The Therapeutic Potential of Butyrate in Insulin Resistance
Butyrate and Insulin Resistance: Background and Objectives
Butyrate, a short-chain fatty acid produced by gut microbiota through fermentation of dietary fibers, has emerged as a promising therapeutic agent in the context of insulin resistance and metabolic disorders. The exploration of butyrate's potential in addressing insulin resistance has gained significant momentum in recent years, driven by the growing global prevalence of metabolic syndrome and type 2 diabetes.
The historical context of butyrate research dates back to the early 20th century when it was first identified as a metabolic byproduct of intestinal bacteria. However, its specific role in metabolic health remained largely unexplored until the late 1990s and early 2000s. This period saw a surge in research linking gut microbiota composition to various aspects of human health, including metabolic regulation.
As the understanding of the gut-brain axis and the intricate relationship between gut health and systemic metabolism evolved, butyrate's potential as a therapeutic agent began to crystallize. The recognition of its diverse physiological effects, including anti-inflammatory properties, regulation of gene expression, and modulation of energy metabolism, has positioned butyrate at the forefront of metabolic research.
The primary objective of investigating butyrate's therapeutic potential in insulin resistance is to develop novel, targeted interventions for metabolic disorders. This goal is particularly crucial given the limitations of current treatment options and the increasing burden of metabolic diseases worldwide. Researchers aim to elucidate the mechanisms by which butyrate influences insulin sensitivity, glucose homeostasis, and overall metabolic health.
Key areas of focus include understanding butyrate's effects on pancreatic β-cell function, hepatic glucose production, and skeletal muscle insulin sensitivity. Additionally, there is significant interest in exploring how butyrate modulates the gut microbiome composition and function, potentially leading to indirect metabolic benefits.
The technological evolution in this field has been marked by advancements in analytical techniques, including high-throughput sequencing, metabolomics, and advanced imaging modalities. These tools have enabled researchers to delve deeper into the molecular mechanisms underlying butyrate's effects and to track its distribution and activity in various tissues.
As research progresses, the overarching goal is to translate these findings into practical therapeutic strategies. This may involve developing butyrate-based pharmaceuticals, designing targeted dietary interventions to enhance endogenous butyrate production, or engineering probiotic strains capable of producing therapeutic levels of butyrate in the gut.
Market Analysis for Butyrate-Based Therapeutics
The market for butyrate-based therapeutics targeting insulin resistance is experiencing significant growth, driven by the increasing prevalence of metabolic disorders and the growing understanding of butyrate's potential in metabolic health. The global market for insulin resistance therapeutics is projected to reach substantial figures in the coming years, with butyrate-based treatments poised to capture a notable share.
Demand for novel insulin resistance treatments is rising due to the escalating rates of obesity, type 2 diabetes, and metabolic syndrome worldwide. Butyrate, as a short-chain fatty acid with demonstrated benefits in improving insulin sensitivity and glucose homeostasis, is attracting considerable attention from both researchers and pharmaceutical companies.
The market landscape for butyrate-based therapeutics is characterized by a mix of established pharmaceutical companies and innovative biotech firms. Several key players are investing in research and development to create butyrate-based formulations that can effectively target insulin resistance. These efforts are focused on overcoming challenges such as butyrate's rapid metabolism and developing delivery systems that can maintain therapeutic concentrations in the body.
Current market offerings primarily include dietary supplements containing butyrate or its precursors. However, the pharmaceutical-grade butyrate therapeutics market is still in its nascent stages, with several products in various phases of clinical trials. This presents significant opportunities for companies to establish a strong market position with FDA-approved butyrate-based drugs for insulin resistance.
The potential market for butyrate-based therapeutics extends beyond insulin resistance to encompass related metabolic disorders. This broader application potential enhances the market attractiveness for investors and pharmaceutical companies. Additionally, the growing trend towards personalized medicine and targeted therapies is likely to drive demand for butyrate-based treatments tailored to specific patient populations.
Geographically, North America and Europe are expected to dominate the market initially, given their advanced healthcare infrastructure and higher awareness of metabolic health. However, rapidly developing economies in Asia-Pacific, particularly China and India, represent significant growth opportunities due to their large patient populations and increasing healthcare expenditure.
