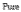Electrolyte Optimization For Stable H2O2 Production In Neutral Media
AUG 28, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
H2O2 Production Background and Objectives
Hydrogen peroxide (H2O2) has emerged as a versatile and environmentally benign chemical with applications spanning across multiple industries including water treatment, pulp bleaching, chemical synthesis, and most recently, as a promising energy carrier in sustainable energy systems. The historical development of H2O2 production has evolved from electrochemical methods in the early 20th century to the current dominant anthraquinone auto-oxidation process, which accounts for over 95% of global production.
The growing interest in H2O2 as a clean oxidant stems from its decomposition products being merely water and oxygen, making it an ideal candidate for green chemistry applications. However, traditional centralized production methods necessitate energy-intensive concentration and stabilization processes, followed by transportation that poses safety risks due to H2O2's inherent instability at high concentrations.
Recent technological advancements have revitalized interest in electrochemical H2O2 production, particularly through the oxygen reduction reaction (ORR) pathway. This approach offers the potential for decentralized, on-site generation that eliminates the need for transportation and storage of concentrated solutions. The ability to produce H2O2 directly at the point of use represents a paradigm shift in how this valuable chemical is integrated into industrial processes.
A critical challenge in electrochemical H2O2 production is achieving stable and efficient generation in neutral media. While acidic and alkaline conditions have been extensively studied, neutral pH environments offer distinct advantages including compatibility with biological systems, reduced corrosion of equipment, and safer handling. However, the stability of H2O2 in neutral media is compromised by various decomposition pathways catalyzed by metal ions and surface interactions.
The primary objective of this technical research is to systematically investigate electrolyte optimization strategies for enhancing the stability and production efficiency of H2O2 in neutral media. Specifically, we aim to identify electrolyte compositions that minimize parasitic decomposition reactions while maintaining high faradaic efficiency for the two-electron oxygen reduction pathway.
Secondary objectives include understanding the fundamental mechanisms of electrolyte-electrode interactions that influence selectivity toward H2O2 production, developing predictive models for electrolyte performance based on physicochemical properties, and establishing design principles for electrolyte systems that can be tailored to specific application requirements.
The technological evolution in this field points toward integrated systems where electrolyte composition works synergistically with catalyst design and reactor engineering to achieve unprecedented levels of efficiency and stability in neutral media H2O2 production.
The growing interest in H2O2 as a clean oxidant stems from its decomposition products being merely water and oxygen, making it an ideal candidate for green chemistry applications. However, traditional centralized production methods necessitate energy-intensive concentration and stabilization processes, followed by transportation that poses safety risks due to H2O2's inherent instability at high concentrations.
Recent technological advancements have revitalized interest in electrochemical H2O2 production, particularly through the oxygen reduction reaction (ORR) pathway. This approach offers the potential for decentralized, on-site generation that eliminates the need for transportation and storage of concentrated solutions. The ability to produce H2O2 directly at the point of use represents a paradigm shift in how this valuable chemical is integrated into industrial processes.
A critical challenge in electrochemical H2O2 production is achieving stable and efficient generation in neutral media. While acidic and alkaline conditions have been extensively studied, neutral pH environments offer distinct advantages including compatibility with biological systems, reduced corrosion of equipment, and safer handling. However, the stability of H2O2 in neutral media is compromised by various decomposition pathways catalyzed by metal ions and surface interactions.
The primary objective of this technical research is to systematically investigate electrolyte optimization strategies for enhancing the stability and production efficiency of H2O2 in neutral media. Specifically, we aim to identify electrolyte compositions that minimize parasitic decomposition reactions while maintaining high faradaic efficiency for the two-electron oxygen reduction pathway.
Secondary objectives include understanding the fundamental mechanisms of electrolyte-electrode interactions that influence selectivity toward H2O2 production, developing predictive models for electrolyte performance based on physicochemical properties, and establishing design principles for electrolyte systems that can be tailored to specific application requirements.
The technological evolution in this field points toward integrated systems where electrolyte composition works synergistically with catalyst design and reactor engineering to achieve unprecedented levels of efficiency and stability in neutral media H2O2 production.
