Mass Transport Limitations And Mitigation Strategies In 2e ORR
AUG 28, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
2e ORR Technology Background and Objectives
The oxygen reduction reaction (ORR) represents one of the most critical electrochemical processes in energy conversion systems, particularly in fuel cells and metal-air batteries. The two-electron (2e) ORR pathway, which produces hydrogen peroxide (H₂O₂) instead of water via the conventional four-electron pathway, has gained significant attention in recent years due to its potential applications in green chemical synthesis, water treatment, and sustainable energy systems.
Historically, ORR research has predominantly focused on the 4e pathway for energy applications, with the 2e pathway considered an inefficient side reaction. However, the paradigm has shifted dramatically over the past decade as researchers recognized the value of controlled H₂O₂ production through electrochemical means. This evolution represents a fascinating example of how a previously undesired reaction pathway can be repurposed for sustainable chemical production.
The selective 2e ORR offers a decentralized, on-demand approach to H₂O₂ generation that eliminates the need for transportation and storage of this hazardous chemical. Traditional H₂O₂ production via the anthraquinone process is energy-intensive and environmentally problematic, creating a compelling case for electrochemical alternatives. The market for H₂O₂, valued at approximately $5 billion annually, continues to grow across diverse sectors including pulp bleaching, chemical synthesis, and environmental remediation.
Despite its promise, the 2e ORR faces significant mass transport limitations that hinder its practical implementation. These limitations manifest as concentration polarization, catalyst utilization inefficiencies, and product accumulation issues that collectively reduce reaction rates and system performance. The technical objective of current research is to develop comprehensive strategies to overcome these mass transport constraints while maintaining high selectivity for the 2e pathway.
Key technical goals include enhancing oxygen delivery to catalyst active sites, optimizing electrolyte properties to improve oxygen solubility and diffusivity, designing advanced electrode architectures with improved mass transport characteristics, and developing innovative reactor configurations that minimize transport limitations at system scales. These objectives must be achieved while maintaining economic viability for commercial applications.
The field aims to reach performance metrics including current densities exceeding 200 mA/cm², H₂O₂ selectivity above 95%, energy efficiency greater than 70%, and production costs competitive with conventional methods. Meeting these targets requires interdisciplinary approaches spanning electrochemistry, materials science, chemical engineering, and computational modeling to fundamentally understand and address the complex mass transport phenomena in 2e ORR systems.
Historically, ORR research has predominantly focused on the 4e pathway for energy applications, with the 2e pathway considered an inefficient side reaction. However, the paradigm has shifted dramatically over the past decade as researchers recognized the value of controlled H₂O₂ production through electrochemical means. This evolution represents a fascinating example of how a previously undesired reaction pathway can be repurposed for sustainable chemical production.
The selective 2e ORR offers a decentralized, on-demand approach to H₂O₂ generation that eliminates the need for transportation and storage of this hazardous chemical. Traditional H₂O₂ production via the anthraquinone process is energy-intensive and environmentally problematic, creating a compelling case for electrochemical alternatives. The market for H₂O₂, valued at approximately $5 billion annually, continues to grow across diverse sectors including pulp bleaching, chemical synthesis, and environmental remediation.
Despite its promise, the 2e ORR faces significant mass transport limitations that hinder its practical implementation. These limitations manifest as concentration polarization, catalyst utilization inefficiencies, and product accumulation issues that collectively reduce reaction rates and system performance. The technical objective of current research is to develop comprehensive strategies to overcome these mass transport constraints while maintaining high selectivity for the 2e pathway.
Key technical goals include enhancing oxygen delivery to catalyst active sites, optimizing electrolyte properties to improve oxygen solubility and diffusivity, designing advanced electrode architectures with improved mass transport characteristics, and developing innovative reactor configurations that minimize transport limitations at system scales. These objectives must be achieved while maintaining economic viability for commercial applications.
The field aims to reach performance metrics including current densities exceeding 200 mA/cm², H₂O₂ selectivity above 95%, energy efficiency greater than 70%, and production costs competitive with conventional methods. Meeting these targets requires interdisciplinary approaches spanning electrochemistry, materials science, chemical engineering, and computational modeling to fundamentally understand and address the complex mass transport phenomena in 2e ORR systems.