Challenges in the market include regulatory hurdles, the need for extensive clinical trials to prove efficacy and safety, and potential competition from other emerging therapies for insulin resistance. Despite these challenges, the unique mechanism of action of butyrate and its potential for minimal side effects compared to some existing treatments position it favorably in the market.
Current Challenges in Butyrate Research and Application
Despite the promising therapeutic potential of butyrate in insulin resistance, several challenges persist in its research and application. One of the primary obstacles is the limited bioavailability of butyrate due to its rapid absorption and metabolism in the upper gastrointestinal tract. This necessitates the development of novel delivery systems to ensure sufficient concentrations reach the target tissues, particularly in the context of systemic insulin resistance.
Another significant challenge lies in the complex interplay between butyrate, the gut microbiome, and host metabolism. While butyrate is known to be produced by certain gut bacteria, the precise mechanisms by which it influences insulin sensitivity and glucose homeostasis are not fully elucidated. This complexity makes it difficult to design targeted interventions and predict outcomes in diverse patient populations.
The dosage and duration of butyrate supplementation for optimal therapeutic effects remain unclear. Current studies show variability in dosing regimens, making it challenging to establish standardized protocols for clinical applications. Moreover, the long-term effects of butyrate supplementation on gut microbiota composition and overall metabolic health are not well understood, raising concerns about potential unintended consequences of prolonged use.
Translating preclinical findings to human studies presents another hurdle. While animal models have shown promising results, human clinical trials are limited in number and scale. The heterogeneity of insulin resistance phenotypes in humans further complicates the generalizability of research findings and the development of targeted therapies.
Additionally, there are technical challenges in accurately measuring butyrate levels in various biological samples, particularly in tissues beyond the gut. This limitation hampers the ability to correlate butyrate concentrations with metabolic outcomes and establish clear dose-response relationships.
The potential for adverse effects and contraindications of butyrate supplementation in certain patient populations, such as those with compromised gut barrier function or specific metabolic disorders, requires careful investigation. Safety profiles and potential drug interactions need to be thoroughly evaluated before widespread clinical application can be recommended.
Lastly, the regulatory landscape for butyrate as a therapeutic agent is complex, with varying classifications across different jurisdictions. This regulatory uncertainty poses challenges for pharmaceutical development and clinical translation, potentially slowing the progress from bench to bedside in the field of butyrate research for insulin resistance.
Existing Butyrate Delivery Methods and Formulations
01 Butyrate as a treatment for insulin resistance
Butyrate, a short-chain fatty acid, has been found to improve insulin sensitivity and reduce insulin resistance. It may work by modulating glucose metabolism, enhancing mitochondrial function, and reducing inflammation in insulin-sensitive tissues. Butyrate supplementation or increasing dietary sources of butyrate could potentially be used as a therapeutic approach for managing insulin resistance and related metabolic disorders.- Butyrate as a treatment for insulin resistance: Butyrate, a short-chain fatty acid, has been found to improve insulin sensitivity and reduce insulin resistance. It can be administered orally or through other routes to help manage metabolic disorders associated with insulin resistance, such as type 2 diabetes and obesity.
- Butyrate-producing bacteria for metabolic health: Probiotic bacteria capable of producing butyrate in the gut have shown potential in improving insulin sensitivity and overall metabolic health. These bacteria can be administered as supplements or through dietary interventions to increase butyrate production in the intestines.
- Butyrate in combination with other compounds: Combining butyrate with other bioactive compounds or pharmaceuticals has demonstrated synergistic effects in addressing insulin resistance. These combinations may include antidiabetic drugs, other short-chain fatty acids, or natural extracts that complement butyrate's action on metabolic pathways.
- Butyrate derivatives for enhanced efficacy: Modified forms of butyrate, such as butyrate esters or prodrugs, have been developed to improve bioavailability and efficacy in treating insulin resistance. These derivatives aim to overcome limitations of butyrate absorption and metabolism while maintaining its beneficial effects on insulin sensitivity.