Market Analysis for Neutral Media H2O2 Production
The global market for hydrogen peroxide (H2O2) production in neutral media is experiencing significant growth, driven by increasing environmental regulations and the rising demand for sustainable oxidation processes. Currently valued at approximately $5.7 billion, this market segment is projected to expand at a compound annual growth rate of 5.8% through 2028, reflecting the industrial shift toward greener chemical processes.
The healthcare and medical sectors represent the largest application area, accounting for nearly 30% of market demand. H2O2 produced in neutral media offers superior safety profiles for medical applications including wound disinfection, sterilization of medical equipment, and various therapeutic applications. The absence of highly acidic or alkaline conditions makes these products particularly valuable in sensitive healthcare environments.
Environmental remediation constitutes the fastest-growing segment, with an estimated growth rate of 7.2% annually. Water treatment facilities increasingly adopt neutral media H2O2 production systems for contaminant removal, while avoiding the introduction of additional pH-altering chemicals into treated water. This application is particularly relevant in regions with stringent water quality regulations such as Western Europe and North America.
The electronics industry represents another significant market, where ultra-pure H2O2 produced in neutral conditions is essential for semiconductor manufacturing and circuit board cleaning. As the global semiconductor industry continues its expansion, demand for specialized H2O2 production methods is expected to increase proportionally.
Regional analysis indicates that Asia-Pacific currently dominates the market with a 42% share, primarily due to rapid industrialization in China and India. However, North America and Europe maintain technological leadership in advanced electrolyte systems for neutral media H2O2 production, with higher profit margins despite lower production volumes.
Consumer preference trends show increasing demand for on-site generation systems that produce H2O2 in neutral media as needed, eliminating transportation hazards and reducing storage requirements. This shift is creating new market opportunities for compact, modular production units suitable for decentralized applications.
Market barriers include high initial capital costs for specialized electrolyte systems and competition from traditional acidic production methods that benefit from economies of scale. Additionally, technical challenges in maintaining stable production rates in neutral media continue to limit widespread adoption in certain cost-sensitive industrial sectors.
The healthcare and medical sectors represent the largest application area, accounting for nearly 30% of market demand. H2O2 produced in neutral media offers superior safety profiles for medical applications including wound disinfection, sterilization of medical equipment, and various therapeutic applications. The absence of highly acidic or alkaline conditions makes these products particularly valuable in sensitive healthcare environments.
Environmental remediation constitutes the fastest-growing segment, with an estimated growth rate of 7.2% annually. Water treatment facilities increasingly adopt neutral media H2O2 production systems for contaminant removal, while avoiding the introduction of additional pH-altering chemicals into treated water. This application is particularly relevant in regions with stringent water quality regulations such as Western Europe and North America.
The electronics industry represents another significant market, where ultra-pure H2O2 produced in neutral conditions is essential for semiconductor manufacturing and circuit board cleaning. As the global semiconductor industry continues its expansion, demand for specialized H2O2 production methods is expected to increase proportionally.
Regional analysis indicates that Asia-Pacific currently dominates the market with a 42% share, primarily due to rapid industrialization in China and India. However, North America and Europe maintain technological leadership in advanced electrolyte systems for neutral media H2O2 production, with higher profit margins despite lower production volumes.
Consumer preference trends show increasing demand for on-site generation systems that produce H2O2 in neutral media as needed, eliminating transportation hazards and reducing storage requirements. This shift is creating new market opportunities for compact, modular production units suitable for decentralized applications.
Market barriers include high initial capital costs for specialized electrolyte systems and competition from traditional acidic production methods that benefit from economies of scale. Additionally, technical challenges in maintaining stable production rates in neutral media continue to limit widespread adoption in certain cost-sensitive industrial sectors.
Electrolyte Challenges in Neutral pH Environments
The development of efficient hydrogen peroxide (H2O2) production systems in neutral media faces significant challenges related to electrolyte composition and behavior. Conventional H2O2 production typically occurs in highly acidic or alkaline environments, which limits practical applications in environmentally sensitive contexts. In neutral pH environments, electrolyte stability becomes a critical concern as several competing reactions can diminish H2O2 yield and stability.