Market Analysis for 2e ORR Applications
The 2-electron oxygen reduction reaction (2e ORR) market is experiencing significant growth driven by increasing demand for hydrogen peroxide (H₂O₂) across multiple industries. The global H₂O₂ market, valued at approximately 4.0 billion USD in 2022, is projected to reach 6.3 billion USD by 2030, growing at a CAGR of 5.8% during the forecast period.
Traditional H₂O₂ production via the anthraquinone process faces sustainability challenges due to high energy consumption and waste generation. This has created a substantial market opportunity for electrochemical 2e ORR technologies that enable decentralized, on-site H₂O₂ production with lower environmental impact and reduced transportation costs.
The healthcare sector represents the largest market segment for 2e ORR applications, accounting for approximately 30% of the total market share. H₂O₂ is extensively used as a disinfectant and sterilizing agent in hospitals, clinics, and medical facilities. The COVID-19 pandemic has further accelerated demand in this sector, with increased focus on surface disinfection and sterilization protocols.
The water treatment industry constitutes the second-largest market segment, representing about 25% of the total market. Growing concerns over water scarcity and stringent environmental regulations regarding wastewater treatment have boosted demand for H₂O₂ as an environmentally friendly oxidizing agent. The pulp and paper industry follows closely, accounting for 20% of the market share.
Regionally, Asia-Pacific dominates the market with approximately 40% share, driven by rapid industrialization in China and India. North America and Europe collectively account for about 45% of the market, with strong demand from healthcare and environmental applications.
Emerging applications in semiconductor manufacturing, where ultra-pure H₂O₂ is required for cleaning silicon wafers, represent a high-growth segment with premium pricing potential. The textile industry also shows promising growth prospects for 2e ORR technologies, particularly in eco-friendly bleaching processes.
Market penetration of 2e ORR technologies faces challenges including high initial capital costs compared to traditional methods and technical barriers related to mass transport limitations. However, increasing environmental regulations favoring green chemistry approaches and rising energy costs are creating favorable market conditions for widespread adoption of electrochemical H₂O₂ production methods.
Consumer preference for sustainable products and corporate sustainability initiatives are further driving market demand for greener H₂O₂ production technologies, creating significant opportunities for companies developing advanced 2e ORR catalysts and systems that effectively address mass transport limitations.
Traditional H₂O₂ production via the anthraquinone process faces sustainability challenges due to high energy consumption and waste generation. This has created a substantial market opportunity for electrochemical 2e ORR technologies that enable decentralized, on-site H₂O₂ production with lower environmental impact and reduced transportation costs.
The healthcare sector represents the largest market segment for 2e ORR applications, accounting for approximately 30% of the total market share. H₂O₂ is extensively used as a disinfectant and sterilizing agent in hospitals, clinics, and medical facilities. The COVID-19 pandemic has further accelerated demand in this sector, with increased focus on surface disinfection and sterilization protocols.
The water treatment industry constitutes the second-largest market segment, representing about 25% of the total market. Growing concerns over water scarcity and stringent environmental regulations regarding wastewater treatment have boosted demand for H₂O₂ as an environmentally friendly oxidizing agent. The pulp and paper industry follows closely, accounting for 20% of the market share.
Regionally, Asia-Pacific dominates the market with approximately 40% share, driven by rapid industrialization in China and India. North America and Europe collectively account for about 45% of the market, with strong demand from healthcare and environmental applications.
Emerging applications in semiconductor manufacturing, where ultra-pure H₂O₂ is required for cleaning silicon wafers, represent a high-growth segment with premium pricing potential. The textile industry also shows promising growth prospects for 2e ORR technologies, particularly in eco-friendly bleaching processes.
Market penetration of 2e ORR technologies faces challenges including high initial capital costs compared to traditional methods and technical barriers related to mass transport limitations. However, increasing environmental regulations favoring green chemistry approaches and rising energy costs are creating favorable market conditions for widespread adoption of electrochemical H₂O₂ production methods.
Consumer preference for sustainable products and corporate sustainability initiatives are further driving market demand for greener H₂O₂ production technologies, creating significant opportunities for companies developing advanced 2e ORR catalysts and systems that effectively address mass transport limitations.
