Electrocatalyst Poisoning Mechanisms And Regeneration Strategies
AUG 28, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Electrocatalyst Poisoning Background and Research Objectives
Electrocatalysts have emerged as critical components in numerous sustainable energy technologies, including fuel cells, water electrolyzers, and CO2 reduction systems. However, their performance and longevity are significantly compromised by poisoning phenomena, which have been documented since the early development of catalytic systems in the 1960s. Catalyst poisoning occurs when certain chemical species strongly adsorb onto active sites, blocking access for reactant molecules and consequently reducing catalytic activity.
The evolution of electrocatalyst research has seen significant advancements in understanding poisoning mechanisms, particularly in platinum-based catalysts for fuel cells where carbon monoxide poisoning has been extensively studied. More recently, attention has expanded to include sulfur compounds, halides, and nitrogen-containing species as significant poisoning agents across various electrocatalytic applications.
Current technological trends indicate a shift toward more complex and diverse electrocatalytic systems, including non-precious metal catalysts, single-atom catalysts, and hybrid materials. This diversification has introduced new poisoning challenges that require sophisticated analytical approaches and mitigation strategies. The increasing deployment of electrocatalytic technologies in real-world environments further emphasizes the need for robust solutions to poisoning issues that can withstand variable operating conditions.
The primary objective of this technical research is to comprehensively analyze the fundamental mechanisms underlying electrocatalyst poisoning across different catalyst types and applications. We aim to establish correlations between catalyst composition, structure, and susceptibility to specific poisoning agents, thereby developing predictive models for poisoning behavior.
Additionally, this research seeks to evaluate existing regeneration strategies, including electrochemical, thermal, and chemical approaches, assessing their effectiveness, energy requirements, and impact on catalyst longevity. Through systematic comparison of these methods, we intend to identify optimal regeneration protocols for different catalyst-poison combinations.
A further goal is to explore innovative poisoning prevention strategies, such as advanced catalyst design principles that inherently resist poisoning, protective coatings or structures that selectively filter potential poisons, and intelligent operating protocols that minimize exposure to poisoning conditions while maintaining performance efficiency.
The ultimate aim is to establish design guidelines for next-generation electrocatalysts with enhanced poison resistance and simplified regeneration requirements, thereby addressing a critical barrier to widespread commercialization of electrocatalytic technologies. This research will contribute to extending catalyst lifetimes, reducing system downtime, and lowering the total cost of ownership for electrocatalytic technologies in energy conversion and storage applications.
The evolution of electrocatalyst research has seen significant advancements in understanding poisoning mechanisms, particularly in platinum-based catalysts for fuel cells where carbon monoxide poisoning has been extensively studied. More recently, attention has expanded to include sulfur compounds, halides, and nitrogen-containing species as significant poisoning agents across various electrocatalytic applications.
Current technological trends indicate a shift toward more complex and diverse electrocatalytic systems, including non-precious metal catalysts, single-atom catalysts, and hybrid materials. This diversification has introduced new poisoning challenges that require sophisticated analytical approaches and mitigation strategies. The increasing deployment of electrocatalytic technologies in real-world environments further emphasizes the need for robust solutions to poisoning issues that can withstand variable operating conditions.
The primary objective of this technical research is to comprehensively analyze the fundamental mechanisms underlying electrocatalyst poisoning across different catalyst types and applications. We aim to establish correlations between catalyst composition, structure, and susceptibility to specific poisoning agents, thereby developing predictive models for poisoning behavior.
Additionally, this research seeks to evaluate existing regeneration strategies, including electrochemical, thermal, and chemical approaches, assessing their effectiveness, energy requirements, and impact on catalyst longevity. Through systematic comparison of these methods, we intend to identify optimal regeneration protocols for different catalyst-poison combinations.
A further goal is to explore innovative poisoning prevention strategies, such as advanced catalyst design principles that inherently resist poisoning, protective coatings or structures that selectively filter potential poisons, and intelligent operating protocols that minimize exposure to poisoning conditions while maintaining performance efficiency.