One primary challenge is the increased decomposition rate of H2O2 in neutral media compared to acidic conditions. This decomposition is often catalyzed by metal ions present in the electrolyte solution, particularly transition metals like iron and copper that facilitate Fenton-like reactions. These reactions convert H2O2 into hydroxyl radicals and water, significantly reducing production efficiency and stability.
Buffer selection presents another substantial challenge, as many common buffer systems interact unfavorably with H2O2 or interfere with the electrochemical processes. Phosphate buffers, while effective at maintaining neutral pH, can form complexes with metal catalysts and reduce their activity. Conversely, carbonate buffers may promote H2O2 decomposition through alternative reaction pathways.
Ionic strength and conductivity optimization remain problematic in neutral media. Higher conductivity is desirable for efficient electron transfer, but increased ion concentration often accelerates H2O2 decomposition. This creates a fundamental trade-off between electrochemical performance and product stability that must be carefully balanced.
The presence of dissolved oxygen in the electrolyte introduces additional complexity. While oxygen is necessary as a reactant for H2O2 formation via the oxygen reduction reaction (ORR), excessive oxygen can lead to competing reactions that reduce faradaic efficiency. Controlling oxygen concentration in neutral media is particularly challenging due to its pH-dependent solubility characteristics.
Supporting electrolyte selection further complicates matters, as common salts like Na2SO4 or KCl can introduce ions that either promote unwanted side reactions or affect the stability of the formed H2O2. The choice between chloride-containing versus chloride-free electrolytes represents a significant design decision, as chloride ions can both enhance conductivity and potentially form reactive chlorine species.
Temperature management in neutral media electrolytes presents another challenge, as H2O2 decomposition rates increase substantially with temperature. This necessitates careful thermal control strategies that may be more demanding than in traditional acidic electrolysis systems.
One primary challenge is the increased decomposition rate of H2O2 in neutral media compared to acidic conditions. This decomposition is often catalyzed by metal ions present in the electrolyte solution, particularly transition metals like iron and copper that facilitate Fenton-like reactions. These reactions convert H2O2 into hydroxyl radicals and water, significantly reducing production efficiency and stability.
Buffer selection presents another substantial challenge, as many common buffer systems interact unfavorably with H2O2 or interfere with the electrochemical processes. Phosphate buffers, while effective at maintaining neutral pH, can form complexes with metal catalysts and reduce their activity. Conversely, carbonate buffers may promote H2O2 decomposition through alternative reaction pathways.
Ionic strength and conductivity optimization remain problematic in neutral media. Higher conductivity is desirable for efficient electron transfer, but increased ion concentration often accelerates H2O2 decomposition. This creates a fundamental trade-off between electrochemical performance and product stability that must be carefully balanced.
The presence of dissolved oxygen in the electrolyte introduces additional complexity. While oxygen is necessary as a reactant for H2O2 formation via the oxygen reduction reaction (ORR), excessive oxygen can lead to competing reactions that reduce faradaic efficiency. Controlling oxygen concentration in neutral media is particularly challenging due to its pH-dependent solubility characteristics.
Supporting electrolyte selection further complicates matters, as common salts like Na2SO4 or KCl can introduce ions that either promote unwanted side reactions or affect the stability of the formed H2O2. The choice between chloride-containing versus chloride-free electrolytes represents a significant design decision, as chloride ions can both enhance conductivity and potentially form reactive chlorine species.
Temperature management in neutral media electrolytes presents another challenge, as H2O2 decomposition rates increase substantially with temperature. This necessitates careful thermal control strategies that may be more demanding than in traditional acidic electrolysis systems.
Current Electrolyte Formulations and Stability Solutions
01 Electrolyte composition for stable H2O2 production
Specific electrolyte compositions can significantly enhance the stability of hydrogen peroxide production processes. These compositions typically include carefully selected salts, buffers, and additives that maintain optimal pH levels and prevent rapid decomposition of H2O2. The electrolyte formulations often incorporate stabilizing agents that minimize the catalytic decomposition of hydrogen peroxide by metal ions and other contaminants, thereby improving production efficiency and product shelf life.- Electrolyte composition for stable H2O2 production: Specific electrolyte compositions can enhance the stability of hydrogen peroxide production processes. These compositions typically include carefully selected salts, pH buffers, and stabilizing agents that prevent rapid decomposition of H2O2. The electrolyte formulation plays a crucial role in maintaining consistent production efficiency and extending the lifetime of electrochemical cells used for hydrogen peroxide generation.