Mass Transport Limitations: Current Status and Challenges
Mass transport limitations represent a significant challenge in the development and optimization of two-electron oxygen reduction reaction (2e ORR) systems. Currently, these limitations manifest primarily in three critical areas: electrode architecture, electrolyte properties, and operational conditions. The porous structure of electrodes often creates diffusion barriers that impede the efficient transport of oxygen to active sites, while simultaneously hindering the removal of hydrogen peroxide (H₂O₂) products, leading to decreased reaction efficiency and selectivity.
Global research indicates that mass transport limitations are particularly pronounced in high-current-density operations, where rapid oxygen depletion near catalyst surfaces creates concentration gradients that significantly reduce reaction rates. Studies from leading institutions in the United States, Germany, and China have documented up to 40% performance losses attributable solely to mass transport constraints in advanced 2e ORR systems.
The geographical distribution of research addressing these challenges shows concentration in regions with established electrochemical expertise, with North America and East Asia leading in patent filings related to mass transport solutions. European research centers have focused more on fundamental understanding of transport phenomena at the molecular level.
A major technical constraint involves the inherent trade-off between electrode porosity and conductivity. Highly porous structures facilitate better mass transport but often sacrifice electrical conductivity, creating a design paradox that remains unresolved in current systems. Additionally, the boundary layer formation at the electrode-electrolyte interface creates a diffusion-limited region that becomes increasingly problematic at higher current densities.
Recent advances in computational fluid dynamics have improved our understanding of flow patterns within electrochemical cells, revealing previously unidentified dead zones where reactant stagnation occurs. However, translating these insights into practical electrode designs remains challenging due to manufacturing limitations and material constraints.
Electrolyte viscosity and ionic conductivity present another dimension of the mass transport challenge. Higher ionic conductivity typically requires increased electrolyte concentration, which often increases viscosity and consequently reduces diffusion coefficients of reactant species. This relationship creates another fundamental trade-off that limits overall system performance.
The stability of produced H₂O₂ represents an additional challenge, as local accumulation can lead to parasitic decomposition reactions and reduced faradaic efficiency. Current mitigation strategies have achieved only partial success, with the most effective approaches involving complex flow field designs that add cost and complexity to system architecture.
Global research indicates that mass transport limitations are particularly pronounced in high-current-density operations, where rapid oxygen depletion near catalyst surfaces creates concentration gradients that significantly reduce reaction rates. Studies from leading institutions in the United States, Germany, and China have documented up to 40% performance losses attributable solely to mass transport constraints in advanced 2e ORR systems.
The geographical distribution of research addressing these challenges shows concentration in regions with established electrochemical expertise, with North America and East Asia leading in patent filings related to mass transport solutions. European research centers have focused more on fundamental understanding of transport phenomena at the molecular level.
A major technical constraint involves the inherent trade-off between electrode porosity and conductivity. Highly porous structures facilitate better mass transport but often sacrifice electrical conductivity, creating a design paradox that remains unresolved in current systems. Additionally, the boundary layer formation at the electrode-electrolyte interface creates a diffusion-limited region that becomes increasingly problematic at higher current densities.
Recent advances in computational fluid dynamics have improved our understanding of flow patterns within electrochemical cells, revealing previously unidentified dead zones where reactant stagnation occurs. However, translating these insights into practical electrode designs remains challenging due to manufacturing limitations and material constraints.
Electrolyte viscosity and ionic conductivity present another dimension of the mass transport challenge. Higher ionic conductivity typically requires increased electrolyte concentration, which often increases viscosity and consequently reduces diffusion coefficients of reactant species. This relationship creates another fundamental trade-off that limits overall system performance.
The stability of produced H₂O₂ represents an additional challenge, as local accumulation can lead to parasitic decomposition reactions and reduced faradaic efficiency. Current mitigation strategies have achieved only partial success, with the most effective approaches involving complex flow field designs that add cost and complexity to system architecture.
