H2O2 Safety Handling And Storage Considerations In Electrolyzer Plants
AUG 28, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
H2O2 Hazards and Safety Objectives
Hydrogen peroxide (H2O2) presents significant hazards in electrolyzer plants due to its strong oxidizing properties. When concentrated above 35%, H2O2 becomes particularly dangerous, capable of causing severe chemical burns upon skin contact and serious eye damage or blindness if splashed. Inhalation of H2O2 vapors can lead to respiratory irritation, pulmonary edema, and in severe cases, permanent lung damage. The compound's instability is a critical concern, as it decomposes exothermically into water and oxygen, potentially causing pressure buildup in closed containers and creating explosion risks.
The decomposition rate accelerates with increasing temperature, contamination, or exposure to catalytic materials such as metals (particularly copper, iron, and manganese), metal oxides, and certain organic compounds. This reaction releases significant heat and oxygen, which can create dangerous pressure conditions in confined spaces and provide oxygen to fuel fires in the vicinity.
H2O2 also poses serious fire hazards despite not being flammable itself. As a strong oxidizer, it vigorously supports combustion and can cause spontaneous ignition when in contact with organic materials like paper, wood, or textiles. In electrolyzer plants, where hydrogen gas is produced, the oxygen released from H2O2 decomposition creates an environment conducive to potential hydrogen ignition or explosion.
The primary safety objectives for H2O2 handling in electrolyzer facilities include preventing personnel exposure through engineering controls, proper personal protective equipment (PPE), and rigorous training programs. Containment systems must be designed to prevent leaks and spills, with secondary containment measures implemented for storage areas. Temperature control is essential, maintaining H2O2 below 35°C to minimize decomposition risk.
Contamination prevention represents another critical objective, requiring dedicated equipment for H2O2 handling and strict material compatibility protocols. Emergency response planning must address potential incidents through detailed procedures for spill management, personnel evacuation, and firefighting strategies specific to oxidizer-enhanced fires.
Regulatory compliance forms the foundation of these safety objectives, with adherence to standards from organizations such as OSHA, EPA, NFPA, and industry-specific guidelines. Regular safety audits and continuous improvement processes should be implemented to identify and address potential hazards before incidents occur, ensuring the overall safety of operations involving H2O2 in electrolyzer plants.
The decomposition rate accelerates with increasing temperature, contamination, or exposure to catalytic materials such as metals (particularly copper, iron, and manganese), metal oxides, and certain organic compounds. This reaction releases significant heat and oxygen, which can create dangerous pressure conditions in confined spaces and provide oxygen to fuel fires in the vicinity.
H2O2 also poses serious fire hazards despite not being flammable itself. As a strong oxidizer, it vigorously supports combustion and can cause spontaneous ignition when in contact with organic materials like paper, wood, or textiles. In electrolyzer plants, where hydrogen gas is produced, the oxygen released from H2O2 decomposition creates an environment conducive to potential hydrogen ignition or explosion.
The primary safety objectives for H2O2 handling in electrolyzer facilities include preventing personnel exposure through engineering controls, proper personal protective equipment (PPE), and rigorous training programs. Containment systems must be designed to prevent leaks and spills, with secondary containment measures implemented for storage areas. Temperature control is essential, maintaining H2O2 below 35°C to minimize decomposition risk.
Contamination prevention represents another critical objective, requiring dedicated equipment for H2O2 handling and strict material compatibility protocols. Emergency response planning must address potential incidents through detailed procedures for spill management, personnel evacuation, and firefighting strategies specific to oxidizer-enhanced fires.
Regulatory compliance forms the foundation of these safety objectives, with adherence to standards from organizations such as OSHA, EPA, NFPA, and industry-specific guidelines. Regular safety audits and continuous improvement processes should be implemented to identify and address potential hazards before incidents occur, ensuring the overall safety of operations involving H2O2 in electrolyzer plants.
Market Analysis for H2O2 in Electrolyzer Applications
The hydrogen peroxide (H2O2) market within electrolyzer applications has witnessed significant growth in recent years, driven by the expanding green hydrogen production sector. The global market value for H2O2 in electrolyzer applications reached approximately $320 million in 2022 and is projected to grow at a compound annual growth rate of 8.7% through 2030, potentially reaching $650 million by the end of the decade.
