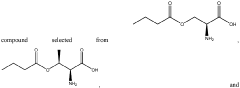
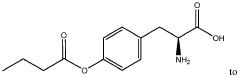
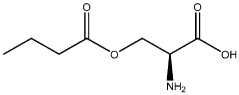
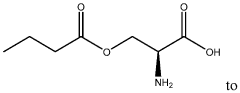
The Role of Microbiota-Derived Butyrate in Mitigating Chronic Diseases: A Comprehensive Review
Butyrate and Chronic Disease: Background and Objectives
The field of microbiome research has witnessed significant advancements in recent years, with a growing focus on the role of microbial metabolites in human health. Among these metabolites, butyrate has emerged as a key player in maintaining gut health and potentially mitigating chronic diseases. This comprehensive review aims to explore the intricate relationship between microbiota-derived butyrate and its impact on various chronic conditions.
Butyrate, a short-chain fatty acid produced by bacterial fermentation of dietary fiber in the colon, has garnered attention for its multifaceted effects on human physiology. Historically, butyrate was primarily recognized for its role in providing energy to colonocytes. However, recent research has unveiled its broader implications in modulating immune responses, maintaining intestinal barrier integrity, and influencing systemic metabolic processes.
The prevalence of chronic diseases has been on the rise globally, posing significant challenges to healthcare systems and individuals' quality of life. Conditions such as inflammatory bowel disease (IBD), type 2 diabetes, obesity, and cardiovascular diseases have been linked to alterations in gut microbiota composition and function. This has led researchers to investigate the potential of microbiota-derived metabolites, particularly butyrate, in alleviating or preventing these chronic conditions.
The objectives of this report are multifold. Firstly, it aims to provide a comprehensive overview of the current understanding of butyrate production by gut microbiota, including the key bacterial species involved and the dietary factors that influence butyrate synthesis. Secondly, it seeks to elucidate the mechanisms by which butyrate exerts its beneficial effects on various physiological systems, with a focus on its anti-inflammatory, immunomodulatory, and metabolic properties.
Furthermore, this review will critically examine the existing evidence linking butyrate to the prevention and management of specific chronic diseases. It will explore both preclinical and clinical studies that have investigated the therapeutic potential of butyrate supplementation or interventions aimed at enhancing endogenous butyrate production. The review will also address the challenges and limitations in translating these findings into clinical applications.
By synthesizing the current state of knowledge on microbiota-derived butyrate and its role in chronic diseases, this review aims to identify gaps in our understanding and highlight promising avenues for future research. It will discuss the potential of butyrate as a biomarker for disease risk assessment and as a therapeutic target for developing novel interventions to combat chronic diseases.
Market Analysis: Microbiome-Based Therapeutics
The microbiome-based therapeutics market has experienced significant growth in recent years, driven by increasing recognition of the gut microbiome's role in human health. This market segment is poised for continued expansion, with a particular focus on butyrate-producing bacteria and their potential in mitigating chronic diseases.
The global microbiome therapeutics market is projected to grow substantially over the next decade. This growth is fueled by rising prevalence of chronic diseases, growing awareness of the microbiome's impact on health, and advancements in microbiome research and technology. Chronic conditions such as inflammatory bowel disease, diabetes, and obesity represent key target areas for microbiome-based interventions.
Butyrate, a short-chain fatty acid produced by certain gut bacteria, has emerged as a promising therapeutic target. Research has demonstrated its potential in alleviating inflammation, improving gut barrier function, and modulating metabolism. This has led to increased interest in developing therapies that either directly deliver butyrate or enhance its production in the gut.
Several pharmaceutical and biotech companies are actively developing microbiome-based therapeutics targeting butyrate production. These range from probiotic formulations containing butyrate-producing bacteria to prebiotic compounds that selectively promote the growth of these beneficial microbes. Additionally, some firms are exploring synthetic biology approaches to engineer bacteria for enhanced butyrate production.
