Flow Cell Design Considerations For Industrial H2O2 Electrosynthesis
AUG 28, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
H2O2 Electrosynthesis Background and Objectives
Hydrogen peroxide (H2O2) has emerged as a versatile and environmentally benign oxidizing agent with applications spanning across multiple industries including pulp and paper bleaching, wastewater treatment, chemical synthesis, and medical disinfection. Traditionally, H2O2 production has relied on the anthraquinone auto-oxidation process, which despite its industrial maturity, presents significant drawbacks including high energy consumption, substantial waste generation, and centralized production necessitating hazardous transportation.
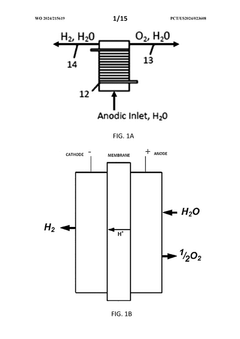
Electrochemical synthesis of H2O2 has gained considerable attention as a promising alternative due to its potential for on-site generation, reduced environmental impact, and integration with renewable energy sources. The electrochemical approach primarily involves the two-electron oxygen reduction reaction (2e- ORR) or water oxidation pathways, offering direct conversion without the need for complex chemical intermediates or catalysts used in conventional methods.
The evolution of H2O2 electrosynthesis technology can be traced back to the early 20th century, but significant advancements have only materialized in the past two decades with the development of novel electrode materials, improved understanding of reaction mechanisms, and innovative cell designs. Recent breakthroughs in selective catalysts and membrane technology have further accelerated progress in this field.
Despite these advances, industrial-scale implementation of electrochemical H2O2 production faces substantial challenges related to production rates, energy efficiency, and system stability. Current laboratory-scale demonstrations typically achieve H2O2 concentrations below 1 wt%, whereas industrial applications often require concentrations of 3-8 wt% or higher, highlighting the significant gap between research achievements and industrial requirements.
The flow cell design represents a critical component in bridging this gap, as it directly influences mass transport phenomena, reaction kinetics, current distribution, and ultimately the production efficiency and economic viability of the process. Optimizing flow cell architecture requires balancing multiple parameters including electrode configuration, electrolyte flow patterns, pressure distribution, and heat management.
This technical research report aims to comprehensively examine the design considerations for industrial-scale H2O2 electrosynthesis flow cells, with specific objectives including: identifying critical design parameters that influence performance metrics such as production rate, energy efficiency, and stability; evaluating existing flow cell architectures and their suitability for scale-up; analyzing the interplay between cell design and catalyst performance; and proposing innovative design strategies to overcome current limitations in achieving commercially viable electrochemical H2O2 production systems.
Electrochemical synthesis of H2O2 has gained considerable attention as a promising alternative due to its potential for on-site generation, reduced environmental impact, and integration with renewable energy sources. The electrochemical approach primarily involves the two-electron oxygen reduction reaction (2e- ORR) or water oxidation pathways, offering direct conversion without the need for complex chemical intermediates or catalysts used in conventional methods.
The evolution of H2O2 electrosynthesis technology can be traced back to the early 20th century, but significant advancements have only materialized in the past two decades with the development of novel electrode materials, improved understanding of reaction mechanisms, and innovative cell designs. Recent breakthroughs in selective catalysts and membrane technology have further accelerated progress in this field.
Despite these advances, industrial-scale implementation of electrochemical H2O2 production faces substantial challenges related to production rates, energy efficiency, and system stability. Current laboratory-scale demonstrations typically achieve H2O2 concentrations below 1 wt%, whereas industrial applications often require concentrations of 3-8 wt% or higher, highlighting the significant gap between research achievements and industrial requirements.
The flow cell design represents a critical component in bridging this gap, as it directly influences mass transport phenomena, reaction kinetics, current distribution, and ultimately the production efficiency and economic viability of the process. Optimizing flow cell architecture requires balancing multiple parameters including electrode configuration, electrolyte flow patterns, pressure distribution, and heat management.
