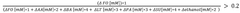Butyrate as a Prebiotic: Enhancing Digestive Health
Butyrate Research Background and Objectives
Butyrate, a short-chain fatty acid (SCFA), has emerged as a crucial component in maintaining digestive health and overall well-being. The research on butyrate as a prebiotic has gained significant momentum in recent years, driven by the growing understanding of the gut microbiome's role in human health. This field of study aims to explore the potential of butyrate in enhancing digestive health, particularly through its prebiotic properties.
The historical context of butyrate research dates back to the early 20th century when it was first identified as a metabolic product of gut bacteria. However, it wasn't until the late 1990s and early 2000s that researchers began to fully appreciate its significance in human health. The advent of advanced sequencing technologies and improved analytical methods has since accelerated our understanding of butyrate's role in the gut ecosystem.
The primary objective of current butyrate research is to elucidate its mechanisms of action as a prebiotic and its potential applications in improving digestive health. This includes investigating how butyrate influences the composition and function of the gut microbiome, its effects on intestinal barrier integrity, and its role in modulating the immune system within the gut.
Another key focus is on understanding the optimal methods for delivering butyrate to the colon, where it exerts its primary effects. This involves exploring various forms of butyrate supplementation, including resistant starch and other dietary fibers that can be fermented by gut bacteria to produce butyrate.
Researchers are also investigating the broader health implications of butyrate beyond digestive health. This includes its potential role in preventing and managing various chronic diseases, such as inflammatory bowel disease, colorectal cancer, and metabolic disorders. The anti-inflammatory and epigenetic properties of butyrate are of particular interest in this context.
The evolving trend in butyrate research is towards personalized approaches, recognizing that individual variations in gut microbiome composition may influence the effectiveness of butyrate-based interventions. This has led to increased interest in developing tailored prebiotic strategies that can optimize butyrate production for individual patients.
As the field progresses, there is a growing emphasis on translating laboratory findings into practical clinical applications. This includes the development of novel prebiotic formulations, functional foods, and targeted therapies that can harness the health-promoting properties of butyrate. The ultimate goal is to leverage this research to improve digestive health outcomes and overall quality of life for individuals suffering from gastrointestinal disorders and related conditions.
Market Analysis for Prebiotic Supplements
The prebiotic supplement market has experienced significant growth in recent years, driven by increasing consumer awareness of gut health and its impact on overall well-being. The global prebiotic market size was valued at approximately $6.2 billion in 2022 and is projected to expand at a compound annual growth rate (CAGR) of around 14% from 2023 to 2030. This growth is attributed to the rising prevalence of digestive disorders, growing interest in preventive healthcare, and the increasing adoption of functional foods and dietary supplements.
Within the prebiotic market, butyrate-based supplements are gaining traction due to their potential to enhance digestive health and improve gut microbiome composition. Butyrate, a short-chain fatty acid produced by gut bacteria during fermentation of dietary fibers, has shown promising results in supporting intestinal health and reducing inflammation. The market for butyrate-specific prebiotic supplements is still in its early stages but is expected to grow rapidly as more research emerges supporting its benefits.
Consumer demographics for prebiotic supplements, including butyrate-based products, span across various age groups. However, the primary target market consists of health-conscious adults aged 25-54, who are more likely to invest in preventive health measures. Additionally, there is a growing demand among the elderly population, as digestive issues become more prevalent with age.
Geographically, North America and Europe currently dominate the prebiotic supplement market, accounting for over 60% of the global market share. However, the Asia-Pacific region is expected to witness the fastest growth in the coming years, driven by increasing disposable incomes, changing dietary habits, and growing health awareness in countries like China and India.
The distribution channels for prebiotic supplements are diverse, with online retail platforms experiencing the highest growth rate. E-commerce has become increasingly popular for supplement purchases, offering convenience and a wide product selection. Traditional brick-and-mortar pharmacies and health food stores continue to play a significant role, particularly for consumers seeking personalized advice from healthcare professionals.