The ultimate aim is to establish design guidelines for next-generation electrocatalysts with enhanced poison resistance and simplified regeneration requirements, thereby addressing a critical barrier to widespread commercialization of electrocatalytic technologies. This research will contribute to extending catalyst lifetimes, reducing system downtime, and lowering the total cost of ownership for electrocatalytic technologies in energy conversion and storage applications.
Market Demand Analysis for Poison-Resistant Electrocatalysts
The global market for poison-resistant electrocatalysts is experiencing robust growth, driven primarily by the increasing adoption of fuel cells and electrolyzers across various industries. Current market estimates value this specialized segment at approximately 2.3 billion USD in 2023, with projections indicating a compound annual growth rate of 8.7% through 2030.
The automotive sector represents the largest demand driver, as fuel cell electric vehicles (FCEVs) continue to gain traction in commercial transportation fleets. Major automotive manufacturers have identified catalyst poisoning as a critical barrier to widespread FCEV adoption, creating urgent market pull for more resilient catalyst technologies. Fleet operators specifically cite catalyst longevity and reduced maintenance requirements as key purchasing factors.
Industrial hydrogen production presents another significant market opportunity. Green hydrogen initiatives worldwide are accelerating the deployment of PEM electrolyzers, which face similar poisoning challenges from feedwater contaminants. The European Hydrogen Strategy alone aims to install 40GW of electrolyzer capacity by 2030, creating substantial demand for poison-resistant catalysts that can maintain efficiency under variable operating conditions.
Stationary power generation applications, particularly in remote or backup power scenarios, constitute a growing market segment with specific requirements for catalyst durability. These systems often operate in environments with variable air quality, making resistance to airborne contaminants a critical performance parameter.
Regional analysis reveals Asia-Pacific as the fastest-growing market, with China, Japan, and South Korea making significant investments in hydrogen infrastructure. North America and Europe maintain strong demand driven by stringent emissions regulations and renewable energy initiatives.
Customer requirements analysis indicates five primary market demands: extended catalyst lifetime (>10,000 operating hours without significant degradation), reduced sensitivity to common contaminants (particularly CO, sulfur compounds, and ammonia), simplified regeneration protocols, decreased precious metal loading to reduce costs, and compatibility with existing system architectures to minimize redesign requirements.
Market surveys indicate customers are willing to pay a premium of 15-25% for catalysts demonstrating superior poison resistance, provided they deliver at least a 30% improvement in operational lifetime. This price sensitivity varies by application, with critical infrastructure and transportation applications showing greater willingness to prioritize performance over initial cost.
The competitive landscape remains fragmented, with specialized catalyst manufacturers competing alongside major chemical companies expanding into this high-growth segment. Recent market entrants include several technology startups commercializing novel catalyst architectures specifically designed to address poisoning mechanisms.
The automotive sector represents the largest demand driver, as fuel cell electric vehicles (FCEVs) continue to gain traction in commercial transportation fleets. Major automotive manufacturers have identified catalyst poisoning as a critical barrier to widespread FCEV adoption, creating urgent market pull for more resilient catalyst technologies. Fleet operators specifically cite catalyst longevity and reduced maintenance requirements as key purchasing factors.
Industrial hydrogen production presents another significant market opportunity. Green hydrogen initiatives worldwide are accelerating the deployment of PEM electrolyzers, which face similar poisoning challenges from feedwater contaminants. The European Hydrogen Strategy alone aims to install 40GW of electrolyzer capacity by 2030, creating substantial demand for poison-resistant catalysts that can maintain efficiency under variable operating conditions.
Stationary power generation applications, particularly in remote or backup power scenarios, constitute a growing market segment with specific requirements for catalyst durability. These systems often operate in environments with variable air quality, making resistance to airborne contaminants a critical performance parameter.
Regional analysis reveals Asia-Pacific as the fastest-growing market, with China, Japan, and South Korea making significant investments in hydrogen infrastructure. North America and Europe maintain strong demand driven by stringent emissions regulations and renewable energy initiatives.