This technical research report aims to comprehensively examine the design considerations for industrial-scale H2O2 electrosynthesis flow cells, with specific objectives including: identifying critical design parameters that influence performance metrics such as production rate, energy efficiency, and stability; evaluating existing flow cell architectures and their suitability for scale-up; analyzing the interplay between cell design and catalyst performance; and proposing innovative design strategies to overcome current limitations in achieving commercially viable electrochemical H2O2 production systems.
Market Analysis for Industrial H2O2 Production
The global hydrogen peroxide (H2O2) market has been experiencing steady growth, driven primarily by its versatile applications across multiple industries. As of recent market analyses, the global H2O2 market is valued at approximately 3.5 billion USD, with projections indicating growth to reach 5.2 billion USD by 2027, representing a compound annual growth rate (CAGR) of 5.8%.
The industrial production of H2O2 serves diverse sectors, with pulp and paper bleaching accounting for the largest market share at 35%, followed by chemical synthesis (25%), wastewater treatment (15%), mining applications (10%), and textile industry (8%). The remaining percentage is distributed among various applications including electronics manufacturing, food processing, and healthcare.
Regionally, Asia-Pacific dominates the H2O2 market with over 40% share, attributed to rapid industrialization in China and India. North America and Europe follow with approximately 25% and 20% market shares respectively, while Latin America and Middle East & Africa constitute the remaining portion.
Traditional H2O2 production methods, particularly the anthraquinone auto-oxidation (AO) process, have dominated the market for decades. However, this process faces significant challenges including high energy consumption, substantial capital investment requirements, and environmental concerns related to organic solvents usage. These limitations have created a growing demand for alternative production methods, with electrochemical synthesis emerging as a promising approach.
The market for electrochemical H2O2 production systems is currently in its nascent stage but demonstrates significant growth potential. Early adopters are primarily found in specialized chemical manufacturing, water treatment facilities requiring on-site H2O2 generation, and research institutions. The decentralized production capability of electrochemical systems addresses logistical challenges associated with H2O2 transportation and storage.
Key market drivers for flow cell-based H2O2 electrosynthesis include increasing environmental regulations favoring greener production methods, rising demand for on-site generation capabilities, and the potential for cost reduction in small to medium-scale applications. Additionally, the integration of renewable energy sources with electrochemical production systems aligns with global sustainability initiatives, further enhancing market appeal.
Market barriers include technological maturity concerns, competition from established production methods with economies of scale advantages, and initial capital investment requirements for new infrastructure. Despite these challenges, the market trajectory indicates growing acceptance of electrochemical H2O2 production technologies, particularly as flow cell designs continue to improve in efficiency and scalability.
The industrial production of H2O2 serves diverse sectors, with pulp and paper bleaching accounting for the largest market share at 35%, followed by chemical synthesis (25%), wastewater treatment (15%), mining applications (10%), and textile industry (8%). The remaining percentage is distributed among various applications including electronics manufacturing, food processing, and healthcare.
Regionally, Asia-Pacific dominates the H2O2 market with over 40% share, attributed to rapid industrialization in China and India. North America and Europe follow with approximately 25% and 20% market shares respectively, while Latin America and Middle East & Africa constitute the remaining portion.
Traditional H2O2 production methods, particularly the anthraquinone auto-oxidation (AO) process, have dominated the market for decades. However, this process faces significant challenges including high energy consumption, substantial capital investment requirements, and environmental concerns related to organic solvents usage. These limitations have created a growing demand for alternative production methods, with electrochemical synthesis emerging as a promising approach.
The market for electrochemical H2O2 production systems is currently in its nascent stage but demonstrates significant growth potential. Early adopters are primarily found in specialized chemical manufacturing, water treatment facilities requiring on-site H2O2 generation, and research institutions. The decentralized production capability of electrochemical systems addresses logistical challenges associated with H2O2 transportation and storage.