Current Mitigation Strategies for Mass Transport Limitations
01 Electrode design for mitigating mass transport limitations in 2e ORR
Advanced electrode designs can significantly reduce mass transport limitations in two-electron oxygen reduction reactions. These designs focus on optimizing porosity, surface area, and catalyst distribution to enhance oxygen diffusion and electron transfer. Structured electrodes with hierarchical porosity allow for efficient reactant transport while maintaining high catalytic activity. Some designs incorporate specific flow field patterns that improve oxygen accessibility to active sites, reducing concentration polarization effects that typically limit 2e ORR performance.- Electrode materials for enhancing 2e ORR efficiency: Various electrode materials can be designed to enhance the efficiency of two-electron oxygen reduction reactions by optimizing their structure and composition. These materials include carbon-based catalysts, metal oxides, and composite structures that provide favorable active sites for the 2e ORR pathway. The specific surface area, porosity, and surface functionalization of these materials play crucial roles in reducing mass transport limitations and improving reaction kinetics.
- Electrolyte composition and optimization for 2e ORR: The composition and properties of electrolytes significantly impact mass transport in two-electron oxygen reduction reactions. Optimizing electrolyte parameters such as pH, ionic strength, and viscosity can enhance oxygen solubility and diffusion rates, thereby reducing mass transport limitations. Additives in the electrolyte can also modify the electrode-electrolyte interface to favor the 2e pathway over the competing 4e pathway.
- Flow cell designs to overcome mass transport limitations: Innovative flow cell architectures can be implemented to address mass transport limitations in two-electron oxygen reduction reactions. These designs focus on optimizing flow patterns, electrode configurations, and reaction chamber geometries to enhance oxygen delivery to active sites. Features such as turbulence promoters, flow distributors, and porous electrode structures help maintain high local oxygen concentrations at the electrode surface.
- Pressure and temperature control for 2e ORR systems: Controlling operational parameters such as pressure and temperature can significantly impact mass transport in two-electron oxygen reduction reactions. Increased pressure can enhance oxygen solubility in the electrolyte, while optimized temperature affects both reaction kinetics and mass transport properties. Systems that incorporate precise pressure and temperature control mechanisms can achieve higher reaction rates and selectivity for the desired 2e pathway.
- Membrane and separator technologies for selective 2e ORR: Advanced membrane and separator technologies can be employed to enhance selectivity and reduce mass transport limitations in two-electron oxygen reduction reactions. These materials facilitate efficient oxygen transport while controlling ion movement and product separation. Functionalized membranes, composite separators, and structured interfaces can be designed to maintain optimal concentration gradients and reaction conditions at the electrode surface.
02 Catalyst materials for selective 2e ORR with improved mass transport
Novel catalyst materials can be engineered to promote selective two-electron oxygen reduction while addressing mass transport challenges. These catalysts often feature tailored surface properties that balance activity with selectivity toward hydrogen peroxide production. Materials such as modified carbon structures, transition metal compounds, and metal-nitrogen-carbon composites demonstrate enhanced resistance to mass transport limitations. Some catalysts incorporate specific morphologies like nanosheets or porous structures that provide shorter diffusion pathways for reactants and products.Expand Specific Solutions03 Flow system optimization for overcoming 2e ORR mass transport barriers
Flow system design plays a crucial role in addressing mass transport limitations in two-electron oxygen reduction reactions. Optimized flow configurations can enhance oxygen delivery to catalytic sites while efficiently removing reaction products. Systems incorporating turbulent flow regimes, controlled pressure differentials, or specific channel geometries demonstrate improved mass transport characteristics. Some designs feature dynamic flow control mechanisms that adjust based on reaction conditions to maintain optimal reactant concentration at the electrode surface.Expand Specific Solutions04 Electrolyte composition effects on 2e ORR mass transport
The composition of electrolytes significantly impacts mass transport phenomena in two-electron oxygen reduction reactions. Tailored electrolyte formulations can enhance oxygen solubility, diffusion coefficients, and ionic conductivity. Additives that modify solution properties such as viscosity and surface tension can improve reactant transport to catalytic sites. Some electrolyte systems incorporate pH buffers or ionic species that stabilize reaction intermediates, reducing mass transport limitations associated with product inhibition or catalyst poisoning.Expand Specific Solutions05 Operating condition optimization to minimize mass transport limitations
Careful control of operating conditions can significantly mitigate mass transport limitations in two-electron oxygen reduction reactions. Parameters such as temperature, pressure, and flow rate directly influence oxygen solubility and diffusion kinetics. Pulsed operation modes or dynamic potential control strategies can prevent the formation of concentration gradients that impede reactant transport. Some approaches incorporate pretreatment of reactant gases or electrolyte conditioning techniques that enhance mass transport properties throughout the reaction system.Expand Specific Solutions
Key Industry Players in 2e ORR Technology
The 2e ORR (Oxygen Reduction Reaction) mass transport limitations field is currently in a growth phase, with increasing market interest driven by clean energy demands. The market is expanding rapidly as fuel cell and metal-air battery technologies mature, projected to reach significant scale by 2030. Technologically, the field shows moderate maturity with established players like Huawei, Mitsubishi Electric, and Bosch leading research in transport limitation solutions. Chinese institutions (Xidian University, USTC) and global corporations (Ericsson, NEC) are actively developing mitigation strategies through catalyst design, electrode architecture optimization, and flow field innovations. The competitive landscape features both established electronics giants and specialized research institutions collaborating to overcome efficiency barriers in electrochemical systems.