Key market trends influencing the prebiotic supplement industry include the rising demand for natural and organic products, increased focus on personalized nutrition, and the integration of prebiotics into functional foods and beverages. There is also a growing interest in synbiotic products that combine prebiotics with probiotics, offering a more comprehensive approach to gut health.
Current Challenges in Butyrate Supplementation
Despite the promising potential of butyrate as a prebiotic for enhancing digestive health, several challenges currently hinder its widespread adoption and effective supplementation. One of the primary obstacles is the rapid absorption of butyrate in the upper gastrointestinal tract, which limits its availability to the colon where it exerts its beneficial effects. This premature absorption reduces the efficacy of oral butyrate supplementation and necessitates the development of targeted delivery systems.
Another significant challenge lies in the organoleptic properties of butyrate. Its strong, unpleasant odor and taste make it difficult to incorporate into palatable dietary supplements or functional foods. This sensory issue not only affects consumer acceptance but also poses formulation challenges for manufacturers seeking to create appealing products.
The stability of butyrate under various environmental conditions presents another hurdle. Butyrate is susceptible to degradation when exposed to heat, light, and certain pH levels, which complicates its storage, processing, and incorporation into diverse food matrices. This instability can lead to reduced shelf life and diminished efficacy of butyrate-containing products.
Furthermore, there is a lack of standardization in butyrate supplementation protocols. The optimal dosage, frequency, and duration of supplementation remain unclear, as does the most effective form of butyrate (e.g., sodium butyrate, tributyrin, or butyrate-producing bacteria). This uncertainty hampers the development of evidence-based recommendations for both clinical and consumer use.
The bioavailability of butyrate is another area of concern. Even when butyrate reaches the colon, its absorption and utilization by colonic cells can be influenced by various factors, including the presence of other dietary components and the individual's gut microbiome composition. Understanding and optimizing these factors to enhance butyrate bioavailability remains a challenge.
Regulatory hurdles also pose significant obstacles to butyrate supplementation. The classification of butyrate as a food ingredient, dietary supplement, or potential therapeutic agent varies across different regulatory jurisdictions, affecting its market availability and claims that can be made about its benefits.
Lastly, while the short-term effects of butyrate supplementation have been studied, there is limited long-term safety and efficacy data. This gap in knowledge raises concerns about potential side effects or interactions with other medications, particularly in vulnerable populations or those with pre-existing gastrointestinal conditions.
Existing Butyrate Delivery Methods
01 Butyrate supplementation for digestive health
Butyrate supplements can be used to improve digestive health by supporting the growth of beneficial gut bacteria, reducing inflammation in the intestinal lining, and enhancing the gut barrier function. These supplements may be formulated as oral capsules, tablets, or powders for easy consumption.- Butyrate supplementation for digestive health: Butyrate supplements can be used to improve digestive health by supporting the growth of beneficial gut bacteria, reducing inflammation in the intestinal lining, and enhancing the integrity of the gut barrier. These supplements may be formulated as oral capsules, tablets, or powders for easy consumption.
- Butyrate-producing probiotic strains: Specific probiotic strains capable of producing butyrate in the gut can be used to promote digestive health. These strains may be incorporated into various food products, dietary supplements, or pharmaceutical formulations to increase butyrate levels in the intestines naturally.
- Butyrate-enriched food products: Food products enriched with butyrate or its precursors can be developed to support digestive health. These may include functional foods, beverages, or snacks formulated to deliver butyrate directly to the gut or to promote its production by gut bacteria.
- Butyrate in combination with other digestive health ingredients: Formulations combining butyrate with other ingredients known to support digestive health, such as prebiotics, other short-chain fatty acids, or specific amino acids, can be developed to provide synergistic benefits for gut health and function.
- Targeted delivery systems for butyrate: Development of targeted delivery systems to ensure that butyrate reaches the desired areas of the intestines for maximum effectiveness. This may involve encapsulation technologies, pH-sensitive coatings, or other methods to protect butyrate from degradation in the upper gastrointestinal tract.