Customer requirements analysis indicates five primary market demands: extended catalyst lifetime (>10,000 operating hours without significant degradation), reduced sensitivity to common contaminants (particularly CO, sulfur compounds, and ammonia), simplified regeneration protocols, decreased precious metal loading to reduce costs, and compatibility with existing system architectures to minimize redesign requirements.
Market surveys indicate customers are willing to pay a premium of 15-25% for catalysts demonstrating superior poison resistance, provided they deliver at least a 30% improvement in operational lifetime. This price sensitivity varies by application, with critical infrastructure and transportation applications showing greater willingness to prioritize performance over initial cost.
The competitive landscape remains fragmented, with specialized catalyst manufacturers competing alongside major chemical companies expanding into this high-growth segment. Recent market entrants include several technology startups commercializing novel catalyst architectures specifically designed to address poisoning mechanisms.
Current Challenges in Electrocatalyst Durability
Electrocatalyst durability remains a critical challenge in the advancement of electrochemical energy conversion and storage technologies. Despite significant progress in developing high-performance electrocatalysts, their practical implementation is severely hindered by rapid activity degradation during operation. This degradation is particularly pronounced in harsh electrochemical environments characterized by extreme pH conditions, high potentials, and the presence of contaminants.
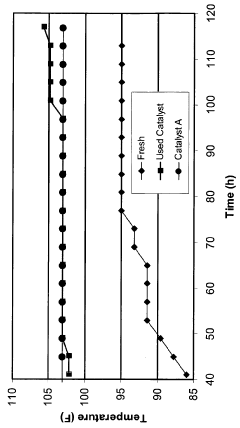
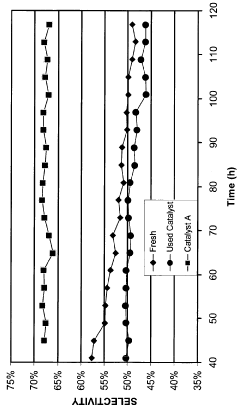
One of the primary durability challenges is catalyst poisoning, where specific chemical species irreversibly adsorb onto active sites, blocking access to reactants. In proton exchange membrane fuel cells (PEMFCs), platinum-based catalysts suffer from severe CO poisoning, with CO binding energy approximately 100 kJ/mol stronger than hydrogen, effectively rendering catalytic sites inactive. Similarly, in water electrolysis systems, transition metal catalysts experience performance losses due to poisoning by sulfur compounds and metal ions from feedwater impurities.
Surface restructuring presents another significant challenge, occurring when the catalyst's surface atoms rearrange under reaction conditions, often leading to decreased active site density. This phenomenon is particularly prevalent in nanostructured catalysts with high surface energy, where the thermodynamic drive toward lower energy configurations results in particle agglomeration and Ostwald ripening. For instance, in oxygen reduction reaction (ORR) catalysts, potential cycling can induce platinum dissolution and redeposition, fundamentally altering the catalyst's morphology and activity.
Leaching of active components constitutes a third major durability issue, especially in non-precious metal catalysts. Under operating conditions, metal atoms can dissolve into the electrolyte, resulting in irreversible activity loss. This is particularly problematic in alkaline environments where many transition metals exhibit increased solubility. Studies have shown that up to 40% of active metal content can be lost within the first 100 hours of operation in certain systems.
The harsh oxidative environments in many electrochemical devices accelerate catalyst degradation through oxidative etching and corrosion processes. Carbon supports, widely used for their conductivity and high surface area, are susceptible to electrochemical oxidation above 0.9V vs. RHE, leading to catalyst detachment and agglomeration. This support corrosion is estimated to account for approximately 30-40% of overall performance degradation in PEMFCs over their operational lifetime.
Addressing these durability challenges requires multifaceted approaches, including the development of poisoning-resistant catalyst compositions, core-shell structures to protect active sites, and alternative support materials with enhanced stability. Recent advances in in-situ and operando characterization techniques have enabled deeper insights into degradation mechanisms, paving the way for more rational design strategies to enhance electrocatalyst longevity.