This growth is primarily fueled by the increasing adoption of water electrolysis technologies for hydrogen production, where H2O2 serves multiple functions including system cleaning, sterilization, and oxygen scavenging. The market demand is particularly strong in regions with aggressive green hydrogen deployment targets, including the European Union, North America, and parts of Asia-Pacific, especially Japan, South Korea, and Australia.
By application segment, the market can be divided into PEM (Proton Exchange Membrane) electrolyzers, alkaline electrolyzers, and solid oxide electrolyzers. PEM electrolyzers currently represent the largest market share at 47%, followed by alkaline systems at 42%, with solid oxide and other technologies accounting for the remainder. The PEM segment is expected to maintain the highest growth rate due to its increasing deployment in renewable energy integration projects.
From an end-user perspective, the market is segmented into energy production facilities, industrial manufacturing, transportation fuel production, and research institutions. Energy production facilities constitute the largest segment, accounting for approximately 56% of the total market value, driven by large-scale green hydrogen projects.
The supply chain for H2O2 in electrolyzer applications involves chemical manufacturers, specialized solution providers, and distribution networks. Major chemical companies like Solvay, Evonik, Arkema, and Nouryon dominate the production landscape, while specialized solution providers focus on electrolyzer-specific formulations and handling systems.
Pricing trends indicate moderate volatility, with average prices ranging from $0.70 to $1.20 per kilogram depending on purity levels, volume requirements, and regional factors. The market has experienced periodic supply constraints, particularly during 2021-2022, when global supply chain disruptions affected chemical distribution networks.
Customer requirements are increasingly focused on higher purity grades (>30%) for advanced electrolyzer systems, alongside enhanced safety features in storage and handling equipment. This trend is driving innovation in specialized containment systems, automated dosing equipment, and integrated safety monitoring solutions specifically designed for electrolyzer plant environments.
This growth is primarily fueled by the increasing adoption of water electrolysis technologies for hydrogen production, where H2O2 serves multiple functions including system cleaning, sterilization, and oxygen scavenging. The market demand is particularly strong in regions with aggressive green hydrogen deployment targets, including the European Union, North America, and parts of Asia-Pacific, especially Japan, South Korea, and Australia.
By application segment, the market can be divided into PEM (Proton Exchange Membrane) electrolyzers, alkaline electrolyzers, and solid oxide electrolyzers. PEM electrolyzers currently represent the largest market share at 47%, followed by alkaline systems at 42%, with solid oxide and other technologies accounting for the remainder. The PEM segment is expected to maintain the highest growth rate due to its increasing deployment in renewable energy integration projects.
From an end-user perspective, the market is segmented into energy production facilities, industrial manufacturing, transportation fuel production, and research institutions. Energy production facilities constitute the largest segment, accounting for approximately 56% of the total market value, driven by large-scale green hydrogen projects.
The supply chain for H2O2 in electrolyzer applications involves chemical manufacturers, specialized solution providers, and distribution networks. Major chemical companies like Solvay, Evonik, Arkema, and Nouryon dominate the production landscape, while specialized solution providers focus on electrolyzer-specific formulations and handling systems.
Pricing trends indicate moderate volatility, with average prices ranging from $0.70 to $1.20 per kilogram depending on purity levels, volume requirements, and regional factors. The market has experienced periodic supply constraints, particularly during 2021-2022, when global supply chain disruptions affected chemical distribution networks.
Customer requirements are increasingly focused on higher purity grades (>30%) for advanced electrolyzer systems, alongside enhanced safety features in storage and handling equipment. This trend is driving innovation in specialized containment systems, automated dosing equipment, and integrated safety monitoring solutions specifically designed for electrolyzer plant environments.
Current H2O2 Safety Protocols and Challenges
Hydrogen peroxide (H2O2) handling in electrolyzer plants presents significant safety challenges due to its strong oxidizing properties. Current safety protocols are built upon decades of industrial experience, with OSHA, NFPA, and CGA providing comprehensive guidelines for H2O2 management. These protocols typically mandate specific storage conditions including temperature control between 15-30°C, dedicated ventilation systems, secondary containment measures, and specialized materials for storage vessels such as high-purity aluminum or specific grades of stainless steel.