02 Probiotic formulations producing butyrate
Probiotic formulations containing specific bacterial strains capable of producing butyrate in the gut can be developed to promote digestive health. These probiotics may be combined with prebiotics to enhance their effectiveness in supporting the growth of beneficial gut bacteria and improving overall gut health.Expand Specific Solutions03 Butyrate-producing dietary fibers
Dietary fibers that can be fermented by gut bacteria to produce butyrate can be incorporated into food products or supplements. These fibers may include resistant starches, inulin, and other complex carbohydrates that promote the growth of butyrate-producing bacteria in the colon.Expand Specific Solutions04 Butyrate-based compositions for treating gastrointestinal disorders
Pharmaceutical compositions containing butyrate or its derivatives can be developed for treating various gastrointestinal disorders, such as inflammatory bowel disease, irritable bowel syndrome, and colitis. These compositions may be formulated for targeted delivery to specific regions of the digestive tract.Expand Specific Solutions05 Synbiotic formulations with butyrate-producing components
Synbiotic formulations combining probiotics, prebiotics, and butyrate-producing components can be developed to enhance digestive health. These formulations may include specific bacterial strains, dietary fibers, and butyrate precursors to promote a healthy gut microbiome and improve overall digestive function.Expand Specific Solutions
Key Players in Prebiotic and Butyrate Research
The research on butyrate as a prebiotic for enhancing digestive health is in a growth phase, with increasing market size and technological advancements. The global prebiotic market is expanding rapidly, driven by growing consumer awareness of gut health. Technologically, the field is progressing from early-stage research to more advanced applications. Companies like Nestlé, Morinaga Milk Industry, and DSM IP Assets are leading in product development, while academic institutions such as Zhejiang University and Ghent University are contributing to fundamental research. The involvement of pharmaceutical companies like Baxter International indicates the potential for medical applications, suggesting a maturing technology landscape with diverse industry participation.
Société des Produits Nestlé SA
Morinaga Milk Industry Co., Ltd.
Innovations in Butyrate-Producing Prebiotics
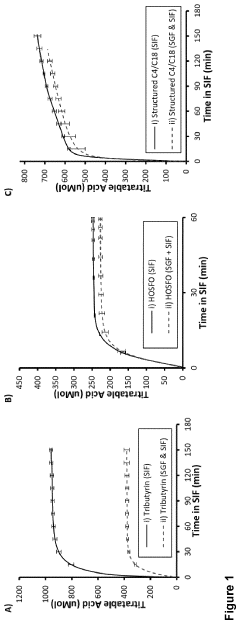
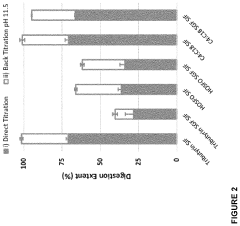
- Development of defined consortia of anaerobic bacterial strains, specifically including Agathobacter rectalis, Anaerostipes caccae, and Anaerobutyricum hallii, which are selected to enhance butyrate production through continuous co-culture fermentations, achieving metabolic equilibrium and stability, and are designed to treat conditions related to intestinal dysbiosis.
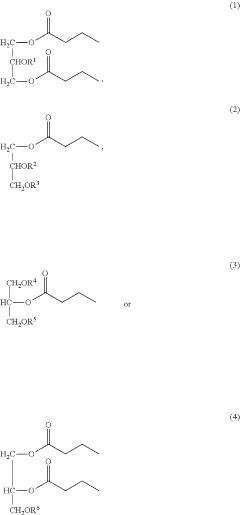
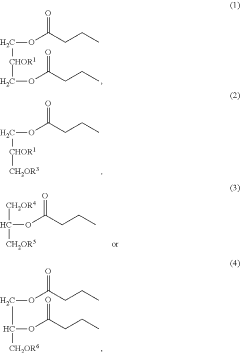
- Development of butyrate moiety containing triglycerides with improved organoleptic properties, such as 1,3-dibutyryl-2-palmitoylglycerol, which are synthesized through interesterification of tributyrin with high oleic sunflower oil, providing a dairy-free, cholesterol-free, and vegan alternative with reduced bitterness and odor, allowing effective delivery of butyric acid to the intestinal compartment.