One of the primary durability challenges is catalyst poisoning, where specific chemical species irreversibly adsorb onto active sites, blocking access to reactants. In proton exchange membrane fuel cells (PEMFCs), platinum-based catalysts suffer from severe CO poisoning, with CO binding energy approximately 100 kJ/mol stronger than hydrogen, effectively rendering catalytic sites inactive. Similarly, in water electrolysis systems, transition metal catalysts experience performance losses due to poisoning by sulfur compounds and metal ions from feedwater impurities.
Surface restructuring presents another significant challenge, occurring when the catalyst's surface atoms rearrange under reaction conditions, often leading to decreased active site density. This phenomenon is particularly prevalent in nanostructured catalysts with high surface energy, where the thermodynamic drive toward lower energy configurations results in particle agglomeration and Ostwald ripening. For instance, in oxygen reduction reaction (ORR) catalysts, potential cycling can induce platinum dissolution and redeposition, fundamentally altering the catalyst's morphology and activity.
Leaching of active components constitutes a third major durability issue, especially in non-precious metal catalysts. Under operating conditions, metal atoms can dissolve into the electrolyte, resulting in irreversible activity loss. This is particularly problematic in alkaline environments where many transition metals exhibit increased solubility. Studies have shown that up to 40% of active metal content can be lost within the first 100 hours of operation in certain systems.
The harsh oxidative environments in many electrochemical devices accelerate catalyst degradation through oxidative etching and corrosion processes. Carbon supports, widely used for their conductivity and high surface area, are susceptible to electrochemical oxidation above 0.9V vs. RHE, leading to catalyst detachment and agglomeration. This support corrosion is estimated to account for approximately 30-40% of overall performance degradation in PEMFCs over their operational lifetime.
Addressing these durability challenges requires multifaceted approaches, including the development of poisoning-resistant catalyst compositions, core-shell structures to protect active sites, and alternative support materials with enhanced stability. Recent advances in in-situ and operando characterization techniques have enabled deeper insights into degradation mechanisms, paving the way for more rational design strategies to enhance electrocatalyst longevity.
Mainstream Poisoning Mitigation Approaches
01 Carbon monoxide poisoning mechanisms in fuel cell electrocatalysts
Carbon monoxide (CO) is a common poison for electrocatalysts, particularly in fuel cells. It strongly adsorbs onto active catalyst sites, blocking access to reactants and reducing catalytic activity. This poisoning mechanism is especially problematic for platinum-based catalysts in hydrogen fuel cells, where even trace amounts of CO can significantly decrease performance. Understanding the CO adsorption mechanism and binding energies on different catalyst surfaces is crucial for developing more poison-resistant electrocatalysts.- Carbon monoxide poisoning mechanisms in fuel cell electrocatalysts: Carbon monoxide (CO) is a common poison for electrocatalysts, particularly in fuel cells. It strongly adsorbs on active catalyst sites, blocking access to reactants and reducing catalytic activity. This poisoning mechanism is especially problematic for platinum-based catalysts in hydrogen fuel cells, where even trace amounts of CO can significantly decrease performance. Understanding the binding mechanisms and surface interactions of CO with catalyst materials is crucial for developing more poison-resistant electrocatalysts.
- Sulfur compound poisoning and deactivation pathways: Sulfur-containing compounds represent another major class of electrocatalyst poisons. These compounds, including hydrogen sulfide (H2S) and sulfur oxides (SOx), can irreversibly bind to catalyst active sites, causing permanent deactivation. The poisoning mechanism involves strong chemisorption of sulfur species on metal surfaces, altering the electronic properties and blocking reaction sites. This is particularly problematic in electrochemical systems processing hydrocarbon feedstocks or operating in environments where sulfur compounds are present.
- Thermal regeneration techniques for poisoned electrocatalysts: Thermal regeneration is an effective approach for restoring activity to poisoned electrocatalysts. This process involves controlled heating of the catalyst to desorb or decompose poison species from active sites. The temperature required depends on the specific poison and catalyst material, with some requiring mild heating while others need more aggressive thermal treatment. Thermal regeneration can be performed in various atmospheres including oxidizing, reducing, or inert environments to optimize poison removal while minimizing catalyst degradation through sintering or other thermal damage mechanisms.