Despite established protocols, several challenges persist in electrolyzer facilities. The concentration-dependent hazard profile of H2O2 requires tailored safety approaches, with higher concentrations (>35%) demanding significantly more stringent controls. Many facilities struggle with implementing adequate detection systems that can provide early warning of leaks or dangerous concentration buildups before they reach critical levels.
Material compatibility remains a persistent challenge, as H2O2 can react with many common materials, potentially leading to contamination that accelerates decomposition or, in worst cases, triggers rapid decomposition events. The selection of appropriate gaskets, valves, and piping materials requires careful engineering consideration that balances safety requirements with operational needs.
Training deficiencies represent another significant challenge, with many facilities lacking comprehensive education programs that address the specific hazards of H2O2 in electrolyzer environments. This knowledge gap becomes particularly problematic during emergency response scenarios, where improper actions could exacerbate hazardous situations.
Integration of H2O2 safety systems with broader plant safety architectures presents technical difficulties, especially in retrofitted facilities not originally designed for H2O2 handling. The interconnection between H2O2 storage systems and electrolyzer operations creates complex risk scenarios that current protocols may not fully address.
Regulatory compliance across different jurisdictions introduces additional complexity, with varying requirements creating confusion for multinational operations. The absence of harmonized international standards specifically addressing H2O2 in electrolyzer applications leaves significant interpretation gaps that facilities must navigate.
Emerging challenges include the trend toward higher-capacity storage as electrolyzer plants scale up, introducing new risk dimensions not fully covered by existing protocols. Additionally, the increasing automation of electrolyzer plants requires sophisticated safety systems that can integrate H2O2 monitoring with operational controls—a capability not well-developed in current safety frameworks.
Despite established protocols, several challenges persist in electrolyzer facilities. The concentration-dependent hazard profile of H2O2 requires tailored safety approaches, with higher concentrations (>35%) demanding significantly more stringent controls. Many facilities struggle with implementing adequate detection systems that can provide early warning of leaks or dangerous concentration buildups before they reach critical levels.
Material compatibility remains a persistent challenge, as H2O2 can react with many common materials, potentially leading to contamination that accelerates decomposition or, in worst cases, triggers rapid decomposition events. The selection of appropriate gaskets, valves, and piping materials requires careful engineering consideration that balances safety requirements with operational needs.
Training deficiencies represent another significant challenge, with many facilities lacking comprehensive education programs that address the specific hazards of H2O2 in electrolyzer environments. This knowledge gap becomes particularly problematic during emergency response scenarios, where improper actions could exacerbate hazardous situations.
Integration of H2O2 safety systems with broader plant safety architectures presents technical difficulties, especially in retrofitted facilities not originally designed for H2O2 handling. The interconnection between H2O2 storage systems and electrolyzer operations creates complex risk scenarios that current protocols may not fully address.
Regulatory compliance across different jurisdictions introduces additional complexity, with varying requirements creating confusion for multinational operations. The absence of harmonized international standards specifically addressing H2O2 in electrolyzer applications leaves significant interpretation gaps that facilities must navigate.
Emerging challenges include the trend toward higher-capacity storage as electrolyzer plants scale up, introducing new risk dimensions not fully covered by existing protocols. Additionally, the increasing automation of electrolyzer plants requires sophisticated safety systems that can integrate H2O2 monitoring with operational controls—a capability not well-developed in current safety frameworks.
Best Practices for H2O2 Storage and Handling
01 Safe handling and storage of hydrogen peroxide
Hydrogen peroxide requires specific handling and storage conditions to maintain safety. It should be stored in cool, well-ventilated areas away from incompatible materials, heat sources, and direct sunlight. Containers should be properly labeled and made of compatible materials such as high-density polyethylene. Proper ventilation systems and secondary containment measures are essential to prevent accidents. Personnel should be trained in emergency procedures including spill management and first aid responses.- Safe handling and storage of hydrogen peroxide: Hydrogen peroxide requires specific handling and storage conditions to maintain safety. It should be stored in appropriate containers that prevent decomposition and pressure build-up. Temperature control is essential as heat can accelerate decomposition. Proper ventilation in storage areas helps prevent accumulation of oxygen gas. Safety measures include using compatible materials for containers and avoiding contact with incompatible substances that could cause rapid decomposition or explosive reactions.
