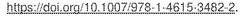Carbon Based Catalysts Versus Metal Catalysts For H2O2 Yield
AUG 28, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Carbon and Metal Catalysts for H2O2 Production: Background and Objectives
Hydrogen peroxide (H2O2) has emerged as a crucial chemical with extensive applications in environmental remediation, chemical synthesis, and industrial processes. The evolution of H2O2 production technologies has witnessed significant transformations over the past century, from traditional anthraquinone auto-oxidation processes to more sustainable direct synthesis methods. This technological progression has been driven by increasing demands for environmentally friendly oxidants and the need for more efficient production methods.
The direct synthesis of H2O2 from hydrogen and oxygen represents a particularly promising approach, offering potential advantages in terms of process simplification, reduced waste generation, and improved energy efficiency. Within this context, catalyst development has become a central focus of research efforts, with both metal-based and carbon-based catalysts showing distinctive characteristics and performance profiles.
Metal catalysts, particularly those based on palladium, have historically dominated the field of H2O2 synthesis. The evolution of these catalysts has progressed from simple monometallic systems to sophisticated bimetallic and alloy configurations designed to enhance selectivity and stability. Recent advancements have focused on controlling particle size, morphology, and electronic properties to optimize catalytic performance.
In parallel, carbon-based catalysts have gained increasing attention as potential alternatives to traditional metal systems. The emergence of carbon materials as catalysts represents a paradigm shift in catalyst design philosophy, moving from metal-centric approaches to exploiting the unique properties of carbon nanostructures. This trend aligns with broader sustainability goals in chemical manufacturing and green chemistry principles.
The technical objectives in this field are multifaceted, encompassing improvements in H2O2 yield, selectivity, catalyst stability, and economic viability. Specifically, researchers aim to develop catalysts that can achieve high H2O2 selectivity (>90%) under mild reaction conditions, maintain activity over extended operation periods, and utilize earth-abundant materials to reduce dependency on precious metals.
Current technological trajectories suggest a convergence of approaches, with hybrid systems incorporating both carbon and metal components emerging as promising candidates. These hybrid catalysts potentially combine the selectivity advantages of metal catalysts with the stability and sustainability benefits of carbon materials. Additionally, the integration of computational modeling and high-throughput experimental techniques is accelerating catalyst discovery and optimization processes.
The evolution of this technology is increasingly influenced by sustainability considerations, with growing emphasis on catalyst designs that minimize environmental impact, reduce energy consumption, and enable decentralized H2O2 production. This aligns with broader industrial trends toward process intensification and circular economy principles in chemical manufacturing.
The direct synthesis of H2O2 from hydrogen and oxygen represents a particularly promising approach, offering potential advantages in terms of process simplification, reduced waste generation, and improved energy efficiency. Within this context, catalyst development has become a central focus of research efforts, with both metal-based and carbon-based catalysts showing distinctive characteristics and performance profiles.
Metal catalysts, particularly those based on palladium, have historically dominated the field of H2O2 synthesis. The evolution of these catalysts has progressed from simple monometallic systems to sophisticated bimetallic and alloy configurations designed to enhance selectivity and stability. Recent advancements have focused on controlling particle size, morphology, and electronic properties to optimize catalytic performance.
In parallel, carbon-based catalysts have gained increasing attention as potential alternatives to traditional metal systems. The emergence of carbon materials as catalysts represents a paradigm shift in catalyst design philosophy, moving from metal-centric approaches to exploiting the unique properties of carbon nanostructures. This trend aligns with broader sustainability goals in chemical manufacturing and green chemistry principles.
The technical objectives in this field are multifaceted, encompassing improvements in H2O2 yield, selectivity, catalyst stability, and economic viability. Specifically, researchers aim to develop catalysts that can achieve high H2O2 selectivity (>90%) under mild reaction conditions, maintain activity over extended operation periods, and utilize earth-abundant materials to reduce dependency on precious metals.
Current technological trajectories suggest a convergence of approaches, with hybrid systems incorporating both carbon and metal components emerging as promising candidates. These hybrid catalysts potentially combine the selectivity advantages of metal catalysts with the stability and sustainability benefits of carbon materials. Additionally, the integration of computational modeling and high-throughput experimental techniques is accelerating catalyst discovery and optimization processes.