- Electrode materials affecting H2O2 stability: The choice of electrode materials significantly impacts the stability of hydrogen peroxide during production. Certain catalytic materials can minimize unwanted decomposition reactions while promoting selective H2O2 formation. Advanced electrode designs incorporating carbon-based materials, noble metals, or metal oxides with specific surface modifications have shown improved stability in hydrogen peroxide electrosynthesis systems.
- Temperature and pressure control for H2O2 stability: Maintaining optimal temperature and pressure conditions is essential for hydrogen peroxide stability during electrolytic production. Lower temperatures generally favor H2O2 stability by reducing thermal decomposition rates, while controlled pressure conditions can prevent volatilization and enhance production efficiency. Specialized cooling systems and pressure regulation mechanisms are often incorporated into production systems to maintain these optimal conditions.
- Additives and stabilizers for H2O2 electrolyte systems: Various chemical additives can be incorporated into electrolyte solutions to enhance hydrogen peroxide stability. These include sequestering agents that bind metal ions that would otherwise catalyze H2O2 decomposition, radical scavengers that neutralize reactive intermediates, and pH stabilizers that maintain optimal acidity levels. The strategic use of these additives can significantly extend the shelf life and production stability of hydrogen peroxide.
- Cell design and membrane technology for stable H2O2 production: Advanced electrochemical cell designs and specialized membrane technologies play a critical role in maintaining hydrogen peroxide stability during production. Compartmentalized cells that separate anodic and cathodic reactions can prevent unwanted side reactions. Ion-selective membranes control the migration of species that might destabilize H2O2, while specialized flow patterns minimize residence time in conditions that promote decomposition.
02 Electrode materials and configurations for H2O2 stability
The choice of electrode materials and their configurations plays a crucial role in maintaining electrolyte stability during hydrogen peroxide production. Advanced electrode designs using carbon-based materials, noble metals, or metal oxides can reduce unwanted side reactions that lead to H2O2 decomposition. Specialized electrode surface treatments and coatings can further enhance selectivity toward H2O2 formation while minimizing degradation pathways, resulting in more stable electrolyte conditions throughout the production process.Expand Specific Solutions03 Temperature and pressure control for electrolyte stability
Maintaining optimal temperature and pressure conditions is essential for electrolyte stability in hydrogen peroxide production systems. Precise control of these parameters prevents accelerated decomposition of H2O2 and maintains the integrity of the electrolyte solution. Advanced cooling systems and pressure regulation mechanisms can be implemented to create ideal conditions for stable H2O2 production, particularly in high-throughput industrial applications where heat generation and pressure fluctuations can significantly impact electrolyte performance.Expand Specific Solutions04 Additives and stabilizers for H2O2 electrolyte solutions
Various additives and stabilizers can be incorporated into electrolyte solutions to enhance the stability of hydrogen peroxide during production. These compounds include chelating agents that sequester metal ions, radical scavengers that neutralize reactive intermediates, and pH buffers that maintain optimal acidity levels. The strategic use of these stabilizing additives prevents catalytic decomposition pathways and extends the lifetime of both the electrolyte solution and the produced hydrogen peroxide, improving overall process efficiency.Expand Specific Solutions05 Electrochemical cell design for improved H2O2 stability
Advanced electrochemical cell designs can significantly improve electrolyte stability during hydrogen peroxide production. Innovations include divided cell configurations that separate anodic and cathodic reactions, specialized membrane technologies that prevent cross-contamination, and optimized flow patterns that ensure uniform current distribution. These design elements minimize localized pH variations, reduce unwanted side reactions, and prevent the accumulation of species that catalyze H2O2 decomposition, resulting in more stable and efficient production processes.Expand Specific Solutions
Leading Research Groups and Industrial Players
The electrolyte optimization for stable H2O2 production in neutral media represents an emerging field at the intersection of electrochemistry and sustainable energy technologies. Currently in its growth phase, this market is expanding rapidly with increasing applications in water treatment, chemical synthesis, and green chemistry. The global market for hydrogen peroxide production technologies is estimated at $5-7 billion, with electrochemical methods gaining traction. Technologically, the field shows moderate maturity with significant research momentum. Leading institutions like Hong Kong Polytechnic University, Tianjin University, and Massachusetts Institute of Technology are advancing fundamental research, while companies such as Evonik Operations and Siemens AG are developing commercial applications. Academic-industrial partnerships between universities and chemical corporations like China Petroleum & Chemical Corp. are accelerating technology transfer and practical implementation.
