The Science of Butyrate-Induced Immune Regulation
Butyrate Immunology Background and Objectives
Butyrate, a short-chain fatty acid produced by gut microbiota through fermentation of dietary fiber, has emerged as a crucial player in immune regulation. The study of butyrate-induced immune regulation has gained significant attention in recent years due to its potential implications for various health conditions, including inflammatory bowel diseases, autoimmune disorders, and even cancer.
The field of butyrate immunology has its roots in the early observations of the anti-inflammatory properties of dietary fiber. As research progressed, scientists began to unravel the complex interactions between gut microbiota, their metabolites, and the host immune system. Butyrate, along with other short-chain fatty acids, was identified as a key mediator in this intricate relationship.
The evolution of butyrate immunology research has been marked by several key milestones. Initial studies focused on the direct effects of butyrate on colonic epithelial cells, demonstrating its role in maintaining gut barrier integrity. Subsequent investigations revealed butyrate's ability to modulate immune cell function, particularly its impact on regulatory T cells and the suppression of pro-inflammatory cytokines.
Recent technological advancements, such as high-throughput sequencing and metabolomics, have further accelerated our understanding of butyrate's immunomodulatory effects. These tools have enabled researchers to elucidate the complex interplay between gut microbiota composition, butyrate production, and immune responses at a molecular level.
The primary objective of current research in butyrate-induced immune regulation is to develop a comprehensive understanding of the mechanisms by which butyrate influences various components of the immune system. This includes investigating its effects on innate and adaptive immune cells, cytokine production, and signaling pathways involved in immune regulation.
Another crucial goal is to explore the therapeutic potential of butyrate in managing inflammatory and autoimmune conditions. Researchers aim to develop targeted interventions that can harness the immunomodulatory properties of butyrate, either through dietary modifications, probiotic supplementation, or direct administration of butyrate or its derivatives.
Furthermore, there is a growing interest in understanding the role of butyrate in maintaining immune homeostasis and its potential in preventing immune-related disorders. This includes investigating how factors such as diet, lifestyle, and environmental exposures influence butyrate production and, consequently, immune function.
As the field progresses, researchers are also focusing on elucidating the dose-dependent effects of butyrate and identifying optimal strategies for its delivery to target tissues. This involves exploring novel formulations and delivery systems that can enhance butyrate's bioavailability and efficacy in modulating immune responses.
Market Analysis for Butyrate-Based Therapeutics
The market for butyrate-based therapeutics is experiencing significant growth, driven by increasing awareness of the compound's potential in immune regulation and overall health. The global market for microbiome-based therapeutics, which includes butyrate-related products, is projected to reach substantial value in the coming years. This growth is fueled by rising incidences of autoimmune disorders, inflammatory bowel diseases, and other immune-related conditions.
Butyrate's role in immune regulation has garnered attention from both pharmaceutical companies and nutraceutical manufacturers. The market is segmented into various product types, including oral supplements, topical formulations, and prescription medications. Oral supplements currently dominate the market share, appealing to health-conscious consumers seeking natural ways to support their immune system and gut health.
The pharmaceutical sector is investing heavily in research and development of butyrate-based drugs, particularly for treating inflammatory bowel diseases like Crohn's disease and ulcerative colitis. Several clinical trials are underway, exploring butyrate's potential in managing these conditions, which could significantly expand the market in the near future.
Geographically, North America leads the market due to high healthcare expenditure and a strong presence of key players. Europe follows closely, with increasing research activities and growing consumer awareness. The Asia-Pacific region is expected to witness the fastest growth, driven by rising disposable incomes and a growing focus on preventive healthcare.
Key market players include both established pharmaceutical companies and emerging biotech firms. These companies are focusing on developing innovative delivery methods to enhance butyrate's bioavailability and efficacy. Partnerships between academic institutions and industry players are also accelerating research and product development in this field.
Consumer trends indicate a growing preference for natural and preventive health solutions, which bodes well for butyrate-based products. The increasing popularity of personalized nutrition and microbiome testing is also expected to drive demand for targeted butyrate supplements.
However, the market faces challenges, including regulatory hurdles and the need for more extensive clinical evidence to support health claims. Additionally, competition from other emerging immunomodulatory compounds and therapies could impact market growth.