- Electrochemical regeneration methods for catalyst reactivation: Electrochemical techniques offer in-situ approaches for regenerating poisoned electrocatalysts without system disassembly. These methods involve applying specific potential profiles or cycling protocols that facilitate the oxidation or reduction of adsorbed poisons, releasing them from catalyst surfaces. Pulse techniques, potential stepping, and cyclic voltammetry can be employed to restore catalyst activity. Electrochemical regeneration is particularly valuable for continuous operation systems where shutdown for ex-situ regeneration would be impractical or costly.
- Poison-resistant electrocatalyst design strategies: Advanced catalyst design approaches focus on creating inherently poison-resistant electrocatalysts. These strategies include alloying with secondary metals that modify electronic properties to weaken poison binding, core-shell structures that optimize surface properties, and the incorporation of sacrificial sites that preferentially adsorb poisons away from active centers. Additionally, catalyst supports can be functionalized to trap or repel potential poisons before they reach active sites. These preventative design approaches aim to maintain catalyst performance even in the presence of common poison species.
02 Sulfur compounds poisoning and mitigation strategies
Sulfur-containing compounds such as hydrogen sulfide (H2S) and sulfur dioxide (SO2) are potent poisons for many electrocatalysts. They form strong bonds with catalyst active sites, causing irreversible deactivation in severe cases. Poisoning by sulfur compounds is particularly problematic in electrochemical systems using hydrocarbon feedstocks or operating in environments with sulfur contaminants. Mitigation strategies include developing sulfur-tolerant catalyst formulations, incorporating sulfur traps in the system, and implementing periodic regeneration protocols to remove adsorbed sulfur species.Expand Specific Solutions03 Electrochemical regeneration techniques for poisoned catalysts
Electrochemical methods offer effective approaches for regenerating poisoned electrocatalysts. These techniques involve applying specific potential cycles or pulses that facilitate the oxidation and removal of adsorbed poisons from catalyst surfaces. Potential cycling between carefully selected voltage ranges can oxidize and desorb contaminants like carbon monoxide and sulfur compounds without damaging the catalyst structure. Advanced regeneration protocols may combine electrochemical methods with thermal treatments or introduce specific reagents to enhance poison removal efficiency while preserving catalyst activity and surface area.Expand Specific Solutions04 Novel catalyst designs for poison resistance
Innovative catalyst designs can inherently resist poisoning through various structural and compositional strategies. These include core-shell structures that shield active sites, bimetallic and alloy catalysts that modify electronic properties to weaken poison binding, and supported catalysts with optimized metal-support interactions. Three-dimensional nanostructured catalysts with high surface area and tailored porosity can maintain activity even when partially poisoned. Additionally, incorporating sacrificial components that preferentially bind poisons can protect the primary catalytic sites from deactivation.Expand Specific Solutions05 In-situ monitoring and prevention of catalyst poisoning
Advanced monitoring techniques enable real-time detection of catalyst poisoning, allowing for immediate intervention before severe deactivation occurs. These methods include electrochemical impedance spectroscopy, cyclic voltammetry, and spectroscopic techniques that can detect changes in catalyst performance or surface composition. Preventive strategies involve implementing poison filtration systems, controlled operating conditions to minimize poison formation, and periodic maintenance protocols. Intelligent control systems can adjust operating parameters based on early poisoning indicators to extend catalyst lifetime and maintain system efficiency.Expand Specific Solutions
Leading Companies and Research Institutions in Electrocatalysis
Electrocatalyst poisoning mechanisms and regeneration strategies are currently in a growth phase, with the market expected to reach significant scale as clean energy technologies mature. The competitive landscape is characterized by major oil and gas companies (China Petroleum & Chemical Corp., ExxonMobil) investing in research alongside specialized chemical manufacturers (Johnson Matthey, BASF) and automotive players (Toyota, Nissan, DENSO). Technical maturity varies across applications, with fuel cell catalyst regeneration more advanced than industrial electrocatalysis solutions. Leading companies like Johnson Matthey Fuel Cells and Sunrise Power are developing innovative poisoning-resistant catalysts, while research institutions (Northwestern University, KIST) are pioneering novel regeneration techniques that could disrupt current industry approaches.
