Butyrate's Protective Effects Against Obesity and Type 2 Diabetes: Mechanistic Insights and Clinical Implications
Butyrate Research Background and Objectives
Butyrate, a short-chain fatty acid produced by gut microbiota through fermentation of dietary fibers, has emerged as a key player in metabolic health. The research into butyrate's protective effects against obesity and type 2 diabetes has gained significant momentum in recent years, driven by the global rise in metabolic disorders and the growing recognition of the gut microbiome's role in human health.
The evolution of butyrate research can be traced back to the early 2000s when scientists began to uncover the complex interactions between gut bacteria and host metabolism. Initial studies focused on butyrate's role as an energy source for colonic epithelial cells and its potential anti-inflammatory properties. As research progressed, the scope expanded to include butyrate's systemic effects, particularly its impact on glucose homeostasis and lipid metabolism.
A pivotal moment in butyrate research came with the discovery of its role as a histone deacetylase (HDAC) inhibitor, which opened up new avenues for understanding its molecular mechanisms. This finding led to a surge in studies exploring butyrate's epigenetic effects and its potential to modulate gene expression related to metabolic processes.
The technological advancements in microbiome sequencing and metabolomics have further accelerated butyrate research, allowing for more comprehensive analyses of the gut ecosystem and its metabolic outputs. These tools have enabled researchers to establish stronger links between dietary interventions, butyrate production, and metabolic outcomes.
Current research objectives in the field of butyrate and metabolic health are multifaceted. Scientists aim to elucidate the precise mechanisms by which butyrate exerts its protective effects against obesity and type 2 diabetes. This includes investigating its impact on insulin sensitivity, energy expenditure, and adipose tissue function. Additionally, researchers are exploring the potential of butyrate as a therapeutic agent, either through direct supplementation or by modulating the gut microbiome to enhance endogenous butyrate production.
Another key objective is to understand the interplay between butyrate and other gut-derived metabolites in maintaining metabolic health. This holistic approach recognizes that butyrate does not act in isolation but as part of a complex network of microbial metabolites and host factors.
Translational research goals focus on developing effective strategies to harness butyrate's benefits in clinical settings. This includes optimizing dietary interventions, designing targeted probiotic and prebiotic therapies, and exploring novel drug delivery systems for butyrate or its analogs.
As the field progresses, researchers are also aiming to personalize butyrate-based interventions by considering individual variations in gut microbiome composition and metabolic profiles. This tailored approach holds promise for more effective prevention and management of obesity and type 2 diabetes.
Market Analysis for Butyrate-Based Therapies
The market for butyrate-based therapies is experiencing significant growth, driven by the increasing prevalence of obesity and type 2 diabetes worldwide. As research continues to unveil the protective effects of butyrate against these metabolic disorders, the demand for innovative treatments is on the rise.
The global market for obesity and diabetes therapeutics is substantial, with projections indicating continued expansion. Butyrate-based therapies represent a promising segment within this market, offering a novel approach to addressing these health concerns. The potential for these therapies extends beyond traditional pharmaceutical interventions, encompassing nutraceuticals and functional foods.
Consumer awareness of gut health and its connection to overall well-being is growing, creating a favorable environment for butyrate-based products. This trend is particularly evident in developed markets, where health-conscious consumers are seeking natural and preventive solutions to metabolic issues.
The market landscape for butyrate-based therapies is diverse, ranging from dietary supplements to prescription medications. Nutraceutical companies are capitalizing on the growing interest in gut health by introducing butyrate-enriched products. Simultaneously, pharmaceutical firms are investing in research and development to create targeted butyrate delivery systems for clinical applications.
Regulatory environments play a crucial role in shaping the market for butyrate-based therapies. While dietary supplements face less stringent regulations, pharmaceutical applications require extensive clinical trials and regulatory approvals. This dichotomy influences market entry strategies and product development timelines for companies operating in this space.
Geographic variations in market potential are notable. North America and Europe currently lead in terms of market size and adoption rates for butyrate-based therapies. However, emerging economies in Asia-Pacific and Latin America present significant growth opportunities as awareness of metabolic health issues increases and healthcare infrastructure improves.
The competitive landscape is evolving rapidly, with both established pharmaceutical companies and innovative startups vying for market share. Strategic partnerships between research institutions and industry players are accelerating the development of novel butyrate-based solutions, fostering a dynamic and competitive market environment.
Looking ahead, the market for butyrate-based therapies is poised for substantial growth. Factors such as the rising incidence of obesity and type 2 diabetes, increasing research validating butyrate's therapeutic potential, and growing consumer interest in gut health are expected to drive market expansion in the coming years.