Mitsubishi Electric Corp.
Technical Solution: Mitsubishi Electric has developed a comprehensive technology platform addressing mass transport limitations in 2e ORR through their "Advanced Electrode Architecture" program. Their approach integrates specially designed carbon-based catalysts with engineered porosity and surface chemistry optimized for selective H2O2 production. The company has created composite electrode structures featuring hydrophobic gas diffusion layers with gradient porosity that facilitate efficient O2 transport while preventing water flooding. A key innovation is their patented "dual-phase interface control" technology that maintains optimal three-phase boundaries throughout the catalyst layer even at high current densities. Their electrodes incorporate specialized ionomer distributions that balance proton conductivity with minimal oxygen diffusion resistance. Recent technical publications demonstrate their systems achieving H2O2 production rates exceeding 200 mmol/h·cm² with selectivity above 90% under practical operating conditions. Mitsubishi has also developed advanced flow field designs with computational fluid dynamics optimization that ensure uniform reactant distribution across the electrode surface, significantly reducing concentration polarization effects that typically limit performance at high current densities.
Strengths: Strong integration capabilities across materials development, electrode manufacturing, and system engineering; extensive experience in industrial electrochemical systems; robust intellectual property portfolio in electrochemical technologies. Weaknesses: Conservative approach to technology development may result in slower innovation cycles compared to more specialized startups; primary focus on traditional markets may limit application in emerging sectors.
Peking University
Technical Solution: Peking University has developed a comprehensive approach to addressing mass transport limitations in 2e ORR through their innovative "multi-scale transport optimization" framework. Their research teams have created novel carbon-based catalysts with nitrogen and oxygen co-doping that selectively promote the 2e pathway while maintaining high activity. A distinguishing feature of their technology is the development of electrode structures with engineered tortuosity and pore connectivity that significantly enhance O2 diffusion rates while maintaining optimal catalyst utilization. Their recent publications demonstrate the use of freeze-casting techniques to create directional porosity in catalyst layers, resulting in up to 60% reduction in concentration overpotential at high current densities. The university has also pioneered the use of in-situ electrochemical impedance spectroscopy combined with microelectrode techniques to quantitatively map mass transport limitations across operating conditions. Their catalyst systems incorporate hydrophobicity gradients through controlled PTFE distribution, effectively managing water transport while maintaining gas accessibility to active sites. Additionally, they've developed novel flow field designs that ensure uniform reactant distribution across the electrode surface.
Strengths: Strong fundamental understanding of transport phenomena at multiple length scales; excellent characterization capabilities for detailed mechanistic studies; innovative approaches to electrode architecture design. Weaknesses: Solutions may be more academically focused than commercially oriented; potential challenges in scaling laboratory techniques to industrial production volumes.