Despite these challenges, the future outlook for butyrate-based therapeutics remains promising. As research continues to uncover the complex interactions between butyrate and the immune system, new applications and market opportunities are likely to emerge, potentially expanding into areas such as mental health, metabolic disorders, and even cancer therapy.
Current Challenges in Butyrate-Induced Immune Regulation
Despite significant advancements in understanding butyrate-induced immune regulation, several challenges persist in this field. One of the primary obstacles is the complexity of the gut microbiome and its interactions with the immune system. The intricate network of microorganisms in the gut and their metabolites, including butyrate, creates a dynamic environment that is difficult to fully characterize and control in experimental settings.
Another challenge lies in the variability of butyrate production and absorption across individuals. Factors such as diet, genetics, and overall health status can significantly influence the amount of butyrate produced by gut bacteria and its subsequent impact on immune regulation. This variability makes it challenging to develop standardized approaches for harnessing butyrate's immunomodulatory effects.
The precise mechanisms by which butyrate influences different immune cell populations remain incompletely understood. While studies have shown that butyrate can affect T cell differentiation and function, the exact signaling pathways and molecular targets involved in these processes are not fully elucidated. This gap in knowledge hinders the development of targeted therapies that could leverage butyrate's immune-regulatory properties.
Furthermore, the optimal dosage and delivery methods for butyrate as a therapeutic agent are yet to be determined. Butyrate's short half-life and rapid metabolism in the gut pose challenges for maintaining effective concentrations at target sites. Developing stable formulations that can deliver butyrate to specific regions of the gastrointestinal tract or systemically remains a significant hurdle.
The translation of in vitro and animal model findings to human clinical applications presents another major challenge. While numerous studies have demonstrated the immune-regulatory effects of butyrate in cell cultures and animal models, replicating these results in human clinical trials has proven difficult. Differences in gut microbiome composition, immune system dynamics, and metabolic processes between species contribute to this translational gap.
Additionally, the potential long-term effects of butyrate supplementation or manipulation of butyrate-producing bacteria on overall immune function and health are not well understood. Concerns about unintended consequences, such as immune suppression or dysregulation of other metabolic processes, need to be thoroughly addressed before widespread clinical application.
Lastly, the integration of butyrate-induced immune regulation with other immunomodulatory approaches and existing treatment regimens poses a significant challenge. Determining how butyrate interacts with other therapeutic agents and how it can be effectively incorporated into comprehensive treatment strategies for immune-related disorders requires extensive research and clinical validation.
Existing Approaches to Butyrate-Induced Immune Modulation
01 Butyrate as an immune modulator
Butyrate plays a crucial role in regulating immune responses. It has been shown to modulate various aspects of the immune system, including T cell differentiation, cytokine production, and inflammation. Butyrate can enhance the production of regulatory T cells, which are important for maintaining immune tolerance and preventing autoimmune diseases.- Butyrate as an immune modulator: Butyrate plays a crucial role in regulating immune responses. It has been shown to modulate various aspects of the immune system, including T cell differentiation, cytokine production, and inflammation. Butyrate can enhance the production of regulatory T cells, which are important for maintaining immune tolerance and preventing autoimmune diseases.
- Butyrate in gut health and immune function: Butyrate is a short-chain fatty acid produced by gut bacteria during fermentation of dietary fiber. It plays a significant role in maintaining gut health and regulating immune function. Butyrate strengthens the intestinal barrier, reduces inflammation, and promotes the growth of beneficial gut bacteria, all of which contribute to a balanced immune system.
- Butyrate-based therapeutic approaches: Researchers are exploring various therapeutic approaches using butyrate or butyrate-producing bacteria to modulate immune responses. These include the development of butyrate-releasing compounds, probiotic formulations containing butyrate-producing bacteria, and dietary interventions to increase butyrate production in the gut. These approaches show promise in treating inflammatory bowel diseases, allergies, and other immune-related disorders.
- Butyrate's effects on specific immune cell populations: Butyrate has been found to influence various immune cell populations, including dendritic cells, macrophages, and natural killer cells. It can modulate their activation, differentiation, and function. For example, butyrate has been shown to enhance the antigen-presenting capacity of dendritic cells and promote the polarization of macrophages towards an anti-inflammatory phenotype.