Current Understanding of Butyrate Mechanisms
Butyrate, a short-chain fatty acid produced by gut microbiota through fermentation of dietary fibers, has emerged as a key player in metabolic health. Current understanding of butyrate mechanisms reveals its multifaceted role in protecting against obesity and type 2 diabetes. At the molecular level, butyrate acts as a histone deacetylase (HDAC) inhibitor, modulating gene expression and epigenetic regulation. This action influences various metabolic pathways and cellular processes crucial for maintaining metabolic homeostasis.
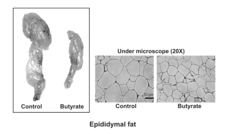
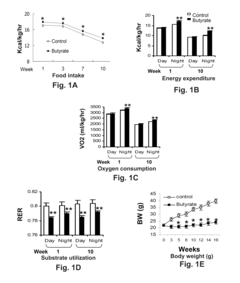
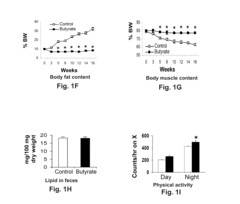
One of the primary mechanisms through which butyrate exerts its protective effects is by enhancing energy expenditure. Studies have shown that butyrate activates brown adipose tissue and promotes the browning of white adipose tissue, leading to increased thermogenesis and fat oxidation. This process is mediated by the upregulation of uncoupling protein 1 (UCP1) and peroxisome proliferator-activated receptor gamma coactivator 1-alpha (PGC-1α), key regulators of mitochondrial biogenesis and energy metabolism.
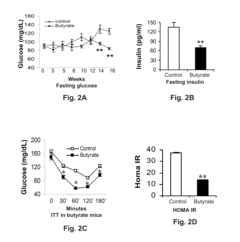
Butyrate also plays a significant role in improving insulin sensitivity and glucose homeostasis. It enhances glucose uptake in skeletal muscle and adipose tissue by promoting the translocation of glucose transporter 4 (GLUT4) to the cell membrane. Additionally, butyrate stimulates the production of glucagon-like peptide-1 (GLP-1) in intestinal L cells, which further improves insulin secretion and sensitivity.
The gut barrier function is another critical aspect influenced by butyrate. By strengthening tight junctions between intestinal epithelial cells, butyrate reduces intestinal permeability and prevents the translocation of lipopolysaccharides (LPS) into the bloodstream. This mechanism is crucial in mitigating low-grade inflammation associated with obesity and type 2 diabetes.
Furthermore, butyrate exhibits anti-inflammatory properties by suppressing the activation of nuclear factor kappa B (NF-κB) and reducing the production of pro-inflammatory cytokines. This anti-inflammatory action contributes to improved insulin sensitivity and overall metabolic health. Butyrate also modulates the gut microbiome composition, promoting the growth of beneficial bacteria and enhancing the production of other short-chain fatty acids, creating a positive feedback loop for metabolic health.
Recent research has uncovered butyrate's role in regulating appetite and food intake through its effects on the central nervous system. By activating the vagus nerve and modulating the production of satiety hormones, butyrate may help reduce caloric intake and promote weight loss. Additionally, butyrate has been shown to improve lipid metabolism by enhancing fatty acid oxidation and reducing lipogenesis in the liver, contributing to its protective effects against obesity-related metabolic disorders.
Existing Butyrate Delivery Methods and Treatments
01 Butyrate as a protective agent for gastrointestinal health
Butyrate has shown protective effects on the gastrointestinal tract, including maintaining gut barrier integrity, reducing inflammation, and promoting the growth of beneficial gut bacteria. It may be used in compositions for treating or preventing gastrointestinal disorders and improving overall gut health.- Butyrate as a protective agent for gastrointestinal health: Butyrate has been found to have protective effects on the gastrointestinal tract. It can help maintain the integrity of the intestinal barrier, reduce inflammation, and promote the growth of beneficial gut bacteria. These effects contribute to overall gut health and may help prevent various gastrointestinal disorders.
- Butyrate's role in metabolic regulation and obesity prevention: Research has shown that butyrate can play a significant role in metabolic regulation. It may help improve insulin sensitivity, regulate energy metabolism, and reduce the risk of obesity. These protective effects make butyrate a potential therapeutic agent for metabolic disorders.
- Neuroprotective effects of butyrate: Butyrate has demonstrated neuroprotective properties in various studies. It may help protect against neurodegenerative diseases by reducing inflammation in the brain, promoting the growth of new neurons, and improving cognitive function. These effects suggest potential applications in the treatment of neurological disorders.