The evolution of this technology is increasingly influenced by sustainability considerations, with growing emphasis on catalyst designs that minimize environmental impact, reduce energy consumption, and enable decentralized H2O2 production. This aligns with broader industrial trends toward process intensification and circular economy principles in chemical manufacturing.
Market Analysis of H2O2 Production Technologies
The global hydrogen peroxide (H2O2) market has been experiencing steady growth, valued at approximately $3.5 billion in 2022 and projected to reach $5.7 billion by 2030, with a compound annual growth rate of 5.8%. This growth is primarily driven by increasing demand across various industries including pulp and paper, textile bleaching, wastewater treatment, mining, and electronics manufacturing.
Traditional H2O2 production technologies have been dominated by the anthraquinone auto-oxidation (AO) process, which accounts for over 95% of global production. This process relies heavily on metal catalysts, particularly palladium-based systems, which despite their efficiency, present significant economic and environmental challenges due to high costs and potential metal leaching.
The emerging carbon-based catalyst technologies for H2O2 production represent a disruptive innovation in this market. These catalysts offer substantial cost advantages, with production expenses potentially 30-40% lower than metal-based alternatives. The elimination of precious metals from the catalyst composition significantly reduces capital investment requirements and mitigates supply chain vulnerabilities associated with rare metal procurement.
Market segmentation analysis reveals distinct adoption patterns across regions. North America and Europe are leading the transition toward carbon-based catalysts, driven by stringent environmental regulations and sustainability initiatives. Asia-Pacific remains the largest consumer of H2O2 overall, with China accounting for approximately 30% of global consumption, though still predominantly using metal-based production technologies.
End-user industry analysis indicates that environmental applications, particularly water treatment, represent the fastest-growing segment for H2O2 demand, expanding at 7.2% annually. This sector shows particular receptiveness to carbon-based catalyst technologies due to their reduced environmental footprint and elimination of metal contamination concerns.
Economic analysis of production technologies reveals that while metal catalysts currently dominate due to established infrastructure, carbon-based alternatives are gaining competitive advantage through lower operational costs and reduced environmental compliance expenses. The total cost of ownership analysis demonstrates that despite higher initial conversion efficiency of metal catalysts, carbon-based systems achieve better long-term economic performance due to extended catalyst lifetime and simplified recovery processes.
Market forecasts suggest that carbon-based catalysts could capture 15-20% of the H2O2 production technology market by 2028, with accelerated adoption in environmentally sensitive applications and regions with aggressive carbon reduction targets. This transition represents a significant market opportunity for technology providers specializing in advanced carbon materials and catalyst design.
Traditional H2O2 production technologies have been dominated by the anthraquinone auto-oxidation (AO) process, which accounts for over 95% of global production. This process relies heavily on metal catalysts, particularly palladium-based systems, which despite their efficiency, present significant economic and environmental challenges due to high costs and potential metal leaching.
The emerging carbon-based catalyst technologies for H2O2 production represent a disruptive innovation in this market. These catalysts offer substantial cost advantages, with production expenses potentially 30-40% lower than metal-based alternatives. The elimination of precious metals from the catalyst composition significantly reduces capital investment requirements and mitigates supply chain vulnerabilities associated with rare metal procurement.
Market segmentation analysis reveals distinct adoption patterns across regions. North America and Europe are leading the transition toward carbon-based catalysts, driven by stringent environmental regulations and sustainability initiatives. Asia-Pacific remains the largest consumer of H2O2 overall, with China accounting for approximately 30% of global consumption, though still predominantly using metal-based production technologies.
End-user industry analysis indicates that environmental applications, particularly water treatment, represent the fastest-growing segment for H2O2 demand, expanding at 7.2% annually. This sector shows particular receptiveness to carbon-based catalyst technologies due to their reduced environmental footprint and elimination of metal contamination concerns.