Key market drivers for flow cell-based H2O2 electrosynthesis include increasing environmental regulations favoring greener production methods, rising demand for on-site generation capabilities, and the potential for cost reduction in small to medium-scale applications. Additionally, the integration of renewable energy sources with electrochemical production systems aligns with global sustainability initiatives, further enhancing market appeal.
Market barriers include technological maturity concerns, competition from established production methods with economies of scale advantages, and initial capital investment requirements for new infrastructure. Despite these challenges, the market trajectory indicates growing acceptance of electrochemical H2O2 production technologies, particularly as flow cell designs continue to improve in efficiency and scalability.
Current Flow Cell Technologies and Challenges
The current landscape of flow cell technologies for hydrogen peroxide electrosynthesis presents a complex array of designs with varying degrees of efficiency and industrial applicability. Traditional flow cells typically employ parallel plate configurations with electrodes separated by ion-exchange membranes. These designs have demonstrated moderate success in laboratory settings but face significant challenges when scaled to industrial production levels.
A major limitation in existing flow cell designs is the trade-off between mass transport and energy efficiency. Conventional parallel plate reactors often suffer from concentration polarization near electrode surfaces, resulting in reduced reaction rates and increased energy consumption. This phenomenon becomes particularly problematic at higher current densities required for industrial-scale production.
Membrane-electrode assembly (MEA) configurations have emerged as a promising alternative, offering improved mass transport characteristics by minimizing the gap between electrodes. However, these systems frequently encounter issues related to membrane fouling and degradation when exposed to the harsh oxidative environment of H2O2 production. The limited lifetime of membranes represents a significant economic barrier to widespread industrial adoption.
Gas diffusion electrodes (GDEs) incorporated into flow cell designs have shown potential for enhancing oxygen reduction reaction (ORR) kinetics—a critical pathway for H2O2 synthesis. These electrodes facilitate the three-phase boundary necessary for efficient oxygen reduction but present challenges in maintaining consistent wetting properties and preventing flooding during extended operation.
Microfluidic flow cells have gained attention for their precise control over reaction conditions and enhanced mass transfer capabilities. Their high surface-area-to-volume ratio theoretically allows for improved reaction kinetics. However, the inherent complexity of fabrication and difficulties in scaling these systems to industrial throughput levels have limited their practical application beyond laboratory demonstrations.
From a materials perspective, current flow cell technologies struggle with electrode stability. Carbon-based electrodes offer good selectivity but suffer from oxidative degradation, while metal-based alternatives may provide better durability but often at the cost of reduced selectivity toward H2O2 formation over competing water oxidation reactions.
Hydrodynamic challenges persist across all flow cell designs, with issues of flow distribution, pressure drop, and bubble management significantly impacting performance. Uneven flow distribution leads to inefficient utilization of electrode surface area, while gas evolution during electrolysis can disrupt flow patterns and increase electrical resistance within the cell.
The integration of advanced monitoring and control systems remains underdeveloped in current industrial flow cells, limiting the ability to maintain optimal operating conditions in response to changing process parameters. This technological gap represents a significant barrier to achieving consistent, high-efficiency H2O2 production at industrial scales.
A major limitation in existing flow cell designs is the trade-off between mass transport and energy efficiency. Conventional parallel plate reactors often suffer from concentration polarization near electrode surfaces, resulting in reduced reaction rates and increased energy consumption. This phenomenon becomes particularly problematic at higher current densities required for industrial-scale production.
Membrane-electrode assembly (MEA) configurations have emerged as a promising alternative, offering improved mass transport characteristics by minimizing the gap between electrodes. However, these systems frequently encounter issues related to membrane fouling and degradation when exposed to the harsh oxidative environment of H2O2 production. The limited lifetime of membranes represents a significant economic barrier to widespread industrial adoption.