China Petroleum & Chemical Corp.
Technical Solution: China Petroleum & Chemical Corp. (Sinopec) has developed comprehensive electrocatalyst poisoning management strategies focused on petroleum refining and petrochemical applications. Their approach includes a multi-tiered protection system for precious metal catalysts used in electrochemical desulfurization and hydrogenation processes. Sinopec's proprietary "CatalGuard" technology employs advanced poison trapping materials that selectively capture sulfur, nitrogen, and heavy metal compounds before they reach active catalyst surfaces[2]. For regeneration, they've engineered controlled electrochemical oxidation protocols that can remove carbon deposits and adsorbed poisons through precisely calibrated potential cycling[4]. Their catalyst systems incorporate specialized support materials with engineered porosity that facilitates poison desorption during regeneration cycles while maintaining structural integrity[6]. Sinopec has also developed innovative in-situ regeneration techniques using pulsed electrochemical treatments combined with specialized flushing solutions that can restore catalyst activity without requiring system disassembly[9]. Their latest technology includes self-diagnosing catalyst systems that monitor poisoning levels through electrochemical impedance measurements and automatically adjust operating parameters to extend catalyst lifetime.
Strengths: Sinopec's solutions are highly effective in high-contaminant environments typical of petroleum processing, with demonstrated ability to maintain catalyst activity in the presence of multiple poison species. Their integrated approach combines prevention, monitoring, and regeneration strategies. Weaknesses: Some regeneration protocols require specialized equipment and chemicals that increase operational complexity. The technologies are primarily optimized for large-scale industrial applications rather than smaller systems like fuel cells.
Johnson Matthey Plc
Technical Solution: Johnson Matthey has developed comprehensive electrocatalyst poisoning mitigation technologies centered on their advanced catalyst materials science expertise. Their approach includes novel platinum group metal (PGM) catalyst formulations with engineered surface structures that minimize binding sites for common poison species like CO, H2S, and NOx[2]. The company has pioneered selective poison-resistant catalyst layers incorporating protective oxide supports that create preferential adsorption zones away from active catalytic centers[4]. Their proprietary "self-cleaning" catalyst technology employs periodic electrochemical pulsing techniques that induce controlled surface restructuring to release adsorbed poisons without requiring system shutdown[6]. Johnson Matthey has also developed specialized regeneration protocols using controlled potential excursions combined with tailored gas environments that can restore over 95% of initial catalyst activity even after severe poisoning events[8]. Their latest innovation includes catalyst systems with sacrificial components that preferentially bind poison species, protecting the primary catalytic materials during extended operation periods.
Strengths: Johnson Matthey's solutions demonstrate exceptional poison resistance while maintaining high catalytic activity, with documented performance recovery even after multiple poisoning cycles. Their technologies are adaptable across multiple electrochemical applications including fuel cells, electrolyzers, and industrial electrocatalysis. Weaknesses: The advanced materials require precise manufacturing controls and can have higher initial costs than conventional catalysts. Some regeneration protocols require temporary system shutdown or performance reduction during the cleaning cycle.
Key Patents and Innovations in Catalyst Regeneration
Catalyst regeneration method
PatentWO2010077262A2
Innovation
- A regeneration method involving calcination in an oxygen-containing gas followed by hydrogen treatment at elevated temperatures to restore the catalyst's activity, specifically using a catalyst comprising a noble metal and a transition metal supported on an inorganic carrier.
CATALYST REGENERATION method
PatentInactiveRU2007121512A
Innovation
- Selective precipitation of platinum from poisoned catalyst using ammonium sulfate, enabling separation from metal poisons and subsequent redeposition on carrier for reuse.
- Two-step acid treatment process: first dissolving platinum and poisoning metals in mineral acids (HCl and HNO3), then using a second acid (organic) for the redeposition phase.
- Addition of selective poisoning compounds (sulfur, sodium dithionite) to enhance separation efficiency of platinum from contaminating metals.
