration may carry risks of local irritation or systemic effects.
Interactions with other medications and dietary components should also be thoroughly investigated. Butyrate may influence the absorption or metabolism of certain drugs, potentially altering their efficacy or safety profiles. Additionally, its effects on nutrient absorption and energy metabolism warrant careful consideration, particularly in patients with metabolic disorders or nutritional deficiencies.
Regulatory considerations play a significant role in the safety assessment of butyrate interventions. Establishing clear guidelines for the production, quality control, and clinical use of butyrate-based therapies is essential to ensure consistent safety standards across different applications and patient populations. This includes developing standardized protocols for monitoring adverse events and establishing appropriate dosing regimens based on robust clinical evidence.