The market for microbiome-based therapeutics faces some challenges, including regulatory hurdles and the complexity of microbiome interactions. However, ongoing clinical trials and positive preliminary results are bolstering investor confidence in this sector. Collaborations between academic institutions and industry players are accelerating the translation of microbiome research into clinical applications.
Consumer interest in microbiome health is also driving market growth in the nutraceutical and functional food sectors. Products claiming to support gut health and boost butyrate production are gaining traction, although regulatory oversight of these claims varies by region.
In conclusion, the market for microbiome-based therapeutics, particularly those targeting butyrate production, shows strong growth potential. As research continues to elucidate the role of butyrate in chronic disease mitigation, we can expect to see an increasing number of innovative products and therapies entering the market in the coming years.
Current Understanding of Microbiota-Derived Butyrate
Microbiota-derived butyrate has emerged as a key player in maintaining gut health and mitigating chronic diseases. Current understanding of this short-chain fatty acid has expanded significantly in recent years, revealing its multifaceted role in human physiology. Butyrate is primarily produced by anaerobic bacteria in the colon through the fermentation of dietary fibers and resistant starches.
Research has shown that butyrate serves as the primary energy source for colonocytes, the cells lining the colon. This function is crucial for maintaining the integrity of the intestinal barrier, which is essential in preventing the translocation of harmful substances into the bloodstream. Additionally, butyrate has been found to possess potent anti-inflammatory properties, modulating the immune response in the gut and potentially throughout the body.
Recent studies have highlighted butyrate's epigenetic effects, particularly its ability to inhibit histone deacetylases (HDACs). This mechanism allows butyrate to influence gene expression, potentially altering cellular processes involved in inflammation, metabolism, and even cancer development. The impact of butyrate on metabolic health has also gained attention, with evidence suggesting its role in improving insulin sensitivity and glucose homeostasis.
The gut-brain axis has become a focal point in understanding the far-reaching effects of butyrate. Research indicates that butyrate can influence neurological function and behavior, possibly through its interaction with the enteric nervous system and vagus nerve signaling. This connection has opened new avenues for investigating butyrate's potential in neurological and psychiatric disorders.
In the context of chronic diseases, butyrate has shown promise in alleviating symptoms and potentially preventing the progression of conditions such as inflammatory bowel disease, obesity, type 2 diabetes, and certain cancers. Its ability to modulate the immune system, reduce oxidative stress, and promote a healthy gut microbiome composition contributes to its therapeutic potential.
However, it is important to note that while the current understanding of butyrate is promising, many aspects of its mechanisms and long-term effects require further investigation. The complex interplay between butyrate, the gut microbiome, and host physiology presents challenges in fully elucidating its role in health and disease. Ongoing research aims to clarify these relationships and explore targeted approaches to harness butyrate's beneficial effects.
Existing Approaches to Enhance Butyrate Production
01 Butyrate-producing microbiota modulation
Methods for modulating butyrate-producing microbiota in the gut to mitigate various health conditions. This involves administering specific probiotic strains or prebiotic compounds that promote the growth of butyrate-producing bacteria, thereby increasing butyrate production in the intestinal environment.- Butyrate-producing microbiota modulation: Methods for modulating butyrate-producing microbiota in the gut to mitigate various health conditions. This involves administering specific bacterial strains or prebiotics to enhance butyrate production, which can help in managing inflammatory disorders, metabolic diseases, and improving overall gut health.
- Butyrate-based pharmaceutical compositions: Development of pharmaceutical compositions containing butyrate or its derivatives for therapeutic applications. These formulations aim to deliver butyrate effectively to target tissues, potentially addressing conditions such as inflammatory bowel diseases, colorectal cancer, and metabolic disorders.
- Dietary interventions for butyrate production: Strategies involving dietary modifications or supplements to enhance the production of butyrate by gut microbiota. This includes the use of specific fibers, resistant starches, or other compounds that can be fermented by butyrate-producing bacteria in the colon.