Gas diffusion electrodes (GDEs) incorporated into flow cell designs have shown potential for enhancing oxygen reduction reaction (ORR) kinetics—a critical pathway for H2O2 synthesis. These electrodes facilitate the three-phase boundary necessary for efficient oxygen reduction but present challenges in maintaining consistent wetting properties and preventing flooding during extended operation.
Microfluidic flow cells have gained attention for their precise control over reaction conditions and enhanced mass transfer capabilities. Their high surface-area-to-volume ratio theoretically allows for improved reaction kinetics. However, the inherent complexity of fabrication and difficulties in scaling these systems to industrial throughput levels have limited their practical application beyond laboratory demonstrations.
From a materials perspective, current flow cell technologies struggle with electrode stability. Carbon-based electrodes offer good selectivity but suffer from oxidative degradation, while metal-based alternatives may provide better durability but often at the cost of reduced selectivity toward H2O2 formation over competing water oxidation reactions.
Hydrodynamic challenges persist across all flow cell designs, with issues of flow distribution, pressure drop, and bubble management significantly impacting performance. Uneven flow distribution leads to inefficient utilization of electrode surface area, while gas evolution during electrolysis can disrupt flow patterns and increase electrical resistance within the cell.
The integration of advanced monitoring and control systems remains underdeveloped in current industrial flow cells, limiting the ability to maintain optimal operating conditions in response to changing process parameters. This technological gap represents a significant barrier to achieving consistent, high-efficiency H2O2 production at industrial scales.
Flow Cell Design Solutions for H2O2 Synthesis
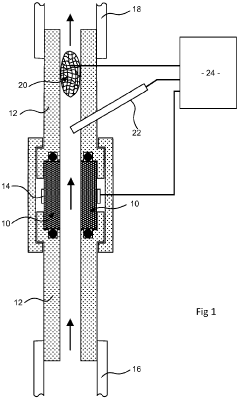
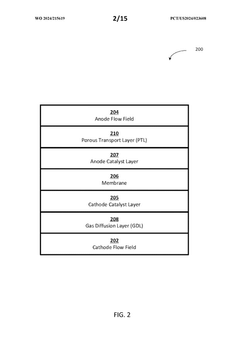
01 Electrode and Membrane Configuration
Flow cell design considerations include the arrangement and configuration of electrodes and membranes to optimize performance. This involves the strategic placement of electrodes, selection of appropriate membrane materials, and design of the interface between these components. Proper configuration enhances ion transport, reduces resistance, and improves overall efficiency of the flow cell system.- Electrode and Membrane Configuration in Flow Cells: The design of electrodes and membranes is critical in flow cell performance. Considerations include electrode material selection, membrane permeability, and their spatial arrangement within the cell. Proper configuration enhances ion transport, reduces internal resistance, and improves overall electrochemical efficiency. Advanced designs incorporate specialized electrode geometries and membrane compositions to optimize reactant flow and product separation.
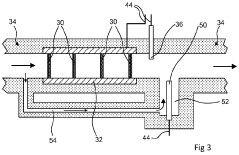
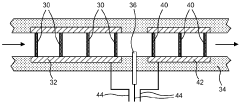
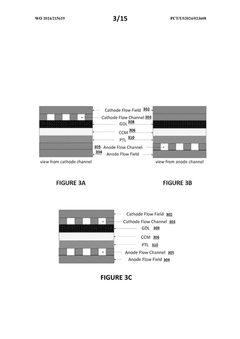
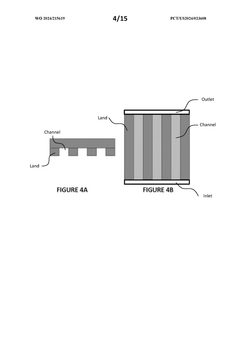
- Flow Distribution and Channel Design: Effective flow distribution within cells is achieved through optimized channel designs that ensure uniform reactant delivery and product removal. Key considerations include channel width, depth, pattern configurations, and the use of flow distributors. These design elements minimize pressure drops, prevent dead zones, and reduce concentration gradients, resulting in improved mass transfer and overall cell performance.