Economic analysis of production technologies reveals that while metal catalysts currently dominate due to established infrastructure, carbon-based alternatives are gaining competitive advantage through lower operational costs and reduced environmental compliance expenses. The total cost of ownership analysis demonstrates that despite higher initial conversion efficiency of metal catalysts, carbon-based systems achieve better long-term economic performance due to extended catalyst lifetime and simplified recovery processes.
Market forecasts suggest that carbon-based catalysts could capture 15-20% of the H2O2 production technology market by 2028, with accelerated adoption in environmentally sensitive applications and regions with aggressive carbon reduction targets. This transition represents a significant market opportunity for technology providers specializing in advanced carbon materials and catalyst design.
Current Challenges in Carbon-Based vs Metal Catalytic Systems
The development of hydrogen peroxide (H2O2) production technologies faces significant challenges in both carbon-based and metal catalytic systems. Currently, the industrial production of H2O2 predominantly relies on the anthraquinone auto-oxidation process, which is energy-intensive and generates considerable waste. This has driven research toward direct synthesis methods using catalysts, where both metal and carbon-based systems present distinct obstacles.
Metal catalysts, particularly palladium-based systems, demonstrate high activity but suffer from poor selectivity toward H2O2. The primary challenge lies in preventing the over-hydrogenation of H2O2 to water, which significantly reduces yield. Additionally, metal catalysts often require acidic conditions and halide promoters to achieve acceptable selectivity, introducing corrosion issues and environmental concerns. The high cost and limited availability of precious metals like palladium and platinum further constrain their industrial application.
Stability represents another critical challenge for metal catalysts. Leaching of metal particles during reaction cycles leads to catalyst deactivation and product contamination. This necessitates frequent catalyst replacement, increasing operational costs and reducing process efficiency. Furthermore, metal catalysts are highly sensitive to reaction conditions, requiring precise control of temperature, pressure, and reactant ratios to maintain performance.
Carbon-based catalysts have emerged as promising alternatives but face their own set of challenges. While they offer superior selectivity compared to metal counterparts, their activity remains substantially lower. The activation of oxygen and hydrogen molecules on carbon surfaces is less efficient, resulting in slower reaction kinetics and reduced productivity. The relationship between carbon structure, functional groups, and catalytic performance remains incompletely understood, hampering rational catalyst design.
The scalability of carbon-based catalytic systems presents additional hurdles. Laboratory-scale successes often fail to translate to industrial settings due to mass transfer limitations and heat management issues. The synthesis of carbon catalysts with consistent properties at scale remains challenging, with batch-to-batch variations affecting performance reliability.
Both catalyst types struggle with hydrogen peroxide decomposition, which occurs through parallel reactions during synthesis. This decomposition is particularly problematic at higher H2O2 concentrations, limiting achievable yields. The development of stabilization strategies that do not compromise catalytic activity represents an ongoing research focus.
Environmental considerations further complicate catalyst selection. While carbon-based catalysts offer advantages in terms of sustainability and reduced environmental impact, their production methods may involve hazardous chemicals or energy-intensive processes. Comprehensive life cycle assessments comparing both catalyst types remain scarce, making sustainability comparisons difficult.
Metal catalysts, particularly palladium-based systems, demonstrate high activity but suffer from poor selectivity toward H2O2. The primary challenge lies in preventing the over-hydrogenation of H2O2 to water, which significantly reduces yield. Additionally, metal catalysts often require acidic conditions and halide promoters to achieve acceptable selectivity, introducing corrosion issues and environmental concerns. The high cost and limited availability of precious metals like palladium and platinum further constrain their industrial application.
Stability represents another critical challenge for metal catalysts. Leaching of metal particles during reaction cycles leads to catalyst deactivation and product contamination. This necessitates frequent catalyst replacement, increasing operational costs and reducing process efficiency. Furthermore, metal catalysts are highly sensitive to reaction conditions, requiring precise control of temperature, pressure, and reactant ratios to maintain performance.
Carbon-based catalysts have emerged as promising alternatives but face their own set of challenges. While they offer superior selectivity compared to metal counterparts, their activity remains substantially lower. The activation of oxygen and hydrogen molecules on carbon surfaces is less efficient, resulting in slower reaction kinetics and reduced productivity. The relationship between carbon structure, functional groups, and catalytic performance remains incompletely understood, hampering rational catalyst design.