- Butyrate's anti-inflammatory and immunomodulatory effects: Butyrate has been shown to possess potent anti-inflammatory and immunomodulatory properties. It can help regulate the immune response, reduce the production of pro-inflammatory cytokines, and promote the development of regulatory T cells. These effects may be beneficial in treating various inflammatory conditions and autoimmune disorders.
- Butyrate as a potential anticancer agent: Studies have indicated that butyrate may have anticancer properties. It has been shown to induce apoptosis in cancer cells, inhibit tumor growth, and enhance the effectiveness of certain chemotherapy drugs. These protective effects suggest that butyrate could be a promising adjunct therapy in cancer treatment.
02 Butyrate's role in metabolic regulation and obesity prevention
Butyrate has demonstrated protective effects against metabolic disorders and obesity. It may help regulate energy metabolism, improve insulin sensitivity, and reduce fat accumulation. Compositions containing butyrate or its derivatives can be used for weight management and metabolic health improvement.Expand Specific Solutions03 Neuroprotective effects of butyrate
Butyrate has shown neuroprotective properties, potentially benefiting cognitive function and protecting against neurodegenerative diseases. It may help reduce neuroinflammation, promote neuroplasticity, and support brain health. Compositions containing butyrate or related compounds can be developed for neurological applications.Expand Specific Solutions04 Butyrate in cancer prevention and treatment
Butyrate has demonstrated potential anticancer effects, including inhibition of tumor growth and promotion of cancer cell apoptosis. It may be used in compositions for cancer prevention or as an adjunct to cancer treatments, particularly for colorectal cancer.Expand Specific Solutions05 Butyrate derivatives and delivery systems for enhanced protective effects
Various butyrate derivatives and delivery systems have been developed to enhance its protective effects and improve its bioavailability. These include encapsulated forms, prodrugs, and novel formulations that can target specific areas of the body or provide controlled release of butyrate.Expand Specific Solutions
Key Players in Butyrate Research and Development
The research on butyrate's protective effects against obesity and Type 2 diabetes is in its early stages, with growing interest from both academic institutions and pharmaceutical companies. The market for potential butyrate-based therapies is expanding, driven by the increasing prevalence of metabolic disorders globally. While the technology is still emerging, several key players are actively involved in research and development. Companies like Nestlé, Sanofi, and Merck & Co. are exploring butyrate's potential, leveraging their expertise in metabolic health. Biotechnology firms such as BioKier and Seed Health are focusing on innovative approaches to butyrate delivery. Academic institutions, including Columbia University and the University of Michigan, are contributing significant research to elucidate butyrate's mechanisms of action. As the field progresses, collaborations between industry and academia are likely to accelerate the development of butyrate-based interventions for obesity and diabetes management.
BioKier, Inc.
Seed Health, Inc.
Breakthrough Studies on Butyrate's Metabolic Effects
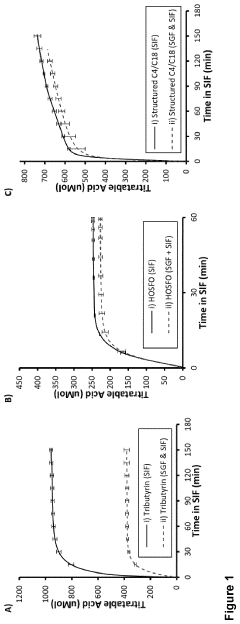
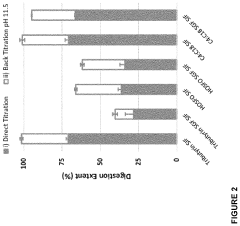
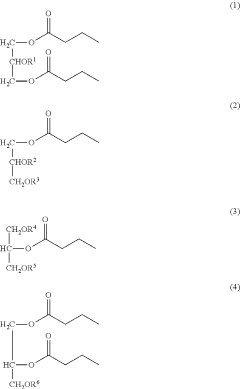
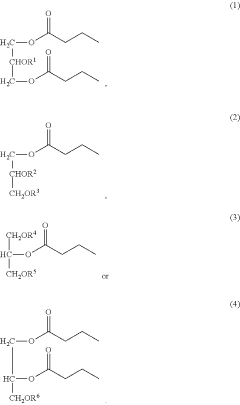
- Development of butyrate moiety containing triglycerides with improved organoleptic properties, synthesized through interesterification of tributyrin and high oleic sunflower oil, which provide a sustained release of butyric acid with reduced bitterness and odor, and are designed to minimize gastric lipolysis, allowing effective intestinal delivery.