- Butyrate in combination therapies: Approaches that combine butyrate or butyrate-producing interventions with other therapeutic agents or strategies. This may include combinations with probiotics, antibiotics, or other drugs to enhance overall efficacy in treating various health conditions.
- Diagnostic methods for butyrate-related conditions: Development of diagnostic tools and methods to assess butyrate levels, butyrate-producing microbial populations, or related biomarkers. These diagnostics can help in identifying individuals who may benefit from butyrate-targeted interventions or in monitoring the effectiveness of such treatments.
02 Butyrate-based pharmaceutical compositions
Development of pharmaceutical compositions containing butyrate or butyrate precursors for therapeutic applications. These compositions are designed to deliver butyrate to specific areas of the gastrointestinal tract, potentially mitigating inflammation and improving gut health.Expand Specific Solutions03 Dietary interventions for butyrate production
Strategies involving dietary modifications or supplements to enhance the production of butyrate by gut microbiota. This includes the use of specific fiber types, resistant starches, or other food components that serve as substrates for butyrate-producing bacteria.Expand Specific Solutions04 Butyrate in combination therapies
Approaches that combine butyrate or butyrate-producing strategies with other therapeutic agents or interventions. This may include combining butyrate with specific drugs, probiotics, or other bioactive compounds to enhance overall efficacy in treating various conditions.Expand Specific Solutions05 Butyrate-mediated microbiome restoration
Methods focused on using butyrate or butyrate-producing bacteria to restore a healthy microbiome balance. This approach aims to address dysbiosis and associated health issues by promoting the growth of beneficial bacteria and improving overall gut ecosystem function.Expand Specific Solutions
Key Players in Microbiome and Metabolite Research
The field of microbiota-derived butyrate in chronic disease mitigation is in a rapidly evolving stage, with growing market potential and increasing technological maturity. The global market for microbiome-based therapeutics is expanding, driven by rising chronic disease prevalence and growing awareness of gut health's importance. Technological advancements are evident in the diverse range of companies involved, from established players like Nestlé SA and DSM IP Assets BV to innovative startups such as Seed Health, Inc. and Pendulum Therapeutics, Inc. Academic institutions like The University of Chicago and Zhejiang University are contributing significantly to research, while pharmaceutical companies like Baxter International, Inc. are exploring clinical applications. The involvement of both Western and Eastern institutions suggests a global focus on this emerging field.
Seed Health, Inc.
Baxter International, Inc.
Breakthrough Studies on Butyrate's Disease-Mitigating Effects
- Development of oral, systemically bioavailable butyrate conjugates with amino acids like serine, threonine, and tyrosine, which enhance bioavailability and allow butyrate to bypass initial metabolism, facilitating its transport into the bloodstream and across the blood-brain barrier.
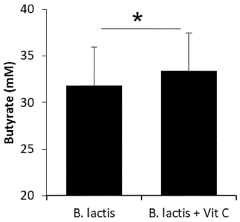
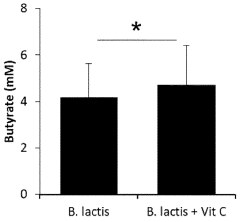
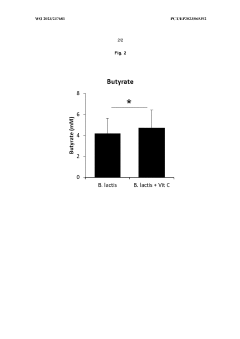
- A combination of vitamin C and Bifidobacterium animalis ssp. lactis, preferably Bifidobacterium animalis ssp. lactis BB-12, is administered simultaneously or sequentially, often in a delayed-release formulation, to directly target the large intestine, enhancing SCFA and butyrate production.
Regulatory Landscape for Microbiome-Based Therapeutics
The regulatory landscape for microbiome-based therapeutics is rapidly evolving as the field advances. Currently, there is no specific regulatory framework dedicated to microbiome-based products in most jurisdictions. Regulatory agencies are adapting existing frameworks to accommodate these novel therapies, often categorizing them as biologics, drugs, or medical devices depending on their intended use and mechanism of action.