- Thermal Management Systems: Thermal management is essential in flow cell design to maintain optimal operating temperatures and prevent performance degradation. Design considerations include cooling channels, heat exchangers, and thermal insulation materials. Advanced systems incorporate temperature sensors and control mechanisms to regulate heat distribution, particularly important in high-power applications where heat generation can significantly impact efficiency and cell lifespan.
- Computational Modeling for Flow Cell Optimization: Computational fluid dynamics and electrochemical modeling are increasingly used to optimize flow cell designs before physical prototyping. These simulation approaches help predict flow patterns, reaction kinetics, and performance under various operating conditions. Advanced modeling techniques incorporate multiphysics simulations that account for fluid dynamics, electrochemistry, and heat transfer simultaneously, enabling more efficient design iterations and performance optimization.
- Scaling and Manufacturing Considerations: Scaling flow cell designs from laboratory to industrial applications requires considerations of manufacturability, cost-effectiveness, and reliability. Design elements include modular components, standardized connections, and materials selection for durability and mass production. Advanced approaches incorporate design for assembly principles, reducing component count and simplifying maintenance while maintaining performance characteristics across different scales of operation.
02 Flow Channel Geometry and Fluid Dynamics
The geometry of flow channels significantly impacts the performance of flow cells. Design considerations include channel width, depth, and shape to ensure uniform flow distribution, minimize pressure drop, and prevent dead zones. Computational fluid dynamics modeling helps optimize these parameters to enhance mass transfer, reduce concentration polarization, and improve overall system efficiency.Expand Specific Solutions03 Thermal Management Systems
Effective thermal management is crucial in flow cell design to maintain optimal operating temperatures and prevent overheating. This includes incorporating cooling channels, heat exchangers, or thermal insulation materials. Proper thermal design ensures consistent performance, extends component lifespan, and enhances safety, particularly in high-power applications where heat generation can be substantial.Expand Specific Solutions04 Sealing and Pressure Distribution
Sealing mechanisms and pressure distribution are critical aspects of flow cell design to prevent leakage and ensure uniform compression. This includes gasket selection, compression systems, and structural support elements. Proper sealing design maintains system integrity under varying operating conditions, prevents cross-contamination between channels, and ensures consistent performance throughout the cell's operational lifetime.Expand Specific Solutions05 Modular and Scalable Architectures
Modern flow cell designs often incorporate modular and scalable architectures to facilitate maintenance, allow for capacity expansion, and enable system customization. This approach includes standardized components, interconnection systems, and stackable units. Modular designs reduce manufacturing complexity, simplify troubleshooting, and provide flexibility to adapt the system to different applications or power requirements.Expand Specific Solutions
Leading Companies in Electrochemical H2O2 Production
The hydrogen peroxide (H2O2) electrosynthesis market is currently in a growth phase, with increasing industrial applications driving demand for more efficient flow cell designs. The market size is expanding as H2O2 gains prominence as a green oxidant in various industries, estimated to reach several billion dollars globally. From a technical maturity perspective, the field shows varied development levels across key players. Research institutions like Technical University of Denmark, Tohoku University, and University of Delaware are advancing fundamental innovations, while industrial entities including Shimadzu Corp., Air Liquide SA, and Solvay SA are focusing on commercial applications. The Korea Institute of Energy Research and McPhy Energy are developing specialized electrolysis technologies, positioning themselves at the intersection of academic research and industrial implementation in this evolving clean technology sector.