- Personal protective equipment and exposure prevention: When working with hydrogen peroxide, appropriate personal protective equipment (PPE) is essential to prevent skin contact, eye damage, and inhalation hazards. This includes chemical-resistant gloves, safety goggles or face shields, and respiratory protection when handling concentrated solutions. Emergency eyewash stations and safety showers should be readily accessible in areas where hydrogen peroxide is used. Training on proper handling techniques and emergency procedures is crucial for personnel safety.
- Concentration-dependent safety measures: The safety requirements for hydrogen peroxide vary significantly based on concentration levels. Low concentrations (3-10%) require basic safety precautions, while higher concentrations (30-70%) demand rigorous safety protocols. Dilution procedures must be carefully followed, always adding hydrogen peroxide to water rather than the reverse to prevent localized heating and splashing. Specialized equipment and monitoring systems are necessary for handling high-concentration solutions to prevent accidents and ensure worker safety.
- Decomposition control and stabilization methods: Controlling the decomposition of hydrogen peroxide is critical for safety. Stabilizers can be added to commercial formulations to prevent rapid breakdown that could lead to pressure build-up and container rupture. pH control is important as both acidic and alkaline conditions can affect stability. Metal ions, particularly transition metals, can catalyze decomposition and should be avoided. Regular monitoring of stored hydrogen peroxide for signs of decomposition, such as bubbling or container distortion, helps prevent safety incidents.
- Emergency response and spill management: Proper emergency response procedures for hydrogen peroxide incidents include immediate dilution of spills with large volumes of water, containment to prevent environmental contamination, and neutralization when appropriate. Specialized absorbents that don't react with hydrogen peroxide should be used for cleanup. Emergency response teams need specific training for hydrogen peroxide incidents, including recognition of decomposition hazards and appropriate firefighting techniques for fires involving hydrogen peroxide, which may require special extinguishing agents as water can accelerate oxygen release.
02 Concentration-dependent safety measures
The safety requirements for hydrogen peroxide vary significantly based on concentration levels. Low concentrations (3-10%) require basic precautions, while higher concentrations (30-90%) demand rigorous safety protocols. Industrial-grade hydrogen peroxide (>50%) is particularly hazardous and requires specialized handling equipment, protective gear, and monitoring systems. Dilution procedures must follow strict guidelines to prevent exothermic reactions that could lead to dangerous situations.Expand Specific Solutions03 Personal protective equipment and exposure prevention
Appropriate personal protective equipment is crucial when handling hydrogen peroxide to prevent skin contact, inhalation, and eye exposure. This includes chemical-resistant gloves, safety goggles or face shields, protective clothing, and respiratory protection depending on concentration and application. Workplace safety measures should include emergency eyewash stations, safety showers, and proper ventilation systems. Regular training on exposure risks and emergency response procedures helps minimize accidents and injuries.Expand Specific Solutions04 Stabilization techniques for safer hydrogen peroxide formulations
Various stabilization methods can enhance the safety profile of hydrogen peroxide formulations. These include adding stabilizing agents such as phosphates, silicates, or organic compounds that prevent rapid decomposition. pH adjustment and chelating agents help control decomposition rates and prevent catalytic breakdown by metal ions. Stabilized formulations reduce risks associated with pressure buildup, container rupture, and unexpected reactivity while extending shelf life and maintaining efficacy for various applications.Expand Specific Solutions05 Safety considerations in specific applications
Different applications of hydrogen peroxide require tailored safety approaches. In medical and dental settings, lower concentrations are used with specific protocols to prevent tissue damage. Industrial applications like pulp bleaching or wastewater treatment require engineered safety systems including automated monitoring and emergency shutdown capabilities. Laboratory use demands proper fume hoods and small-quantity handling procedures. Consumer products containing hydrogen peroxide feature additional safety measures such as child-resistant packaging and clear usage instructions to prevent misuse.Expand Specific Solutions
Leading Manufacturers and Safety Solution Providers
The hydrogen peroxide safety handling and storage market in electrolyzer plants is in a growth phase, driven by increasing adoption of green hydrogen technologies. The market is expanding as companies focus on safety protocols for this hazardous chemical essential to electrolyzer operations. Leading players like Air Products & Chemicals, Siemens Energy, and Danfoss are developing specialized handling systems, while research institutions such as NASA, Tianjin University, and Harvard College contribute advanced safety protocols. Companies including EDAC Labs, Toshiba Energy Systems, and LG Chem are integrating safety considerations into their electrolyzer designs. The technology is maturing with innovations in automated monitoring systems, specialized storage containers, and emergency response protocols, though standardization across the industry remains in development.