The scalability of carbon-based catalytic systems presents additional hurdles. Laboratory-scale successes often fail to translate to industrial settings due to mass transfer limitations and heat management issues. The synthesis of carbon catalysts with consistent properties at scale remains challenging, with batch-to-batch variations affecting performance reliability.
Both catalyst types struggle with hydrogen peroxide decomposition, which occurs through parallel reactions during synthesis. This decomposition is particularly problematic at higher H2O2 concentrations, limiting achievable yields. The development of stabilization strategies that do not compromise catalytic activity represents an ongoing research focus.
Environmental considerations further complicate catalyst selection. While carbon-based catalysts offer advantages in terms of sustainability and reduced environmental impact, their production methods may involve hazardous chemicals or energy-intensive processes. Comprehensive life cycle assessments comparing both catalyst types remain scarce, making sustainability comparisons difficult.
Comparative Analysis of Current Carbon and Metal Catalyst Solutions
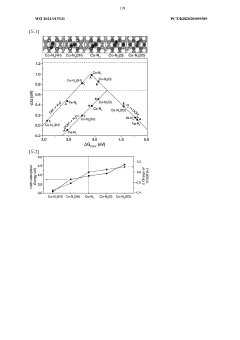
01 Carbon-based catalysts for H2O2 production
Carbon-based materials serve as effective catalysts for hydrogen peroxide production. These catalysts typically include activated carbon, carbon nanotubes, graphene, and other carbon structures that provide high surface area and specific active sites. The carbon materials can be functionalized with oxygen-containing groups to enhance catalytic activity. These catalysts offer advantages such as low cost, environmental friendliness, and tunable surface properties that contribute to improved H2O2 yield in various production processes.- Carbon-based catalysts for H2O2 production: Carbon-based materials serve as effective catalysts for hydrogen peroxide production. These catalysts typically include activated carbon, carbon nanotubes, graphene, and other carbon structures that provide high surface area and specific active sites. The carbon materials can be functionalized with oxygen-containing groups to enhance catalytic activity. These catalysts offer advantages such as low cost, environmental friendliness, and tunable surface properties that contribute to improved H2O2 yield and selectivity in direct synthesis processes.
- Metal-based catalysts for H2O2 synthesis: Various metal catalysts are employed for hydrogen peroxide production, including noble metals (Pd, Pt, Au), transition metals (Fe, Cu, Ni), and their alloys. These metal catalysts can be used in different forms such as nanoparticles, supported structures, or as part of complex catalyst systems. The catalytic performance depends on metal particle size, oxidation state, and dispersion. Metal catalysts typically function by facilitating the selective hydrogenation of oxygen to H2O2 rather than water, with specific metals offering different selectivity and activity profiles.
- Metal-carbon hybrid catalysts for enhanced H2O2 yield: Hybrid catalysts combining metal nanoparticles with carbon supports demonstrate superior performance in hydrogen peroxide synthesis. These catalysts leverage the synergistic effects between the metal active sites and the carbon support structure. Common configurations include palladium on activated carbon, gold-palladium alloys on carbon nanotubes, or other noble metals dispersed on graphene-based materials. The carbon support not only provides high surface area but also influences the electronic properties of the metal particles, leading to improved selectivity, stability, and higher H2O2 yields compared to single-component catalysts.
- Process optimization for H2O2 production using catalysts: Process parameters significantly impact hydrogen peroxide yield when using carbon-based or metal catalysts. Key factors include reaction temperature, pressure, gas composition (H2/O2 ratio), solvent selection, and reactor design. Optimization techniques involve controlling reaction conditions to prevent H2O2 decomposition, using promoters or stabilizers, and implementing continuous flow processes. Advanced reactor designs such as microreactors or membrane reactors can enhance mass transfer and improve safety by keeping hydrogen and oxygen concentrations outside explosive limits, thereby maximizing H2O2 yield and selectivity.