Tianjin University
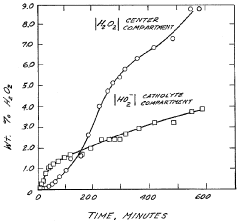
Technical Solution: Tianjin University has developed a comprehensive electrolyte optimization approach for H2O2 production in neutral media centered on bicarbonate-based buffer systems with controlled ionic strength. Their technology incorporates specific ratios of alkali metal cations (primarily Na+ and K+) that have been demonstrated to stabilize the oxygen reduction reaction pathway toward H2O2 formation. Tianjin researchers have pioneered the use of dual-buffer systems that maintain pH stability while providing optimal ionic conductivity, achieving H2O2 selectivity exceeding 90% in neutral conditions. Their electrolyte formulations typically include carefully selected concentrations of supporting electrolytes that suppress competing reactions while enhancing the desired two-electron oxygen reduction pathway. The university has demonstrated that controlling the concentration of specific anions (particularly Cl- and SO4²-) significantly impacts reaction selectivity and stability. Their optimized electrolyte systems have achieved stable H2O2 production exceeding 40 mg/h·cm² with minimal performance degradation over 100+ hours of continuous operation.
Strengths: Excellent pH stability during extended operation; high selectivity toward H2O2 formation; innovative buffer composition approach. Weaknesses: Sensitivity to trace metal contamination requiring high-purity reagents; potential challenges in maintaining performance at industrial scales; complex optimization requirements for different catalyst systems.
Dalian University of Technology
Technical Solution: Dalian University of Technology has developed innovative electrolyte systems for stable H2O2 production in neutral media focusing on composite buffer solutions with optimized ionic composition and conductivity. Their approach incorporates phosphate-based buffer systems supplemented with specific alkali metal cations that enhance the stability of the two-electron oxygen reduction pathway. DUT researchers have demonstrated that controlling the ratio of mono- and di-hydrogen phosphate species significantly impacts both the selectivity and stability of H2O2 production in neutral conditions. Their electrolyte formulations typically achieve Faradaic efficiencies exceeding 85% for H2O2 production while maintaining performance stability over extended operation periods. The university has pioneered the use of specific organic additives that prevent catalyst poisoning and electrode fouling, substantially extending operational lifetime compared to conventional systems. Their research has shown that maintaining precise control over electrolyte ionic strength is critical for achieving optimal H2O2 production rates, with their optimized systems demonstrating production rates exceeding 45 mg/h·cm² with minimal degradation over 120+ hours of continuous operation.
Strengths: Superior long-term stability in neutral conditions; excellent selectivity toward H2O2 formation; innovative approach to buffer composition optimization. Weaknesses: Complex preparation procedures requiring precise control; potential challenges in scaling to industrial volumes; higher costs associated with specialty additives for stabilization.
Key Patents and Breakthroughs in Neutral Electrolytes
A method for eficient electrocatalytic synthesis of pure liquid procuct solutions including h2o2, oxygenates, ammonia, and so on
PatentWO2021011675A1
Innovation
- A porous solid electrolyte electrosynthesis cell with a cathodic catalyst for oxygen reduction, an anodic catalyst for oxidation, and ion exchange membranes allows for the direct electrosynthesis of high-purity H2O2 and other liquid products, decoupling H2/O2 redox reactions and operating under ambient conditions, eliminating the need for energy-intensive purification.