Siemens Energy Global GmbH & Co. KG
Technical Solution: Siemens Energy has developed an integrated H2O2 safety management system for electrolyzer plants that combines advanced materials science with digital monitoring technologies. Their solution features specialized composite storage tanks with multiple containment layers and integrated cooling systems to prevent temperature-induced decomposition. The system employs proprietary stabilization additives that extend H2O2 shelf life while reducing decomposition risks in storage. Siemens' digital twin technology creates virtual models of the entire H2O2 handling system, enabling predictive maintenance and risk assessment through continuous simulation and analysis. Their automated handling system minimizes human contact through robotic transfer mechanisms and closed-loop dispensing systems. The solution integrates with plant-wide safety systems through Siemens' industrial control architecture, providing real-time monitoring and emergency response coordination across the entire facility. Additionally, they've developed specialized venting systems that safely manage oxygen release during normal operations and emergency scenarios.
Strengths: Seamless integration with existing Siemens control systems; advanced digital monitoring capabilities; comprehensive approach combining materials science and automation. Weaknesses: Significant initial capital investment required; potential vendor lock-in with proprietary systems; higher complexity requiring specialized technical expertise for maintenance.
Nippon Shokubai Co., Ltd.
Technical Solution: Nippon Shokubai has developed a specialized H2O2 handling system for electrolyzer plants that focuses on stabilization and decomposition prevention. Their approach utilizes proprietary stabilizers that significantly reduce spontaneous decomposition rates even at higher temperatures encountered in electrolyzer environments. The company has engineered specialized storage vessels with multi-layer construction featuring an inner fluoropolymer lining that prevents catalytic decomposition from metal ion contamination. Their system incorporates advanced temperature control through external cooling jackets that maintain optimal storage conditions between 15-20°C regardless of ambient conditions. Nippon Shokubai's solution includes automated dilution systems that can rapidly reduce H2O2 concentration in emergency situations, coupled with specialized spill containment areas designed to neutralize peroxide through controlled decomposition. Their monitoring system employs multiple redundant sensors measuring concentration, temperature, and pressure with automated alerts and shutdown capabilities. The company has also developed specialized transfer protocols using dedicated pumping systems with materials specifically selected for H2O2 compatibility.
Strengths: Extensive expertise as a major H2O2 manufacturer; specialized knowledge of stabilization chemistry; proven track record in industrial peroxide applications. Weaknesses: Solutions may be optimized for higher concentration H2O2 than typically used in some electrolyzer applications; less integration with broader plant control systems compared to competitors; higher reliance on specialized components that may have limited availability outside Japan.
Critical Safety Systems and Engineering Controls
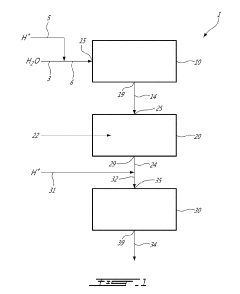
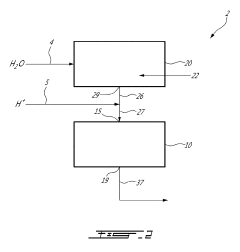
METHOD AND SYSTEM FOR PREPARING A FENTON Fe2+/H2O2 REAGENT
PatentActiveUS20190177195A1
Innovation
- A method and system for in-situ generation of Fenton reagents using an aqueous solution passed through a dispenser containing a hydrogen peroxide generating solid, such as sodium percarbonate, and an iron fibre column, which acidifies the solution to create ferrous ions and hydrogen peroxide, eliminating the need for high-concentration hydrogen peroxide and ferrous sulfate storage and handling.
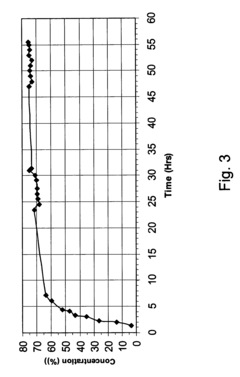
Concentration of hydrogen peroxide
PatentInactiveUS7122166B2
Innovation
- A membrane permeable to water vapor is used to separate water from hydrogen peroxide solutions, allowing water to be removed by a dry gas, thereby increasing the hydrogen peroxide concentration to above 80% by volume at reduced temperatures, using a counter-current continuous process with a selective polymeric membrane.