- Catalyst modifications and additives for improved H2O2 selectivity: Various modifications and additives can enhance catalyst performance in hydrogen peroxide synthesis. These include halide promoters (Br, Cl), acid additives, surface functionalization of carbon supports, and incorporation of secondary metals as promoters. Catalyst pretreatment methods such as oxidation, reduction, or specific synthesis techniques can optimize active site distribution. Additionally, stabilizers can be added to prevent H2O2 decomposition once formed. These modifications aim to increase selectivity toward H2O2 formation over water production, improve catalyst stability, and extend catalyst lifetime under reaction conditions.
02 Metal-based catalysts for H2O2 synthesis
Metal catalysts play a crucial role in hydrogen peroxide synthesis, with noble metals like palladium, platinum, and gold being particularly effective. These metals can be used in various forms including nanoparticles, alloys, and supported structures. The catalytic performance depends on metal particle size, dispersion, and oxidation state. Metal catalysts typically operate via the direct synthesis pathway, where hydrogen and oxygen react on the catalyst surface to form H2O2, offering higher selectivity and yield compared to traditional auto-oxidation processes.Expand Specific Solutions03 Hybrid carbon-metal catalytic systems
Hybrid catalysts combining carbon materials with metal components demonstrate synergistic effects for enhanced H2O2 production. These systems typically feature metal nanoparticles dispersed on carbon supports such as activated carbon, graphene, or carbon nanotubes. The carbon support provides high surface area and stability, while the metal components offer active sites for catalytic reactions. This combination improves catalyst efficiency, selectivity, and longevity, resulting in higher hydrogen peroxide yields compared to single-component catalysts.Expand Specific Solutions04 Process optimization for H2O2 production
Various process parameters significantly impact hydrogen peroxide yield when using carbon and metal catalysts. These include reaction temperature, pressure, reactant concentration, pH, and solvent selection. Optimization techniques involve controlling reaction conditions to minimize side reactions and catalyst deactivation. Advanced reactor designs, continuous flow systems, and precise control of gas-liquid interfaces can substantially improve H2O2 selectivity and production efficiency. Process innovations focus on enhancing mass transfer, reducing decomposition, and maintaining catalyst stability over extended operation periods.Expand Specific Solutions05 Catalyst modifications for improved H2O2 selectivity
Specific modifications to both carbon and metal catalysts can significantly enhance hydrogen peroxide selectivity and yield. These include surface functionalization with oxygen or nitrogen groups, addition of promoters or stabilizers, controlled alloying of metals, and precise engineering of pore structures. Halide additives often serve as selectivity enhancers for metal catalysts. Advanced preparation methods such as controlled deposition, atomic layer deposition, and precise heat treatments help create optimal catalyst structures with increased active sites and improved resistance to deactivation.Expand Specific Solutions
Leading Research Institutions and Companies in Catalytic H2O2 Production
The hydrogen peroxide production landscape is evolving with carbon-based catalysts emerging as promising alternatives to traditional metal catalysts. The market is in a transitional growth phase, with an estimated global H2O2 market of $3.5 billion, expanding at 5-6% annually. Carbon catalysts offer advantages in selectivity and environmental impact, though metal catalysts remain dominant due to established industrial processes. Leading research institutions like Tianjin University, Dalian Institute of Chemical Physics, and Tsinghua University are advancing carbon catalyst technology, while companies including BASF, Sinopec, and LG Electronics are investing in both catalyst types. Metal catalyst technology remains mature, but carbon-based alternatives are rapidly approaching commercial viability with significant sustainability benefits.