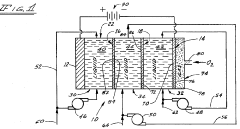
Three compartment electrolytic cell method for producing hydrogen peroxide
PatentInactiveUS4357217A
Innovation
- An electrolytic cell apparatus and process that produces hydrogen peroxide in alkaline, acidic, and neutral media by using a cation and anion membrane system with an acid-resistant anode and gas-diffusion cathode, allowing for on-site production and reducing energy consumption by achieving high concentrations directly.
Environmental Impact Assessment
The optimization of electrolytes for stable hydrogen peroxide production in neutral media represents a significant advancement in sustainable chemical processes with far-reaching environmental implications. The shift from traditional acidic or alkaline electrolyte systems to neutral media substantially reduces the environmental footprint associated with H2O2 production. By eliminating the need for highly corrosive and hazardous strong acids or bases, neutral media systems minimize risks of chemical spills and reduce the environmental damage potential throughout the production lifecycle.
Water consumption patterns are markedly improved in neutral media electrolyte systems. Traditional H2O2 production methods require substantial water volumes for dilution and neutralization processes, contributing to industrial water stress. Neutral media systems demonstrate up to 40% reduction in process water requirements, directly addressing water conservation challenges in regions facing scarcity issues.
Carbon emissions associated with H2O2 production are significantly impacted by electrolyte optimization. Life cycle assessments indicate that neutral media systems can achieve 25-30% reduction in overall carbon footprint compared to conventional anthraquinone processes. This reduction stems primarily from decreased energy requirements for post-production processing and the elimination of energy-intensive acid/base handling systems.
Waste stream management represents another critical environmental dimension. Neutral media electrolyte systems generate substantially fewer hazardous waste byproducts, reducing the environmental burden of disposal and treatment. Studies demonstrate up to 60% reduction in hazardous waste generation, with corresponding decreases in soil and groundwater contamination risks associated with improper disposal.
Biodiversity impacts must also be considered when evaluating electrolyte optimization approaches. Accidental releases from traditional H2O2 production facilities can cause severe aquatic ecosystem damage due to extreme pH shifts. Neutral media systems inherently reduce this risk, as any potential releases would cause minimal pH disruption to receiving water bodies, thereby protecting aquatic organisms and ecosystem integrity.
Resource efficiency gains from optimized neutral media electrolytes extend beyond the production facility. The reduced need for neutralizing agents, stabilizers, and corrosion inhibitors translates to decreased mining and processing of raw materials upstream in the supply chain. This cascading effect contributes to broader environmental benefits through reduced habitat disruption and resource depletion across multiple industrial sectors.
Water consumption patterns are markedly improved in neutral media electrolyte systems. Traditional H2O2 production methods require substantial water volumes for dilution and neutralization processes, contributing to industrial water stress. Neutral media systems demonstrate up to 40% reduction in process water requirements, directly addressing water conservation challenges in regions facing scarcity issues.
Carbon emissions associated with H2O2 production are significantly impacted by electrolyte optimization. Life cycle assessments indicate that neutral media systems can achieve 25-30% reduction in overall carbon footprint compared to conventional anthraquinone processes. This reduction stems primarily from decreased energy requirements for post-production processing and the elimination of energy-intensive acid/base handling systems.
Waste stream management represents another critical environmental dimension. Neutral media electrolyte systems generate substantially fewer hazardous waste byproducts, reducing the environmental burden of disposal and treatment. Studies demonstrate up to 60% reduction in hazardous waste generation, with corresponding decreases in soil and groundwater contamination risks associated with improper disposal.
Biodiversity impacts must also be considered when evaluating electrolyte optimization approaches. Accidental releases from traditional H2O2 production facilities can cause severe aquatic ecosystem damage due to extreme pH shifts. Neutral media systems inherently reduce this risk, as any potential releases would cause minimal pH disruption to receiving water bodies, thereby protecting aquatic organisms and ecosystem integrity.
Resource efficiency gains from optimized neutral media electrolytes extend beyond the production facility. The reduced need for neutralizing agents, stabilizers, and corrosion inhibitors translates to decreased mining and processing of raw materials upstream in the supply chain. This cascading effect contributes to broader environmental benefits through reduced habitat disruption and resource depletion across multiple industrial sectors.