Centre National de la Recherche Scientifique
Technical Solution: CNRS has pioneered innovative flow cell designs for H2O2 electrosynthesis focusing on sustainable catalytic materials and reactor engineering. Their approach utilizes a modular flow cell architecture with carbon-based nanostructured electrodes modified with metal-free nitrogen-doped carbon catalysts that demonstrate high selectivity for the 2-electron oxygen reduction pathway. The flow cell design incorporates precise control of electrolyte hydrodynamics through computational fluid dynamics modeling to optimize mass transport phenomena and minimize parasitic reactions. CNRS researchers have developed specialized serpentine flow field patterns that ensure uniform reactant distribution while minimizing pressure drop across the cell. Their system operates under ambient conditions with carefully controlled pH environments (typically 2-4) to stabilize produced H2O2, achieving faradaic efficiencies exceeding 90% and production rates of 5-7 mmol/h·cm² in laboratory-scale demonstrations.
Strengths: Cutting-edge catalyst development capabilities; sophisticated modeling approaches for flow optimization; strong focus on sustainable materials. Weaknesses: Technology primarily demonstrated at laboratory scale; may face challenges in industrial-scale implementation and long-term stability under continuous operation conditions.
BioIonix, Inc.
Technical Solution: BioIonix has developed specialized flow cell technology for H2O2 electrosynthesis with particular focus on water treatment applications. Their proprietary design features a unique electrode configuration that maximizes oxygen mass transfer to the cathode surface while maintaining optimal current distribution. The flow cells incorporate advanced boron-doped diamond (BDD) electrodes that provide exceptional chemical stability and resistance to fouling in industrial environments. BioIonix's system utilizes a serpentine flow channel architecture that ensures turbulent mixing while minimizing pressure drop, with computational fluid dynamics-optimized flow distributors at inlet and outlet regions. Their technology operates at relatively high current densities (200-400 mA/cm²) while maintaining energy efficiency through careful thermal management and electrode spacing optimization. The company's flow cells are designed for modular scaling, allowing deployment across various production capacities from small decentralized units to large industrial installations.
Strengths: Robust design specifically engineered for industrial water treatment applications; advanced electrode materials with exceptional durability; modular approach facilitates scaling. Weaknesses: Higher energy consumption compared to some competing technologies; system complexity may increase maintenance requirements and operational costs.
Key Electrode Materials and Catalyst Technologies
Electrochemical flow reactors for hydrogen peroxide synthesis
PatentActiveGB2513103B
Innovation
- The method involves using a carbon cathode with an immobilized quinone catalyst, such as anthraquinone, in an electrochemical cell where the cathode is in contact with a solution containing dissolved oxygen, allowing for a two-stage reduction of oxygen to hydrogen peroxide, independent of solution flow rate, and separating the anode and cathode to prevent interference.
Fluid flow pathways in flow fields of electrochemical cells
PatentWO2024215619A2
Innovation
- The introduction of a flow field design featuring a plurality of channels and lands with perforations that create cavities for fluid pathways between adjacent layers, allowing for improved fluid transfer, reduced pressure drops, enhanced heat transfer, and pressure balancing, thereby facilitating more efficient reactant supply and product removal.
Environmental Impact and Sustainability Factors
The electrosynthesis of hydrogen peroxide (H2O2) represents a significant advancement in green chemistry, offering a more sustainable alternative to the traditional anthraquinone process. When evaluating flow cell designs for industrial H2O2 production, environmental impact and sustainability factors must be comprehensively assessed to ensure alignment with global sustainability goals.
Energy efficiency stands as a paramount consideration in H2O2 electrosynthesis. Flow cell designs that minimize energy consumption through optimized electrode configurations and reduced cell resistance directly contribute to lower carbon footprints. Advanced flow cell architectures that operate at high current densities while maintaining energy efficiency can significantly reduce the environmental impact per unit of H2O2 produced.
Water management within flow cell systems presents both challenges and opportunities for sustainability. Designs that minimize water consumption through recirculation systems and efficient separation techniques reduce the overall environmental footprint of the process. Additionally, flow cells capable of utilizing non-potable or reclaimed water sources further enhance sustainability credentials by reducing pressure on freshwater resources.