Dalian Institute of Chemical Physics Chinese Academy of Sci
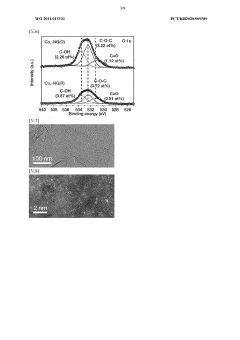
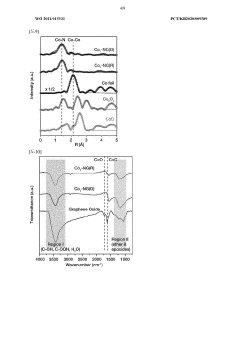
Technical Solution: Dalian Institute has pioneered advanced carbon-based catalysts for H2O2 synthesis through their direct synthesis pathway. Their technology utilizes nitrogen-doped graphene materials with precisely controlled defect structures that serve as active sites for H2O2 formation. The institute has developed a unique approach involving oxygen reduction reaction (ORR) pathways on carbon surfaces, achieving H2O2 selectivity exceeding 90% under optimized conditions. Their catalysts operate at near-ambient temperatures (20-40°C) and moderate pressures (1-5 MPa), significantly reducing energy requirements compared to traditional anthraquinone processes. Recent innovations include carbon materials with hierarchical porosity that enhance mass transfer and reaction kinetics, leading to improved H2O2 yields of up to 25-30% in single-pass operations. The institute has also developed hybrid carbon-metal systems where carbon serves as both support and co-catalyst, creating synergistic effects with precisely dispersed metal nanoparticles.
Strengths: Superior selectivity for H2O2 formation, environmentally benign reaction conditions, lower energy consumption, and excellent stability with minimal deactivation over extended operation periods. Weaknesses: Lower absolute productivity rates compared to some metal catalysts, potential challenges in large-scale manufacturing with consistent quality, and sensitivity to certain impurities in feedstock gases.
China Petroleum & Chemical Corp.
Technical Solution: China Petroleum & Chemical Corp. (Sinopec) has developed a dual-catalyst system combining carbon-based materials with controlled metal loading for direct H2O2 synthesis. Their proprietary technology employs graphene oxide sheets functionalized with oxygen-containing groups that serve as anchoring sites for palladium nanoparticles (2-5 nm diameter). This creates a hybrid catalyst where the carbon material provides selectivity control while the metal component enhances activity. Sinopec's process operates in a continuous flow reactor system at moderate pressures (4-8 MPa) and temperatures (5-25°C), using methanol-water mixtures as solvents to enhance H2O2 stability. Their catalyst formulation achieves H2O2 selectivity of 80-85% with H2 conversion rates of approximately 60-70%. The company has also developed acid-treated carbon supports that inhibit the subsequent decomposition of formed H2O2, significantly improving overall yield. Recent advancements include the incorporation of secondary promoters like halides and phosphates that further enhance selectivity and stability.
Strengths: Balanced approach combining benefits of both carbon and metal catalysts, scalable manufacturing process compatible with existing infrastructure, and good catalyst longevity with regeneration capabilities. Weaknesses: Relatively complex catalyst preparation requiring precise control of metal deposition, moderate sensitivity to catalyst poisoning by sulfur compounds, and higher production costs compared to traditional anthraquinone process for large-scale applications.
Key Patents and Scientific Breakthroughs in H2O2 Catalysis
Catalyst for producing hydrogen peroxide, and preparation method therefor
PatentWO2021015531A1
Innovation
- A catalyst comprising a carbon-based support with a transition metal atom and nitrogen-doped M1-N bond structure, where the transition metal atom is bonded to the carbon-based support, enhancing catalytic activity and stability, and can include cobalt as a catalytic moiety, with a manufacturing method involving preparation of the carbon-based support, addition of the transition metal atom, and nitrogen doping.
Synthesis of h 2o 2 using a rhenium metal-based electrocatalyst and rhenium electrocatalyst
PatentWO2025083671A1
Innovation
- A green synthesis process using an electrocatalyst based on metallic Renio nanoparticles supported on carbon nanotubes for the electrochemical production of hydrogen peroxide, which reduces production costs and environmental impact.
Environmental Impact and Sustainability Assessment
The environmental impact assessment of hydrogen peroxide (H₂O₂) production methods reveals significant differences between carbon-based and metal catalysts. Carbon-based catalysts demonstrate superior environmental credentials due to their composition from abundant, non-toxic elements like carbon, nitrogen, and oxygen, which substantially reduces ecological footprints compared to metal-based alternatives that often contain precious or heavy metals.