- Butyrate's mechanism of action in insulin signaling: Research has elucidated various mechanisms by which butyrate improves insulin sensitivity, including modulation of gene expression, enhancement of mitochondrial function, and reduction of inflammation. Understanding these pathways helps in developing targeted therapies for insulin resistance using butyrate-based approaches.
02 Gut microbiome and butyrate production
The gut microbiome plays a crucial role in producing butyrate through fermentation of dietary fibers. Modulating the gut microbiota composition to increase butyrate-producing bacteria may be an effective strategy to improve insulin sensitivity. Prebiotics, probiotics, and synbiotics that promote butyrate production in the gut could be potential interventions for addressing insulin resistance.Expand Specific Solutions03 Butyrate in combination with other compounds
Combining butyrate with other bioactive compounds or drugs may enhance its effects on insulin sensitivity. Synergistic effects have been observed when butyrate is used in conjunction with certain antidiabetic medications, antioxidants, or other short-chain fatty acids. These combinations could potentially lead to more effective treatments for insulin resistance and related metabolic disorders.Expand Specific Solutions04 Butyrate delivery methods and formulations
Various delivery methods and formulations have been developed to improve the bioavailability and efficacy of butyrate for treating insulin resistance. These include controlled-release formulations, nanoparticle-based delivery systems, and prodrugs of butyrate. Optimizing the delivery of butyrate to target tissues may enhance its therapeutic potential in managing insulin resistance.Expand Specific Solutions05 Mechanisms of butyrate action on insulin signaling
Research has focused on elucidating the molecular mechanisms by which butyrate improves insulin sensitivity. Studies have shown that butyrate can modulate insulin signaling pathways, activate AMPK, reduce endoplasmic reticulum stress, and regulate gene expression through histone deacetylase inhibition. Understanding these mechanisms can lead to the development of more targeted therapies for insulin resistance.Expand Specific Solutions
Key Players in Butyrate and Metabolic Health Research
The therapeutic potential of butyrate in insulin resistance is an emerging field with growing interest from both academic institutions and pharmaceutical companies. The market is in its early stages of development, with a mix of established players like Novartis AG and Nestlé SA, alongside innovative startups such as BioKier, Inc. and Synlogic Operating Co., Inc. The technology is still maturing, with research institutions like The University of Chicago and Nanjing University contributing significantly to the knowledge base. While the market size is currently modest, it shows promise for expansion as more clinical evidence emerges. The involvement of diverse players, from traditional pharmaceutical companies to specialized biotech firms, indicates a competitive landscape with potential for rapid advancements in the near future.
Novartis AG
Morinaga Milk Industry Co., Ltd.
Breakthrough Studies on Butyrate and Insulin Sensitivity
- Delivering butyrate directly to the colon using a colon-targeted delivery system that bypasses the upper digestive system, allowing for a time-released formulation over 0 to 5 hours to stimulate gut hormone secretion from L-cells, thereby increasing GLP-1, GLP-2, and oxyntomodulin production.
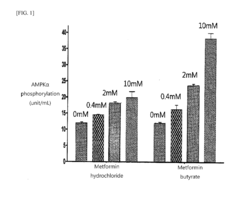
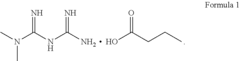
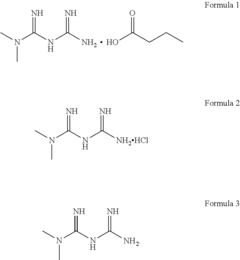
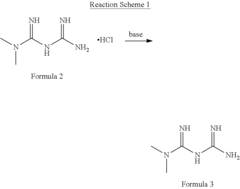
- The development of metformin butyrate, which is synthesized through a method involving the reaction of metformin hydrochloride with a base to form a free base, followed by reaction with butyric acid, offering improved solubility, stability, and anti-adhesive properties, and is suitable for various pharmaceutical formulations.
Regulatory Landscape for Microbiome-Based Therapeutics
The regulatory landscape for microbiome-based therapeutics is rapidly evolving as the potential of butyrate in treating insulin resistance gains recognition. Regulatory agencies worldwide are grappling with the unique challenges posed by these novel therapeutic approaches. The U.S. Food and Drug Administration (FDA) has taken proactive steps to address the regulatory framework for microbiome-based products, including those targeting metabolic disorders like insulin resistance.