Chemical inputs represent another critical environmental consideration. Flow cell designs that eliminate or minimize the need for additional chemicals such as stabilizers, pH adjusters, or separation agents contribute to cleaner production processes. Systems that can operate with minimal chemical additives reduce waste streams and simplify downstream processing, resulting in lower environmental impact throughout the product lifecycle.
Waste stream management capabilities should be integrated into flow cell design considerations. Cells that produce minimal by-products or enable easy separation and recovery of unreacted materials significantly reduce environmental burden. Closed-loop systems that recycle process streams and capture potential contaminants before discharge align with circular economy principles and stringent environmental regulations.
Material selection for flow cell components carries substantial sustainability implications. Designs incorporating abundant, non-toxic materials with long service lives reduce resource depletion and waste generation. The avoidance of rare earth elements, precious metals, or environmentally problematic materials in favor of sustainable alternatives enhances the overall environmental profile of the technology.
Life cycle assessment (LCA) metrics should guide flow cell design optimization. Quantitative evaluation of environmental impacts across the entire production chain—from raw material extraction through manufacturing, operation, and end-of-life disposal—provides valuable insights for sustainable design decisions. Flow cells designed with modular components that facilitate repair, upgrade, and eventual recycling demonstrate superior sustainability performance in comprehensive LCA evaluations.
Regulatory compliance and future-proofing against evolving environmental standards represent strategic sustainability considerations. Flow cell designs that exceed current environmental requirements and anticipate stricter future regulations offer long-term operational security and competitive advantage in increasingly environmentally conscious markets.
Energy efficiency stands as a paramount consideration in H2O2 electrosynthesis. Flow cell designs that minimize energy consumption through optimized electrode configurations and reduced cell resistance directly contribute to lower carbon footprints. Advanced flow cell architectures that operate at high current densities while maintaining energy efficiency can significantly reduce the environmental impact per unit of H2O2 produced.
Water management within flow cell systems presents both challenges and opportunities for sustainability. Designs that minimize water consumption through recirculation systems and efficient separation techniques reduce the overall environmental footprint of the process. Additionally, flow cells capable of utilizing non-potable or reclaimed water sources further enhance sustainability credentials by reducing pressure on freshwater resources.
Chemical inputs represent another critical environmental consideration. Flow cell designs that eliminate or minimize the need for additional chemicals such as stabilizers, pH adjusters, or separation agents contribute to cleaner production processes. Systems that can operate with minimal chemical additives reduce waste streams and simplify downstream processing, resulting in lower environmental impact throughout the product lifecycle.
Waste stream management capabilities should be integrated into flow cell design considerations. Cells that produce minimal by-products or enable easy separation and recovery of unreacted materials significantly reduce environmental burden. Closed-loop systems that recycle process streams and capture potential contaminants before discharge align with circular economy principles and stringent environmental regulations.
Material selection for flow cell components carries substantial sustainability implications. Designs incorporating abundant, non-toxic materials with long service lives reduce resource depletion and waste generation. The avoidance of rare earth elements, precious metals, or environmentally problematic materials in favor of sustainable alternatives enhances the overall environmental profile of the technology.
Life cycle assessment (LCA) metrics should guide flow cell design optimization. Quantitative evaluation of environmental impacts across the entire production chain—from raw material extraction through manufacturing, operation, and end-of-life disposal—provides valuable insights for sustainable design decisions. Flow cells designed with modular components that facilitate repair, upgrade, and eventual recycling demonstrate superior sustainability performance in comprehensive LCA evaluations.
Regulatory compliance and future-proofing against evolving environmental standards represent strategic sustainability considerations. Flow cell designs that exceed current environmental requirements and anticipate stricter future regulations offer long-term operational security and competitive advantage in increasingly environmentally conscious markets.
Scale-up Strategies for Industrial Implementation
Scaling up hydrogen peroxide electrosynthesis from laboratory to industrial scale requires systematic engineering approaches that address multiple technical and economic challenges. The transition demands careful consideration of flow cell design modifications to maintain performance while increasing production capacity.