Regulatory Framework for Prebiotic Claims
The regulatory framework for prebiotic claims is a complex and evolving landscape that plays a crucial role in the research and commercialization of butyrate as a prebiotic for enhancing digestive health. In the United States, the Food and Drug Administration (FDA) oversees the regulation of prebiotics and their associated health claims. The FDA has not established a legal definition for prebiotics, which creates challenges for manufacturers seeking to make specific health claims.
The European Food Safety Authority (EFSA) has been more proactive in defining prebiotics and establishing guidelines for health claims. EFSA defines prebiotics as "non-digestible food ingredients that beneficially affect the host by selectively stimulating the growth and/or activity of one or a limited number of bacteria in the colon, thus improving host health." This definition provides a clearer framework for researchers and manufacturers working with butyrate and other potential prebiotics.
In Japan, the Foods for Specified Health Uses (FOSHU) system allows for more specific health claims related to prebiotics, provided there is sufficient scientific evidence to support such claims. This regulatory environment has fostered significant research and development in the prebiotic field, including studies on butyrate's potential benefits for digestive health.
The International Scientific Association for Probiotics and Prebiotics (ISAPP) has proposed a consensus definition for prebiotics, which has gained traction in the scientific community. This definition emphasizes the selective utilization of prebiotics by host microorganisms, conferring a health benefit. While not legally binding, this definition serves as a valuable reference point for researchers and regulators alike.
For butyrate specifically, regulatory challenges arise due to its dual nature as both a naturally occurring compound in the gut and a potential prebiotic supplement. Researchers must navigate these complexities when designing studies and interpreting results. The regulatory framework often requires extensive clinical trials to substantiate health claims, which can be time-consuming and costly.
As research on butyrate as a prebiotic continues to advance, it is likely that regulatory bodies will refine their approaches to prebiotic claims. This may include more specific guidelines for butyrate-related health claims, potentially opening new avenues for product development and marketing. However, researchers and manufacturers must remain vigilant in adhering to current regulations while also anticipating future changes in the regulatory landscape.
Safety and Efficacy Studies on Butyrate Supplementation
Extensive research has been conducted to evaluate the safety and efficacy of butyrate supplementation in enhancing digestive health. Multiple clinical trials have demonstrated that butyrate supplementation is generally well-tolerated, with minimal adverse effects reported. The most common side effects include mild gastrointestinal discomfort, which typically subsides with continued use.
Safety studies have shown that butyrate supplementation does not significantly alter the overall composition of the gut microbiome, suggesting that it does not disrupt the natural balance of beneficial bacteria. Furthermore, long-term studies have not identified any significant risks associated with prolonged butyrate supplementation, even at higher doses.
Efficacy studies have yielded promising results regarding the potential benefits of butyrate supplementation for digestive health. Several randomized controlled trials have shown that butyrate supplementation can significantly reduce inflammation in the gut, particularly in individuals with inflammatory bowel diseases such as ulcerative colitis and Crohn's disease.
Research has also demonstrated that butyrate supplementation can enhance the integrity of the intestinal barrier, reducing gut permeability and potentially mitigating the risk of leaky gut syndrome. This effect is particularly notable in individuals with compromised gut health or those experiencing chronic digestive issues.
Moreover, studies have indicated that butyrate supplementation may improve bowel regularity and alleviate symptoms of constipation. This effect is attributed to butyrate's ability to stimulate colonic motility and enhance water absorption in the colon.
In terms of metabolic health, butyrate supplementation has shown potential in improving insulin sensitivity and glucose metabolism. Some studies have reported reductions in fasting blood glucose levels and improvements in lipid profiles following butyrate supplementation, suggesting potential benefits for individuals with metabolic disorders.
While the majority of studies have focused on oral supplementation, research is ongoing to explore alternative delivery methods, such as targeted release formulations and rectal administration, to optimize the therapeutic effects of butyrate.
It is important to note that while the current body of evidence is promising, larger-scale, long-term studies are still needed to fully elucidate the safety profile and efficacy of butyrate supplementation across diverse populations and health conditions. Additionally, further research is required to determine optimal dosing regimens and potential interactions with other dietary components or medications.