The FDA has established a dedicated Microbiome Therapeutics Innovation Group to facilitate the development and approval of microbiome-related treatments. This group works closely with researchers and pharmaceutical companies to provide guidance on clinical trial design, safety assessments, and efficacy endpoints specific to microbiome-based therapeutics. For butyrate-focused treatments, the FDA emphasizes the importance of demonstrating a clear mechanism of action and consistent production of therapeutic levels of butyrate in the gut.
In Europe, the European Medicines Agency (EMA) has also recognized the potential of microbiome-based therapies. The EMA has published guidelines on the quality, non-clinical, and clinical aspects of live biotherapeutic products, which include microbiome-based treatments. These guidelines provide a framework for developers working on butyrate-producing therapies for insulin resistance.
Regulatory bodies are particularly focused on ensuring the safety and consistency of microbiome-based products. This includes stringent requirements for strain identification, characterization, and stability testing. For butyrate-producing therapies, regulators are keen to see robust data on the persistence of the introduced microorganisms and their sustained ability to produce therapeutic levels of butyrate in diverse patient populations.
The path to market for microbiome-based therapeutics targeting insulin resistance through butyrate production is becoming clearer, but challenges remain. Regulators are working to strike a balance between fostering innovation and ensuring patient safety. As more clinical data becomes available on the efficacy of butyrate in treating insulin resistance, it is likely that regulatory pathways will continue to be refined and streamlined.
Collaboration between regulatory agencies, researchers, and industry stakeholders is crucial in shaping the regulatory landscape. International harmonization efforts are underway to align regulatory approaches across different regions, facilitating global development and access to these innovative therapies. As the field advances, it is anticipated that more specific guidance will emerge for microbiome-based treatments targeting metabolic disorders, potentially accelerating the development and approval process for butyrate-focused therapies in insulin resistance.
Safety and Efficacy Considerations for Butyrate Supplementation
The safety and efficacy of butyrate supplementation for insulin resistance treatment require careful consideration. Butyrate, a short-chain fatty acid produced by gut microbiota, has shown promising results in preclinical studies for improving insulin sensitivity. However, its therapeutic use in humans necessitates a thorough evaluation of potential risks and benefits.
Safety considerations for butyrate supplementation primarily focus on gastrointestinal side effects. Common adverse reactions include abdominal discomfort, bloating, and diarrhea, particularly at higher doses. These effects are generally mild and transient, but may impact patient compliance and quality of life. Long-term safety data in humans is limited, warranting further investigation into potential chronic effects.
Dosage optimization is crucial for balancing efficacy and safety. Preclinical studies have demonstrated dose-dependent effects of butyrate on insulin sensitivity, but optimal dosing regimens in humans remain to be established. Factors such as individual variability in gut microbiota composition and metabolic status may influence the response to butyrate supplementation, necessitating personalized approaches.
The route of administration is another important consideration. Oral supplementation is the most common and convenient method, but faces challenges in terms of bioavailability and targeted delivery. Enteric-coated formulations and prodrug approaches have been explored to enhance butyrate delivery to the colon, where it exerts its primary effects.
Efficacy assessment of butyrate supplementation in insulin resistance requires robust clinical trials. While animal studies have shown promising results, human data is still limited. Surrogate markers of insulin sensitivity, such as HOMA-IR and glucose tolerance tests, can provide initial insights into efficacy. However, long-term clinical outcomes, including glycemic control and cardiovascular risk reduction, should be evaluated to establish the true therapeutic potential of butyrate.
Potential interactions with other medications and dietary factors must also be considered. Butyrate may influence the absorption and metabolism of certain drugs, and its effects may be modulated by dietary fiber intake and overall gut health. Comprehensive drug-drug and drug-nutrient interaction studies are necessary to ensure safe and effective use in diverse patient populations.
In conclusion, while butyrate shows promise as a therapeutic agent for insulin resistance, careful evaluation of its safety profile and efficacy is essential. Continued research, including well-designed clinical trials and long-term follow-up studies, will be crucial in determining the optimal use of butyrate supplementation in the management of insulin resistance and related metabolic disorders.