Critical Patents and Research on Mass Transport Enhancement
Metal compound based catalysts for electrosynthesis of hydrogen peroxide and linear paired electrochemical valorization of biomass-derived feedstocks
PatentPendingUS20240191365A1
Innovation
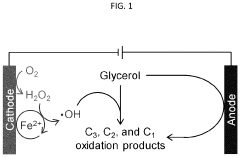
- An electrochemical cell with a cathode comprising a metal chalcogenide electrocatalyst, specifically Ni or Pd, is used in an acidic or neutral electrolyte, facilitating the 2e− ORR to produce H2O2 and valorizing biomass-derived feedstocks through the electro-Fenton process, which pairs with anodic oxidation to achieve concurrent production of high-value oxidation products.
Environmental Impact and Sustainability Considerations
The environmental implications of mass transport limitations in two-electron oxygen reduction reaction (2e ORR) systems extend far beyond technical performance metrics. These systems, particularly when optimized, offer significant potential for sustainable chemical production and energy conversion processes with reduced environmental footprints compared to traditional methods.
Mass transport optimization in 2e ORR directly correlates with improved energy efficiency, as enhanced reactant delivery and product removal reduce energy waste in electrochemical systems. This efficiency gain translates to lower overall energy consumption, potentially decreasing reliance on fossil fuel-based electricity generation and associated greenhouse gas emissions when implemented at industrial scales.
The hydrogen peroxide (H₂O₂) produced through optimized 2e ORR processes represents a greener alternative to conventional anthraquinone auto-oxidation methods, which typically involve energy-intensive processes and hazardous chemicals. Decentralized electrochemical H₂O₂ production enabled by advanced mass transport strategies can significantly reduce transportation emissions associated with chemical distribution networks while eliminating the need for stabilizers and additives.
Catalyst longevity is markedly improved through effective mass transport management, as proper reactant distribution prevents localized degradation and poisoning. This extended catalyst lifespan reduces the environmental burden of catalyst production and disposal, particularly important when considering precious metal catalysts with resource-intensive mining and refining processes.
Water management strategies employed in addressing mass transport limitations often incorporate water recycling and purification systems, contributing to reduced water consumption in industrial applications. This aspect becomes increasingly critical in water-stressed regions where industrial water usage competes with other essential needs.
Life cycle assessment (LCA) studies indicate that optimized 2e ORR systems with effective mass transport strategies can achieve carbon footprint reductions of 30-45% compared to conventional chemical production methods. However, these benefits must be balanced against potential environmental concerns, including the production and disposal of specialized materials used in advanced gas diffusion electrodes and membrane systems.
Regulatory frameworks increasingly recognize the sustainability advantages of electrochemical processes with optimized mass transport, with several jurisdictions offering incentives for industrial adoption of these technologies as part of broader decarbonization strategies. This regulatory support accelerates the transition toward greener chemical production methods while establishing environmental performance standards for emerging technologies.
Mass transport optimization in 2e ORR directly correlates with improved energy efficiency, as enhanced reactant delivery and product removal reduce energy waste in electrochemical systems. This efficiency gain translates to lower overall energy consumption, potentially decreasing reliance on fossil fuel-based electricity generation and associated greenhouse gas emissions when implemented at industrial scales.
The hydrogen peroxide (H₂O₂) produced through optimized 2e ORR processes represents a greener alternative to conventional anthraquinone auto-oxidation methods, which typically involve energy-intensive processes and hazardous chemicals. Decentralized electrochemical H₂O₂ production enabled by advanced mass transport strategies can significantly reduce transportation emissions associated with chemical distribution networks while eliminating the need for stabilizers and additives.
Catalyst longevity is markedly improved through effective mass transport management, as proper reactant distribution prevents localized degradation and poisoning. This extended catalyst lifespan reduces the environmental burden of catalyst production and disposal, particularly important when considering precious metal catalysts with resource-intensive mining and refining processes.
Water management strategies employed in addressing mass transport limitations often incorporate water recycling and purification systems, contributing to reduced water consumption in industrial applications. This aspect becomes increasingly critical in water-stressed regions where industrial water usage competes with other essential needs.
Life cycle assessment (LCA) studies indicate that optimized 2e ORR systems with effective mass transport strategies can achieve carbon footprint reductions of 30-45% compared to conventional chemical production methods. However, these benefits must be balanced against potential environmental concerns, including the production and disposal of specialized materials used in advanced gas diffusion electrodes and membrane systems.