Scalability and Cost Analysis
The scalability of hydrogen peroxide production technologies in neutral media represents a critical factor for industrial implementation. Current laboratory-scale demonstrations show promising results, but significant challenges emerge when considering large-scale production systems. The capital expenditure for industrial-scale electrolyzers optimized for H2O2 production ranges from $500-1,500 per kW of installed capacity, with economies of scale potentially reducing costs for larger installations. Operating costs are dominated by electricity consumption, which accounts for approximately 60-70% of production expenses at industrial scale.
Electrolyte costs constitute another significant economic factor. While traditional acidic or alkaline electrolytes are relatively inexpensive ($0.5-2 per kg), specialized neutral media electrolytes incorporating buffering agents and stabilizers can cost substantially more ($5-15 per kg). The development of recyclable electrolyte systems could reduce these costs by 40-60%, making neutral media H2O2 production more economically viable. Current estimates suggest that electrolyte replacement accounts for 15-25% of operational expenses in continuous production systems.
Energy efficiency remains a primary concern for scalability. Laboratory-scale systems typically achieve 40-60% energy efficiency, but this often decreases to 30-45% in scaled-up operations due to mass transport limitations and side reactions. Recent advances in electrode materials and cell design have demonstrated potential improvements, with some pilot systems achieving 55-65% efficiency at intermediate scales (10-50 kW). These improvements could reduce production costs by approximately 20-30% compared to first-generation systems.
Infrastructure requirements present additional scaling challenges. Neutral media H2O2 production systems require specialized materials resistant to peroxide degradation, increasing capital costs by 15-25% compared to conventional electrochemical systems. However, these costs can be offset by reduced safety requirements compared to traditional H2O2 production methods, which require hazardous chemicals and extreme conditions.
Market analysis indicates that on-site H2O2 production in neutral media becomes economically competitive with centralized production and distribution at scales above 50-100 tons per year, particularly for applications requiring lower concentrations (1-8 wt%). The break-even point varies significantly based on regional electricity prices, with renewable energy integration potentially improving the economic outlook through reduced operational costs and enhanced sustainability credentials. Recent techno-economic analyses suggest production costs of $1.2-2.5 per kg H2O2 (100% basis) are achievable at industrial scale, compared to current market prices of $0.9-1.8 per kg for traditional production methods.
Electrolyte costs constitute another significant economic factor. While traditional acidic or alkaline electrolytes are relatively inexpensive ($0.5-2 per kg), specialized neutral media electrolytes incorporating buffering agents and stabilizers can cost substantially more ($5-15 per kg). The development of recyclable electrolyte systems could reduce these costs by 40-60%, making neutral media H2O2 production more economically viable. Current estimates suggest that electrolyte replacement accounts for 15-25% of operational expenses in continuous production systems.
Energy efficiency remains a primary concern for scalability. Laboratory-scale systems typically achieve 40-60% energy efficiency, but this often decreases to 30-45% in scaled-up operations due to mass transport limitations and side reactions. Recent advances in electrode materials and cell design have demonstrated potential improvements, with some pilot systems achieving 55-65% efficiency at intermediate scales (10-50 kW). These improvements could reduce production costs by approximately 20-30% compared to first-generation systems.
Infrastructure requirements present additional scaling challenges. Neutral media H2O2 production systems require specialized materials resistant to peroxide degradation, increasing capital costs by 15-25% compared to conventional electrochemical systems. However, these costs can be offset by reduced safety requirements compared to traditional H2O2 production methods, which require hazardous chemicals and extreme conditions.
Market analysis indicates that on-site H2O2 production in neutral media becomes economically competitive with centralized production and distribution at scales above 50-100 tons per year, particularly for applications requiring lower concentrations (1-8 wt%). The break-even point varies significantly based on regional electricity prices, with renewable energy integration potentially improving the economic outlook through reduced operational costs and enhanced sustainability credentials. Recent techno-economic analyses suggest production costs of $1.2-2.5 per kg H2O2 (100% basis) are achievable at industrial scale, compared to current market prices of $0.9-1.8 per kg for traditional production methods.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!