- Butyrate in epigenetic regulation of immune responses: Butyrate acts as a histone deacetylase inhibitor, influencing gene expression through epigenetic mechanisms. This property allows butyrate to regulate the expression of genes involved in immune responses, including those related to inflammation, cell cycle regulation, and apoptosis. The epigenetic effects of butyrate contribute to its immunomodulatory properties and potential therapeutic applications.
02 Butyrate in gut health and immune function
Butyrate is a short-chain fatty acid produced by gut bacteria during fermentation of dietary fiber. It plays a vital role in maintaining gut health and regulating immune function. Butyrate strengthens the intestinal barrier, reduces inflammation, and promotes the growth of beneficial bacteria, all of which contribute to a balanced immune system.Expand Specific Solutions03 Butyrate-based therapeutic approaches
Researchers are exploring various therapeutic approaches using butyrate to modulate immune responses. These include developing butyrate-releasing compounds, formulating butyrate-enriched dietary supplements, and designing targeted delivery systems to enhance butyrate's effects on specific immune cell populations. Such approaches show promise in treating inflammatory and autoimmune disorders.Expand Specific Solutions04 Butyrate's effects on specific immune cell types
Butyrate has been found to influence various immune cell types, including macrophages, dendritic cells, and natural killer cells. It can modulate their activation, differentiation, and function. For instance, butyrate has been shown to enhance the antigen-presenting capabilities of dendritic cells and promote the anti-inflammatory phenotype of macrophages.Expand Specific Solutions05 Butyrate in combination with other immune modulators
Research is ongoing to investigate the synergistic effects of combining butyrate with other immune-modulating compounds or therapies. These combinations may enhance the overall immune-regulatory effects and provide more targeted approaches for treating specific immune-related disorders. Potential combinations include butyrate with probiotics, other short-chain fatty acids, or established immunomodulatory drugs.Expand Specific Solutions
Key Players in Butyrate Immunology Research
The field of butyrate-induced immune regulation is in a dynamic growth phase, with increasing market potential as research advances. The global market for immunomodulators is expanding, driven by rising autoimmune disorders and cancer treatments. While the technology is still evolving, several key players are making significant strides. Companies like F. Hoffmann-La Roche Ltd., Bayer AG, and Amgen, Inc. are leveraging their extensive R&D capabilities to explore butyrate's immunomodulatory effects. Emerging biotechnology firms such as Synlogic Operating Co., Inc. and PharmaBiome AG are focusing on innovative approaches to harness butyrate's potential. Academic institutions, including The University of Chicago and Wageningen University, are contributing crucial basic research, fostering a collaborative ecosystem that accelerates progress in this promising field.
Dynavax Technologies Corp.
Synlogic Operating Co., Inc.
Breakthrough Studies in Butyrate Immunology
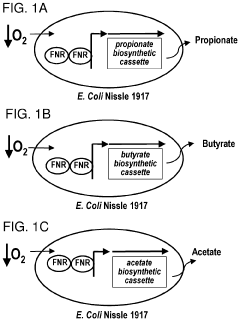
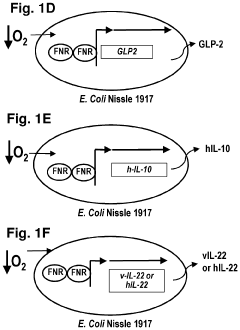
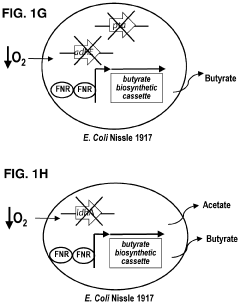
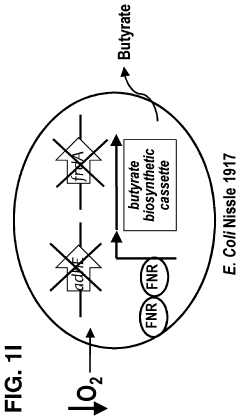
- Genetically engineered bacteria that produce therapeutic molecules like butyrate only in response to specific environmental conditions within the gut, such as low oxygen or inflammatory conditions, are introduced to reduce inflammation and enhance gut barrier function, thereby addressing the limitations of existing treatments.
- Genetically engineered bacteria that produce therapeutic molecules like butyrate only in response to specific environmental conditions within the gut, such as inflammation or low oxygen, enhancing gut barrier function and reducing inflammation without systemic exposure.