Regulatory Compliance Framework for H2O2
The regulatory landscape governing hydrogen peroxide (H2O2) handling in electrolyzer plants is complex and multifaceted, spanning international, national, and local frameworks. At the international level, the Globally Harmonized System of Classification and Labelling of Chemicals (GHS) provides standardized hazard classification and communication requirements for H2O2, which is typically classified as an oxidizing liquid and corrosive substance. These classifications trigger specific labeling, safety data sheet, and handling protocols that must be integrated into facility operations.
In the United States, the Occupational Safety and Health Administration (OSHA) regulates H2O2 under the Hazard Communication Standard (29 CFR 1910.1200) and Process Safety Management standards when quantities exceed threshold limits. The Environmental Protection Agency (EPA) oversees environmental aspects through the Resource Conservation and Recovery Act (RCRA) and the Clean Water Act, particularly regarding discharge permits and spill prevention measures. Additionally, the Department of Transportation (DOT) regulates the transportation of H2O2 under 49 CFR, with specific requirements based on concentration levels.
European regulations are particularly stringent, with H2O2 falling under REACH (Registration, Evaluation, Authorization and Restriction of Chemicals) and the CLP Regulation (Classification, Labelling and Packaging). Facilities handling H2O2 must maintain comprehensive chemical safety assessments and implement appropriate risk management measures. The Seveso III Directive applies to larger quantities, requiring additional safety management systems and emergency planning.
Industry-specific standards complement these regulatory frameworks. The National Fire Protection Association (NFPA) provides guidelines for H2O2 storage and handling in NFPA 400 (Hazardous Materials Code). The Compressed Gas Association (CGA) offers detailed technical specifications for H2O2 systems in electrolyzer applications through publications like G-4.4 (Industrial Practices for Gaseous Oxygen Transmission and Distribution Piping Systems).
Compliance documentation requirements are substantial, including detailed inventory records, safety data sheets, risk assessments, standard operating procedures, training records, and incident reports. Electrolyzer plant operators must implement systematic compliance management systems that address permit acquisition, regular auditing, and continuous monitoring of regulatory changes.
The regulatory landscape continues to evolve, with increasing focus on quantitative risk assessment methodologies and integration with broader process safety management systems. Recent regulatory trends indicate movement toward more harmonized international standards and increased emphasis on cybersecurity for automated H2O2 handling systems in modern electrolyzer facilities.
In the United States, the Occupational Safety and Health Administration (OSHA) regulates H2O2 under the Hazard Communication Standard (29 CFR 1910.1200) and Process Safety Management standards when quantities exceed threshold limits. The Environmental Protection Agency (EPA) oversees environmental aspects through the Resource Conservation and Recovery Act (RCRA) and the Clean Water Act, particularly regarding discharge permits and spill prevention measures. Additionally, the Department of Transportation (DOT) regulates the transportation of H2O2 under 49 CFR, with specific requirements based on concentration levels.
European regulations are particularly stringent, with H2O2 falling under REACH (Registration, Evaluation, Authorization and Restriction of Chemicals) and the CLP Regulation (Classification, Labelling and Packaging). Facilities handling H2O2 must maintain comprehensive chemical safety assessments and implement appropriate risk management measures. The Seveso III Directive applies to larger quantities, requiring additional safety management systems and emergency planning.
Industry-specific standards complement these regulatory frameworks. The National Fire Protection Association (NFPA) provides guidelines for H2O2 storage and handling in NFPA 400 (Hazardous Materials Code). The Compressed Gas Association (CGA) offers detailed technical specifications for H2O2 systems in electrolyzer applications through publications like G-4.4 (Industrial Practices for Gaseous Oxygen Transmission and Distribution Piping Systems).
Compliance documentation requirements are substantial, including detailed inventory records, safety data sheets, risk assessments, standard operating procedures, training records, and incident reports. Electrolyzer plant operators must implement systematic compliance management systems that address permit acquisition, regular auditing, and continuous monitoring of regulatory changes.