Life cycle analyses indicate that carbon catalyst production processes generally consume less energy and generate fewer greenhouse gas emissions. The synthesis of carbon catalysts typically requires lower temperatures and less intensive processing than metal catalysts, resulting in reduced carbon dioxide emissions during the manufacturing phase. Additionally, carbon catalysts often utilize renewable or waste-derived precursors, further enhancing their sustainability profile.
Water consumption and pollution metrics also favor carbon-based systems. Metal catalyst production frequently involves extensive water usage and generates metal-contaminated wastewater requiring specialized treatment. In contrast, carbon catalyst synthesis generally has lower water requirements and produces fewer hazardous effluents, minimizing impacts on aquatic ecosystems.
End-of-life considerations strongly favor carbon catalysts, which typically degrade into environmentally benign compounds or can be repurposed through thermal treatment. Metal catalysts, conversely, require resource-intensive recovery processes to reclaim valuable metals and prevent environmental contamination, adding significant downstream environmental costs.
Regulatory compliance represents another advantage for carbon-based systems. As environmental regulations tighten globally, particularly regarding heavy metal usage and disposal, carbon catalysts face fewer regulatory hurdles and compliance costs. This regulatory landscape is increasingly influencing industrial adoption decisions beyond pure performance metrics.
The sustainability assessment must also consider catalyst longevity and regeneration capabilities. While some metal catalysts demonstrate exceptional durability, carbon catalysts are making significant advances in stability and reusability, narrowing this historical gap. Recent innovations in carbon catalyst design have yielded materials with improved resistance to degradation during H₂O₂ production cycles.
When evaluating total environmental impact, carbon-based catalysts for H₂O₂ production increasingly represent the more sustainable option, particularly as research continues to enhance their performance efficiency. This environmental advantage, combined with improving yield capabilities, positions carbon catalysts as the preferred direction for environmentally responsible hydrogen peroxide production technologies.
Life cycle analyses indicate that carbon catalyst production processes generally consume less energy and generate fewer greenhouse gas emissions. The synthesis of carbon catalysts typically requires lower temperatures and less intensive processing than metal catalysts, resulting in reduced carbon dioxide emissions during the manufacturing phase. Additionally, carbon catalysts often utilize renewable or waste-derived precursors, further enhancing their sustainability profile.
Water consumption and pollution metrics also favor carbon-based systems. Metal catalyst production frequently involves extensive water usage and generates metal-contaminated wastewater requiring specialized treatment. In contrast, carbon catalyst synthesis generally has lower water requirements and produces fewer hazardous effluents, minimizing impacts on aquatic ecosystems.
End-of-life considerations strongly favor carbon catalysts, which typically degrade into environmentally benign compounds or can be repurposed through thermal treatment. Metal catalysts, conversely, require resource-intensive recovery processes to reclaim valuable metals and prevent environmental contamination, adding significant downstream environmental costs.
Regulatory compliance represents another advantage for carbon-based systems. As environmental regulations tighten globally, particularly regarding heavy metal usage and disposal, carbon catalysts face fewer regulatory hurdles and compliance costs. This regulatory landscape is increasingly influencing industrial adoption decisions beyond pure performance metrics.
The sustainability assessment must also consider catalyst longevity and regeneration capabilities. While some metal catalysts demonstrate exceptional durability, carbon catalysts are making significant advances in stability and reusability, narrowing this historical gap. Recent innovations in carbon catalyst design have yielded materials with improved resistance to degradation during H₂O₂ production cycles.
When evaluating total environmental impact, carbon-based catalysts for H₂O₂ production increasingly represent the more sustainable option, particularly as research continues to enhance their performance efficiency. This environmental advantage, combined with improving yield capabilities, positions carbon catalysts as the preferred direction for environmentally responsible hydrogen peroxide production technologies.
Scalability and Industrial Implementation Considerations
The scalability of hydrogen peroxide production processes using different catalyst systems represents a critical factor in determining their industrial viability. Carbon-based catalysts demonstrate several advantages over traditional metal catalysts when considering large-scale implementation. Their inherently lower cost structure provides a significant economic advantage, particularly when production volumes increase to industrial scales. This cost efficiency stems from both the abundance of carbon precursors and the relatively straightforward synthesis procedures compared to the complex purification processes required for precious metal catalysts.