- Chronic administration of dietary sodium butyrate, which inhibits histone deacetylases, enhances PGC-1α activity, leading to increased insulin sensitivity, reduced body weight, and improved metabolic parameters in non-ruminant mammals on a high-fat diet.
Regulatory Landscape for Butyrate as a Therapeutic Agent
The regulatory landscape for butyrate as a therapeutic agent is complex and evolving, reflecting the growing interest in its potential health benefits, particularly in addressing obesity and type 2 diabetes. Currently, butyrate is primarily regulated as a dietary supplement in many jurisdictions, falling under the purview of food safety authorities rather than drug regulatory agencies.
In the United States, the Food and Drug Administration (FDA) classifies butyrate supplements as "generally recognized as safe" (GRAS), allowing their sale without premarket approval. However, manufacturers are prohibited from making specific health claims without substantial scientific evidence and FDA approval. The European Food Safety Authority (EFSA) has similarly recognized butyrate as a safe food additive but maintains strict guidelines on health claims.
As research into butyrate's therapeutic potential advances, there is increasing pressure on regulatory bodies to develop more specific frameworks for its use as a medical intervention. This has led to ongoing discussions about the potential reclassification of certain butyrate formulations as pharmaceuticals, which would require more rigorous clinical trials and regulatory oversight.
Several countries have initiated research programs to evaluate butyrate's efficacy in treating metabolic disorders, potentially paving the way for its approval as a prescribed medication. These efforts are complicated by the diverse forms of butyrate available, including salts, esters, and prodrugs, each potentially requiring separate regulatory considerations.
The regulatory landscape is further influenced by the growing trend towards personalized medicine. As butyrate's effects may vary based on individual gut microbiome compositions, regulators are grappling with how to incorporate this variability into safety and efficacy assessments.
Internationally, there is a lack of harmonization in butyrate regulation, with some countries adopting more permissive stances while others maintain stricter controls. This disparity creates challenges for global research collaboration and market access, potentially slowing the development of butyrate-based therapies.
As the scientific understanding of butyrate's mechanisms in obesity and diabetes prevention deepens, regulatory agencies are likely to face increased pressure to establish clear guidelines for its therapeutic use. This may involve developing new regulatory categories that bridge the gap between dietary supplements and traditional pharmaceuticals, accommodating the unique properties of microbiome-modulating agents like butyrate.
Safety and Long-Term Efficacy Considerations
While butyrate has shown promising protective effects against obesity and type 2 diabetes in preclinical studies, safety and long-term efficacy considerations are crucial for its potential clinical applications. The safety profile of butyrate supplementation needs to be thoroughly evaluated through rigorous clinical trials.
Short-term studies have generally reported good tolerability of butyrate supplementation, with mild gastrointestinal side effects being the most common adverse events. However, long-term safety data is limited, and potential risks associated with prolonged use need to be carefully assessed. Particular attention should be given to the effects of chronic butyrate supplementation on gut microbiota composition and function, as well as potential interactions with other medications or dietary components.
The optimal dosage and formulation of butyrate for therapeutic use in humans remain to be determined. Different delivery methods, such as oral supplements, targeted colonic delivery systems, or prodrugs, may have varying safety profiles and efficacy outcomes. Standardization of butyrate preparations and quality control measures are essential to ensure consistent and reliable results in clinical settings.
Long-term efficacy considerations are equally important. While short-term studies have demonstrated beneficial effects on metabolic parameters, the sustainability of these improvements over extended periods needs to be established. Factors such as adherence to supplementation regimens, potential development of tolerance, and the impact of lifestyle modifications on butyrate's efficacy should be investigated.
Moreover, the heterogeneity of patient populations with obesity and type 2 diabetes necessitates the identification of specific subgroups that may benefit most from butyrate supplementation. Personalized approaches based on individual metabolic profiles, gut microbiota composition, or genetic factors may enhance the long-term efficacy of butyrate-based interventions.
Potential drug-drug interactions and the effects of butyrate on the pharmacokinetics of commonly prescribed medications for obesity and diabetes should also be thoroughly examined. This is particularly important given the complex treatment regimens often required for these conditions.
In conclusion, while butyrate shows promise as a therapeutic agent, comprehensive safety assessments and long-term efficacy studies are essential before its widespread clinical application. Future research should focus on addressing these considerations to fully realize the potential of butyrate in the management of obesity and type 2 diabetes.