In the United States, the Food and Drug Administration (FDA) has taken steps to address the unique challenges posed by microbiome-based therapeutics. The agency has issued guidance documents on the development of live biotherapeutic products (LBPs), which include many microbiome-based therapies. These guidelines outline considerations for chemistry, manufacturing, and controls (CMC), as well as preclinical and clinical study designs.
The European Medicines Agency (EMA) has also recognized the potential of microbiome-based therapies and is working on developing appropriate regulatory pathways. The EMA has established a working group on microbiome-based medicinal products to address specific regulatory challenges and provide guidance to developers.
One of the key regulatory challenges in this field is the standardization of manufacturing processes and quality control measures for complex microbial communities. Regulatory agencies are grappling with how to ensure consistency and safety in products that contain multiple strains of living organisms.
Safety considerations are paramount in the regulatory landscape for microbiome-based therapeutics. Agencies are particularly concerned about the potential for unintended consequences, such as the transfer of antibiotic resistance genes or the overgrowth of opportunistic pathogens. As a result, rigorous safety assessments are required throughout the development process.
The regulatory approach to microbiome-based therapeutics targeting chronic diseases, such as those involving butyrate-producing bacteria, may vary depending on the specific indication and product characteristics. Regulators are likely to consider factors such as the mechanism of action, the level of manipulation of the microbial strains, and the intended route of administration when determining the appropriate regulatory pathway.
As the field continues to advance, it is expected that regulatory frameworks will evolve to better accommodate the unique aspects of microbiome-based therapeutics. This may include the development of new guidelines specifically tailored to these products, as well as the establishment of specialized review processes within regulatory agencies.
Ethical Considerations in Microbiome Manipulation
The manipulation of the human microbiome for therapeutic purposes raises significant ethical considerations that must be carefully addressed. As research into microbiota-derived butyrate and its potential to mitigate chronic diseases advances, it is crucial to navigate the ethical landscape surrounding microbiome interventions.
One primary ethical concern is the long-term effects of altering the microbiome. While short-term benefits may be observed, the complex interactions within the microbial ecosystem make it challenging to predict long-term consequences. Researchers and clinicians must weigh the potential risks against the expected benefits, ensuring that interventions do not inadvertently cause harm or disrupt the delicate balance of the microbiome.
Informed consent is another critical ethical issue. Patients must be fully aware of the nature of microbiome manipulations, including potential risks and uncertainties. This requires clear communication of current scientific understanding and acknowledgment of areas where knowledge is limited. Ensuring that patients can make autonomous decisions based on comprehensive information is paramount.
Privacy and data protection concerns also arise in microbiome research and interventions. The microbiome contains highly personal information, potentially revealing details about an individual's health, lifestyle, and even genetic predispositions. Strict protocols must be in place to protect this sensitive data and prevent its misuse or unauthorized access.
Equity in access to microbiome-based therapies is an important ethical consideration. As these interventions develop, there is a risk that they may only be available to affluent populations, exacerbating health disparities. Efforts should be made to ensure equitable access to microbiome-based treatments across diverse socioeconomic groups.
The concept of personal identity and autonomy may be challenged by microbiome manipulations. If the microbiome significantly influences behavior and mental health, altering it could raise questions about the boundaries of self and the nature of individual agency. This philosophical dimension adds complexity to the ethical debate surrounding microbiome interventions.
Regulatory frameworks must evolve to address the unique challenges posed by microbiome manipulations. Current regulations may not adequately cover the nuances of these interventions, necessitating the development of new guidelines that balance innovation with safety and ethical considerations.
In conclusion, as research into microbiota-derived butyrate and its therapeutic potential progresses, it is imperative that ethical considerations remain at the forefront of scientific and clinical endeavors. A multidisciplinary approach, involving ethicists, scientists, clinicians, and policymakers, is essential to navigate these complex issues and ensure that microbiome manipulations are conducted responsibly and ethically.