Regulatory Landscape for Butyrate-Based Therapies
The regulatory landscape for butyrate-based therapies is complex and evolving, reflecting the growing interest in harnessing the immunomodulatory properties of butyrate for therapeutic purposes. Regulatory bodies, such as the FDA in the United States and the EMA in Europe, are increasingly recognizing the potential of microbiome-based therapies, including those involving butyrate.
Currently, butyrate and its derivatives are classified under different regulatory categories depending on their intended use and formulation. As a naturally occurring substance, butyrate itself is often considered a dietary supplement in many jurisdictions. However, when formulated for specific therapeutic purposes, it may fall under the category of a drug or biologic, subject to more stringent regulatory requirements.
The FDA has established a framework for the development and approval of live biotherapeutic products (LBPs), which includes microbiome-based therapies that may indirectly modulate butyrate levels. This guidance provides a pathway for the clinical development of butyrate-producing bacterial strains as potential therapeutics.
In Europe, the EMA has also recognized the potential of microbiome-based therapies and has been working on developing appropriate regulatory frameworks. The agency has published guidelines on the quality of live biotherapeutic products, which are relevant to butyrate-producing bacterial therapies.
Regulatory challenges specific to butyrate-based therapies include demonstrating consistent production and delivery of butyrate in the gut, as well as establishing clear efficacy endpoints for immune regulation. The variability in individual microbiome compositions adds another layer of complexity to the regulatory process.
Safety considerations are paramount in the regulatory landscape. While butyrate is generally recognized as safe (GRAS) by the FDA when used as a food additive, its use as a therapeutic agent requires additional safety data, particularly regarding long-term effects on immune function and potential interactions with other medications.
As research in butyrate-induced immune regulation advances, regulatory agencies are likely to refine their approaches. There is a growing trend towards adaptive licensing and accelerated approval pathways for innovative therapies, which could benefit the development of butyrate-based treatments for immune-mediated disorders.
Safety and Efficacy Considerations for Butyrate Use
The safety and efficacy of butyrate use in immune regulation are critical considerations for its potential therapeutic applications. Butyrate, a short-chain fatty acid produced by gut microbiota, has shown promising immunomodulatory effects. However, its use as a therapeutic agent requires careful evaluation of both safety and efficacy parameters.
Safety considerations for butyrate use primarily focus on potential adverse effects and toxicity. While butyrate is naturally produced in the gut, exogenous administration may lead to different physiological responses. Studies have shown that butyrate is generally well-tolerated at therapeutic doses, with minimal side effects reported. Common mild side effects include gastrointestinal discomfort, nausea, and flatulence. However, long-term safety data for chronic administration is limited and requires further investigation.
The route of administration plays a crucial role in both safety and efficacy. Oral administration of butyrate faces challenges due to rapid absorption and metabolism in the upper gastrointestinal tract. To overcome this, various formulations have been developed, including enteric-coated tablets and microencapsulation techniques. These formulations aim to deliver butyrate to the colon, where it exerts its primary effects. Topical and rectal administrations have also been explored for localized effects, particularly in inflammatory bowel diseases.
Efficacy considerations for butyrate-induced immune regulation are multifaceted. Butyrate has demonstrated anti-inflammatory properties, primarily through its ability to inhibit histone deacetylases (HDACs) and activate G-protein coupled receptors (GPCRs). These mechanisms lead to the suppression of pro-inflammatory cytokines and the induction of regulatory T cells. However, the optimal dosage and treatment duration for achieving these effects in various immune-mediated conditions remain to be established.
Interindividual variability in response to butyrate treatment is a significant factor affecting efficacy. Differences in gut microbiota composition, genetic factors, and underlying health conditions can influence the response to butyrate supplementation. This variability necessitates personalized approaches and potentially the development of biomarkers to predict treatment efficacy.
The potential for butyrate to interact with other medications and dietary components must also be considered. While butyrate itself has a favorable safety profile, its effects on drug metabolism and absorption are not fully understood. Additionally, its impact on the overall gut microbiome composition and function requires further investigation to ensure that long-term use does not lead to unintended consequences in microbial ecology.
In conclusion, while butyrate shows promise as an immunomodulatory agent, careful consideration of safety and efficacy is essential for its successful therapeutic application. Ongoing research and clinical trials are needed to establish optimal dosing regimens, identify potential contraindications, and develop targeted delivery systems to maximize efficacy while minimizing adverse effects.