Production infrastructure requirements differ substantially between the two catalyst types. Metal catalyst systems often necessitate specialized handling equipment due to their sensitivity to contamination and potential for leaching. In contrast, carbon-based catalysts generally exhibit greater stability in industrial environments, reducing the need for specialized containment systems and simplifying reactor design considerations. This translates to potentially lower capital expenditure requirements for new production facilities.
Reactor engineering considerations also favor carbon-based catalysts in certain applications. Their structural stability under flow conditions makes them particularly suitable for continuous production systems, which are increasingly preferred in modern chemical manufacturing. The mechanical durability of properly engineered carbon catalysts allows for higher throughput without significant performance degradation, addressing a key limitation in scaling up hydrogen peroxide production.
Lifecycle analysis reveals additional advantages for carbon-based systems. While metal catalysts often suffer from progressive deactivation requiring frequent regeneration or replacement, many carbon catalysts demonstrate extended operational lifespans. This reduces downtime and maintenance costs in industrial settings, though comprehensive long-term stability data remains limited for newer carbon catalyst formulations.
Supply chain considerations cannot be overlooked when evaluating industrial implementation. The geopolitical concentration of precious metals used in traditional catalysts introduces supply vulnerability, whereas carbon precursors are widely available globally. This reduces dependency on specific supplier regions and mitigates raw material price volatility risks for manufacturers adopting carbon-based technologies.
Regulatory compliance pathways generally favor carbon-based systems due to reduced environmental concerns regarding heavy metal contamination. This streamlines permitting processes for new production facilities and may reduce compliance costs associated with waste handling and disposal. However, nanoscale carbon materials may face emerging regulatory scrutiny as their environmental fate becomes better understood.
Integration with existing hydrogen peroxide production infrastructure presents both challenges and opportunities. While retrofitting established metal catalyst systems requires significant engineering adaptation, the potential efficiency gains and operational cost reductions make such transitions increasingly economically justifiable as carbon catalyst technology matures.
Production infrastructure requirements differ substantially between the two catalyst types. Metal catalyst systems often necessitate specialized handling equipment due to their sensitivity to contamination and potential for leaching. In contrast, carbon-based catalysts generally exhibit greater stability in industrial environments, reducing the need for specialized containment systems and simplifying reactor design considerations. This translates to potentially lower capital expenditure requirements for new production facilities.
Reactor engineering considerations also favor carbon-based catalysts in certain applications. Their structural stability under flow conditions makes them particularly suitable for continuous production systems, which are increasingly preferred in modern chemical manufacturing. The mechanical durability of properly engineered carbon catalysts allows for higher throughput without significant performance degradation, addressing a key limitation in scaling up hydrogen peroxide production.
Lifecycle analysis reveals additional advantages for carbon-based systems. While metal catalysts often suffer from progressive deactivation requiring frequent regeneration or replacement, many carbon catalysts demonstrate extended operational lifespans. This reduces downtime and maintenance costs in industrial settings, though comprehensive long-term stability data remains limited for newer carbon catalyst formulations.
Supply chain considerations cannot be overlooked when evaluating industrial implementation. The geopolitical concentration of precious metals used in traditional catalysts introduces supply vulnerability, whereas carbon precursors are widely available globally. This reduces dependency on specific supplier regions and mitigates raw material price volatility risks for manufacturers adopting carbon-based technologies.
Regulatory compliance pathways generally favor carbon-based systems due to reduced environmental concerns regarding heavy metal contamination. This streamlines permitting processes for new production facilities and may reduce compliance costs associated with waste handling and disposal. However, nanoscale carbon materials may face emerging regulatory scrutiny as their environmental fate becomes better understood.
Integration with existing hydrogen peroxide production infrastructure presents both challenges and opportunities. While retrofitting established metal catalyst systems requires significant engineering adaptation, the potential efficiency gains and operational cost reductions make such transitions increasingly economically justifiable as carbon catalyst technology matures.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!