A modular approach represents the most promising scale-up strategy, where multiple standardized flow cell units operate in parallel rather than simply enlarging individual cells. This methodology offers several advantages: it maintains the optimal electrode distance and flow dynamics established in smaller units, simplifies maintenance through replacement of individual modules, and allows for incremental capacity expansion. Companies like ThyssenKrupp and Siemens have successfully implemented such modular designs in related electrochemical processes.
Process intensification techniques must accompany physical scaling. This includes optimizing electrode materials with higher surface area-to-volume ratios, such as advanced carbon-based materials or metal meshes that maximize reaction sites while minimizing pressure drop. Three-dimensional electrodes have demonstrated up to 300% increased productivity compared to traditional flat electrodes in pilot studies.
Heat management becomes increasingly critical at industrial scale. As reaction volumes increase, heat dissipation systems must be integrated into the flow cell design to prevent temperature gradients that reduce efficiency and accelerate catalyst degradation. Computational fluid dynamics modeling suggests that strategic placement of cooling channels can maintain temperature variations below 3°C across industrial-scale cells.
Automation and control systems represent another essential component of successful scale-up. Real-time monitoring of key parameters (current density, temperature, flow rates, and H2O2 concentration) coupled with feedback control mechanisms ensures consistent production quality despite variations in operating conditions. Advanced predictive maintenance algorithms can further optimize uptime by anticipating component failures before they occur.
Economic considerations must guide the scale-up process. Capital expenditure optimization requires balancing equipment costs against operational efficiency. Analysis of several commercial implementations indicates that modular designs typically increase initial investment by 15-20% but reduce operational costs by 25-30% over a five-year period through improved maintenance efficiency and production flexibility.
Regulatory compliance and safety systems must be integrated from the earliest design stages. H2O2 concentration monitoring, emergency shutdown protocols, and containment systems must all scale proportionally with production capacity to maintain safe operation at industrial levels.
A modular approach represents the most promising scale-up strategy, where multiple standardized flow cell units operate in parallel rather than simply enlarging individual cells. This methodology offers several advantages: it maintains the optimal electrode distance and flow dynamics established in smaller units, simplifies maintenance through replacement of individual modules, and allows for incremental capacity expansion. Companies like ThyssenKrupp and Siemens have successfully implemented such modular designs in related electrochemical processes.
Process intensification techniques must accompany physical scaling. This includes optimizing electrode materials with higher surface area-to-volume ratios, such as advanced carbon-based materials or metal meshes that maximize reaction sites while minimizing pressure drop. Three-dimensional electrodes have demonstrated up to 300% increased productivity compared to traditional flat electrodes in pilot studies.
Heat management becomes increasingly critical at industrial scale. As reaction volumes increase, heat dissipation systems must be integrated into the flow cell design to prevent temperature gradients that reduce efficiency and accelerate catalyst degradation. Computational fluid dynamics modeling suggests that strategic placement of cooling channels can maintain temperature variations below 3°C across industrial-scale cells.
Automation and control systems represent another essential component of successful scale-up. Real-time monitoring of key parameters (current density, temperature, flow rates, and H2O2 concentration) coupled with feedback control mechanisms ensures consistent production quality despite variations in operating conditions. Advanced predictive maintenance algorithms can further optimize uptime by anticipating component failures before they occur.
Economic considerations must guide the scale-up process. Capital expenditure optimization requires balancing equipment costs against operational efficiency. Analysis of several commercial implementations indicates that modular designs typically increase initial investment by 15-20% but reduce operational costs by 25-30% over a five-year period through improved maintenance efficiency and production flexibility.
Regulatory compliance and safety systems must be integrated from the earliest design stages. H2O2 concentration monitoring, emergency shutdown protocols, and containment systems must all scale proportionally with production capacity to maintain safe operation at industrial levels.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!