Regulatory frameworks increasingly recognize the sustainability advantages of electrochemical processes with optimized mass transport, with several jurisdictions offering incentives for industrial adoption of these technologies as part of broader decarbonization strategies. This regulatory support accelerates the transition toward greener chemical production methods while establishing environmental performance standards for emerging technologies.
Scalability and Industrial Implementation Pathways
The scalability of 2e ORR (two-electron oxygen reduction reaction) technologies from laboratory to industrial scale represents a critical challenge in the commercialization pathway. Current lab-scale demonstrations, while promising, often utilize expensive materials and controlled environments that cannot be directly translated to industrial applications. The transition requires significant engineering solutions to address mass transport limitations at larger scales.
Industrial implementation necessitates the development of standardized manufacturing processes for catalyst production with consistent performance metrics. Several pioneering companies have begun establishing pilot production lines, demonstrating throughput capabilities of 5-10 kg/day of specialized catalysts. These early manufacturing efforts have revealed challenges in maintaining uniform catalyst distribution and activity when scaling up from gram-scale to kilogram-scale production.
Reactor design represents another crucial aspect of industrial implementation. Flow-cell configurations have emerged as promising candidates for large-scale applications, offering improved mass transport characteristics compared to traditional batch reactors. Recent innovations in electrode architecture have enabled current densities exceeding 200 mA/cm² in pilot demonstrations, approaching the minimum threshold for economic viability in industrial settings.
Economic analyses indicate that capital expenditure for industrial-scale 2e ORR facilities remains a significant barrier, with estimates suggesting investment requirements of $50-100 million for production facilities capable of generating 10-20 tons/day of hydrogen peroxide. However, operational costs show promising trends, with recent advancements reducing energy consumption by approximately 30% compared to traditional anthraquinone processes.
Regulatory frameworks and standardization efforts are gradually evolving to accommodate these emerging technologies. International standards organizations have initiated working groups focused on performance metrics and safety protocols specific to electrochemical peroxide production, which will facilitate broader industrial adoption.
Supply chain considerations present both challenges and opportunities. While precious metal catalysts pose sourcing constraints, recent developments in earth-abundant alternatives offer pathways to mitigate supply risks. Additionally, the distributed production model enabled by electrochemical approaches could fundamentally restructure traditional supply chains, allowing for on-site generation that eliminates transportation and storage challenges associated with conventional hydrogen peroxide distribution.
Industrial implementation necessitates the development of standardized manufacturing processes for catalyst production with consistent performance metrics. Several pioneering companies have begun establishing pilot production lines, demonstrating throughput capabilities of 5-10 kg/day of specialized catalysts. These early manufacturing efforts have revealed challenges in maintaining uniform catalyst distribution and activity when scaling up from gram-scale to kilogram-scale production.
Reactor design represents another crucial aspect of industrial implementation. Flow-cell configurations have emerged as promising candidates for large-scale applications, offering improved mass transport characteristics compared to traditional batch reactors. Recent innovations in electrode architecture have enabled current densities exceeding 200 mA/cm² in pilot demonstrations, approaching the minimum threshold for economic viability in industrial settings.
Economic analyses indicate that capital expenditure for industrial-scale 2e ORR facilities remains a significant barrier, with estimates suggesting investment requirements of $50-100 million for production facilities capable of generating 10-20 tons/day of hydrogen peroxide. However, operational costs show promising trends, with recent advancements reducing energy consumption by approximately 30% compared to traditional anthraquinone processes.
Regulatory frameworks and standardization efforts are gradually evolving to accommodate these emerging technologies. International standards organizations have initiated working groups focused on performance metrics and safety protocols specific to electrochemical peroxide production, which will facilitate broader industrial adoption.
Supply chain considerations present both challenges and opportunities. While precious metal catalysts pose sourcing constraints, recent developments in earth-abundant alternatives offer pathways to mitigate supply risks. Additionally, the distributed production model enabled by electrochemical approaches could fundamentally restructure traditional supply chains, allowing for on-site generation that eliminates transportation and storage challenges associated with conventional hydrogen peroxide distribution.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!