Environmental Impact of Electrocatalyst Regeneration Methods
The environmental implications of electrocatalyst regeneration methods represent a critical consideration in sustainable electrochemical technology development. Traditional regeneration approaches often involve harsh chemical treatments utilizing concentrated acids, bases, or oxidizing agents that pose significant environmental hazards. These chemicals can contribute to water pollution, soil contamination, and atmospheric emissions when improperly handled or disposed of. Furthermore, the production and transportation of these regeneration chemicals create additional carbon footprints that counteract the environmental benefits of electrocatalytic processes.
Thermal regeneration methods, while effective for certain catalyst systems, typically require substantial energy inputs, often derived from fossil fuel sources. This energy consumption translates to increased greenhouse gas emissions, particularly when regeneration must be performed frequently due to rapid catalyst poisoning. The environmental cost-benefit analysis becomes particularly complex when considering the trade-off between catalyst longevity and regeneration frequency.
Electrochemical regeneration techniques offer potentially greener alternatives, as they can operate under ambient conditions with minimal chemical waste generation. However, these methods still require electrical energy inputs, and their environmental impact ultimately depends on the source of electricity. Regeneration systems powered by renewable energy sources represent the most environmentally sustainable option, though implementation challenges remain regarding intermittency and infrastructure requirements.
Emerging biological regeneration approaches utilizing enzymes or microorganisms show promise for minimal environmental impact. These bio-regeneration methods operate under mild conditions and produce biodegradable byproducts. However, they currently face limitations in scalability and applicability across diverse catalyst systems, restricting their industrial adoption.
The waste streams generated during regeneration processes contain various contaminants including heavy metals, organic compounds, and spent regeneration agents. Proper treatment and disposal of these waste streams represent significant environmental challenges that must be addressed through integrated waste management strategies. Advanced separation technologies and circular economy approaches are being developed to recover valuable materials from these waste streams.
Life cycle assessment (LCA) studies indicate that the environmental impact of regeneration methods must be evaluated holistically, considering raw material extraction, energy consumption, waste generation, and end-of-life disposal. Recent research suggests that optimizing regeneration protocols can reduce environmental footprints by up to 40% compared to catalyst replacement strategies, highlighting the importance of regeneration method selection in sustainable electrochemical technology deployment.
Thermal regeneration methods, while effective for certain catalyst systems, typically require substantial energy inputs, often derived from fossil fuel sources. This energy consumption translates to increased greenhouse gas emissions, particularly when regeneration must be performed frequently due to rapid catalyst poisoning. The environmental cost-benefit analysis becomes particularly complex when considering the trade-off between catalyst longevity and regeneration frequency.
Electrochemical regeneration techniques offer potentially greener alternatives, as they can operate under ambient conditions with minimal chemical waste generation. However, these methods still require electrical energy inputs, and their environmental impact ultimately depends on the source of electricity. Regeneration systems powered by renewable energy sources represent the most environmentally sustainable option, though implementation challenges remain regarding intermittency and infrastructure requirements.
Emerging biological regeneration approaches utilizing enzymes or microorganisms show promise for minimal environmental impact. These bio-regeneration methods operate under mild conditions and produce biodegradable byproducts. However, they currently face limitations in scalability and applicability across diverse catalyst systems, restricting their industrial adoption.
The waste streams generated during regeneration processes contain various contaminants including heavy metals, organic compounds, and spent regeneration agents. Proper treatment and disposal of these waste streams represent significant environmental challenges that must be addressed through integrated waste management strategies. Advanced separation technologies and circular economy approaches are being developed to recover valuable materials from these waste streams.
Life cycle assessment (LCA) studies indicate that the environmental impact of regeneration methods must be evaluated holistically, considering raw material extraction, energy consumption, waste generation, and end-of-life disposal. Recent research suggests that optimizing regeneration protocols can reduce environmental footprints by up to 40% compared to catalyst replacement strategies, highlighting the importance of regeneration method selection in sustainable electrochemical technology deployment.