The regulatory landscape continues to evolve, with increasing focus on quantitative risk assessment methodologies and integration with broader process safety management systems. Recent regulatory trends indicate movement toward more harmonized international standards and increased emphasis on cybersecurity for automated H2O2 handling systems in modern electrolyzer facilities.
Environmental Impact Assessment
The environmental impact of hydrogen peroxide (H2O2) handling and storage in electrolyzer plants requires comprehensive assessment due to its potential ecological implications. H2O2, while considered a more environmentally friendly oxidant compared to chlorine-based alternatives, still presents significant environmental considerations that must be addressed in facility planning and operations.
Water ecosystems face particular vulnerability to H2O2 releases. Even at relatively low concentrations, hydrogen peroxide can be toxic to aquatic organisms, disrupting ecological balances in receiving water bodies. The rapid decomposition of H2O2 into water and oxygen, while generally beneficial, can temporarily deplete dissolved oxygen levels during large-scale releases, potentially causing stress or mortality to aquatic life.
Soil contamination represents another environmental concern, as concentrated H2O2 spills can alter soil chemistry and affect microbial communities essential for ecosystem functioning. The oxidizing properties of hydrogen peroxide can temporarily sterilize affected soil areas, though these effects typically diminish as the compound decomposes.
Atmospheric impacts must also be considered in environmental assessments. While H2O2 itself does not contribute to air pollution in conventional terms, its handling may involve energy-intensive processes that generate indirect emissions. Additionally, emergency venting systems must be designed to prevent the creation of vapor clouds that could affect local air quality or pose risks to nearby sensitive ecosystems.
Waste management protocols for H2O2 storage facilities require particular attention. Dilution procedures for disposing of expired or contaminated hydrogen peroxide must be carefully controlled to prevent ecological damage. Environmental monitoring systems should be implemented around storage areas to detect leaks before they can impact surrounding ecosystems.
The life cycle assessment of H2O2 in electrolyzer plants reveals both benefits and challenges. While hydrogen peroxide eventually decomposes into environmentally benign substances, its production, transportation, and storage all carry environmental footprints that must be quantified and minimized through efficient facility design and operational practices.
Regulatory compliance frameworks across different jurisdictions increasingly emphasize preventative measures rather than reactive responses to environmental incidents. Electrolyzer plants must develop comprehensive environmental management systems that include regular ecological risk assessments, monitoring programs, and emergency response protocols specifically addressing H2O2-related environmental hazards.
Water ecosystems face particular vulnerability to H2O2 releases. Even at relatively low concentrations, hydrogen peroxide can be toxic to aquatic organisms, disrupting ecological balances in receiving water bodies. The rapid decomposition of H2O2 into water and oxygen, while generally beneficial, can temporarily deplete dissolved oxygen levels during large-scale releases, potentially causing stress or mortality to aquatic life.
Soil contamination represents another environmental concern, as concentrated H2O2 spills can alter soil chemistry and affect microbial communities essential for ecosystem functioning. The oxidizing properties of hydrogen peroxide can temporarily sterilize affected soil areas, though these effects typically diminish as the compound decomposes.
Atmospheric impacts must also be considered in environmental assessments. While H2O2 itself does not contribute to air pollution in conventional terms, its handling may involve energy-intensive processes that generate indirect emissions. Additionally, emergency venting systems must be designed to prevent the creation of vapor clouds that could affect local air quality or pose risks to nearby sensitive ecosystems.
Waste management protocols for H2O2 storage facilities require particular attention. Dilution procedures for disposing of expired or contaminated hydrogen peroxide must be carefully controlled to prevent ecological damage. Environmental monitoring systems should be implemented around storage areas to detect leaks before they can impact surrounding ecosystems.
The life cycle assessment of H2O2 in electrolyzer plants reveals both benefits and challenges. While hydrogen peroxide eventually decomposes into environmentally benign substances, its production, transportation, and storage all carry environmental footprints that must be quantified and minimized through efficient facility design and operational practices.
Regulatory compliance frameworks across different jurisdictions increasingly emphasize preventative measures rather than reactive responses to environmental incidents. Electrolyzer plants must develop comprehensive environmental management systems that include regular ecological risk assessments, monitoring programs, and emergency response protocols specifically addressing H2O2-related environmental hazards.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!