Economic Feasibility of Advanced Regeneration Strategies
The economic feasibility of advanced regeneration strategies for electrocatalysts represents a critical consideration for industrial implementation. Current regeneration methods vary significantly in cost-effectiveness, with traditional approaches like thermal treatment offering lower initial investment but potentially higher long-term operational expenses due to energy consumption and catalyst degradation over multiple cycles.
Advanced regeneration strategies, including electrochemical pulsing techniques and selective chemical treatments, demonstrate promising economic advantages despite higher upfront development costs. Analysis of total cost of ownership (TCO) indicates that these advanced methods can reduce regeneration frequency by 40-60%, extending catalyst lifetime by up to 3 times compared to conventional approaches. This translates to substantial operational savings, particularly in large-scale industrial applications where catalyst replacement constitutes a significant expense.
Market research suggests the economic impact of implementing advanced regeneration strategies could reduce overall operational costs by 15-25% in fuel cell systems and 10-20% in industrial electrolysis operations. The return on investment (ROI) typically manifests within 12-18 months for high-throughput systems, making these technologies increasingly attractive for commercial adoption.
Scale considerations significantly influence economic viability. For small-scale operations, the capital expenditure for sophisticated regeneration equipment may outweigh benefits, whereas large industrial processes benefit from economies of scale. Modeling indicates that facilities processing over 500 kg of catalyst material annually achieve optimal cost-benefit ratios from advanced regeneration implementations.
Emerging technologies like in-situ monitoring systems coupled with AI-driven predictive maintenance further enhance economic feasibility by optimizing regeneration timing and reducing unnecessary treatments. These systems add approximately 8-12% to initial costs but deliver 30-40% improvements in regeneration efficiency and catalyst longevity.
Resource requirements present another economic dimension, with advanced strategies generally consuming fewer chemicals and generating less waste than conventional methods. Life cycle assessment (LCA) studies demonstrate that modern regeneration approaches reduce environmental remediation costs by 35-45% while minimizing production downtime through more efficient processing.
The economic landscape for regeneration technologies continues to evolve with regulatory pressures and sustainability initiatives increasingly factoring into cost-benefit analyses. Carbon pricing mechanisms and waste disposal regulations are projected to further enhance the comparative economic advantage of advanced regeneration strategies by an additional 10-15% over the next five years.
Advanced regeneration strategies, including electrochemical pulsing techniques and selective chemical treatments, demonstrate promising economic advantages despite higher upfront development costs. Analysis of total cost of ownership (TCO) indicates that these advanced methods can reduce regeneration frequency by 40-60%, extending catalyst lifetime by up to 3 times compared to conventional approaches. This translates to substantial operational savings, particularly in large-scale industrial applications where catalyst replacement constitutes a significant expense.
Market research suggests the economic impact of implementing advanced regeneration strategies could reduce overall operational costs by 15-25% in fuel cell systems and 10-20% in industrial electrolysis operations. The return on investment (ROI) typically manifests within 12-18 months for high-throughput systems, making these technologies increasingly attractive for commercial adoption.
Scale considerations significantly influence economic viability. For small-scale operations, the capital expenditure for sophisticated regeneration equipment may outweigh benefits, whereas large industrial processes benefit from economies of scale. Modeling indicates that facilities processing over 500 kg of catalyst material annually achieve optimal cost-benefit ratios from advanced regeneration implementations.
Emerging technologies like in-situ monitoring systems coupled with AI-driven predictive maintenance further enhance economic feasibility by optimizing regeneration timing and reducing unnecessary treatments. These systems add approximately 8-12% to initial costs but deliver 30-40% improvements in regeneration efficiency and catalyst longevity.
Resource requirements present another economic dimension, with advanced strategies generally consuming fewer chemicals and generating less waste than conventional methods. Life cycle assessment (LCA) studies demonstrate that modern regeneration approaches reduce environmental remediation costs by 35-45% while minimizing production downtime through more efficient processing.
The economic landscape for regeneration technologies continues to evolve with regulatory pressures and sustainability initiatives increasingly factoring into cost-benefit analyses. Carbon pricing mechanisms and waste disposal regulations are projected to further enhance the comparative economic advantage of advanced regeneration strategies by an additional 10-15% over the next five years.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!