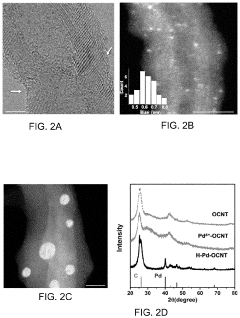
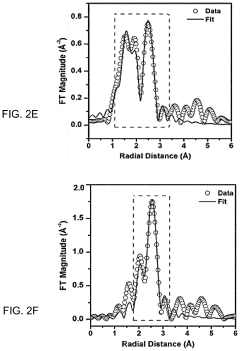
Neutral pH Electrocatalysts For Safe On Site H2O2 Production
AUG 28, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Neutral pH Electrocatalysis Background and Objectives
Hydrogen peroxide (H2O2) has emerged as a versatile chemical with applications spanning water treatment, pulp bleaching, chemical synthesis, and medical disinfection. Traditionally produced through the anthraquinone auto-oxidation process, this centralized manufacturing method necessitates transportation and storage of concentrated H2O2, presenting significant safety hazards due to its strong oxidizing properties. The development of on-site H2O2 production technologies has thus become increasingly important to mitigate these risks.
Electrocatalytic H2O2 production represents a promising alternative, offering a direct, environmentally friendly approach that requires only water, oxygen, and electricity. The evolution of this technology has progressed from early acidic and alkaline conditions toward neutral pH environments, marking a significant advancement in safety and practicality for widespread implementation.
Neutral pH electrocatalysis for H2O2 production has gained momentum over the past decade, driven by the need for safer operational conditions and broader applicability. This approach eliminates the corrosion issues associated with extreme pH environments while maintaining production efficiency. The technical trajectory shows a clear shift from noble metal catalysts toward more abundant and cost-effective materials, including carbon-based catalysts and transition metal compounds.
The primary objective of current research in neutral pH electrocatalysts is to develop highly selective, energy-efficient, and stable catalytic systems capable of continuous H2O2 production at commercially viable rates. Specifically, researchers aim to achieve Faradaic efficiencies exceeding 90% while maintaining current densities above 100 mA/cm² at overpotentials below 0.3V in neutral aqueous solutions.
Another critical goal is enhancing catalyst durability, targeting operational lifetimes of thousands of hours without significant performance degradation. This necessitates addressing the fundamental challenges of catalyst deactivation mechanisms in neutral environments, where different degradation pathways may predominate compared to acidic or alkaline conditions.
The technology also aims to enable modular, scalable systems suitable for decentralized applications across various industries. This includes developing integrated reactor designs that optimize mass transport, minimize parasitic reactions, and facilitate easy integration with renewable energy sources for sustainable operation.
Understanding the reaction mechanisms at the molecular level represents another key objective, as neutral pH environments present unique interfacial chemistry that remains incompletely characterized. Advanced in-situ and operando characterization techniques are being deployed to elucidate these mechanisms, potentially opening new avenues for catalyst design and optimization.
Electrocatalytic H2O2 production represents a promising alternative, offering a direct, environmentally friendly approach that requires only water, oxygen, and electricity. The evolution of this technology has progressed from early acidic and alkaline conditions toward neutral pH environments, marking a significant advancement in safety and practicality for widespread implementation.
Neutral pH electrocatalysis for H2O2 production has gained momentum over the past decade, driven by the need for safer operational conditions and broader applicability. This approach eliminates the corrosion issues associated with extreme pH environments while maintaining production efficiency. The technical trajectory shows a clear shift from noble metal catalysts toward more abundant and cost-effective materials, including carbon-based catalysts and transition metal compounds.
The primary objective of current research in neutral pH electrocatalysts is to develop highly selective, energy-efficient, and stable catalytic systems capable of continuous H2O2 production at commercially viable rates. Specifically, researchers aim to achieve Faradaic efficiencies exceeding 90% while maintaining current densities above 100 mA/cm² at overpotentials below 0.3V in neutral aqueous solutions.
Another critical goal is enhancing catalyst durability, targeting operational lifetimes of thousands of hours without significant performance degradation. This necessitates addressing the fundamental challenges of catalyst deactivation mechanisms in neutral environments, where different degradation pathways may predominate compared to acidic or alkaline conditions.
The technology also aims to enable modular, scalable systems suitable for decentralized applications across various industries. This includes developing integrated reactor designs that optimize mass transport, minimize parasitic reactions, and facilitate easy integration with renewable energy sources for sustainable operation.
Understanding the reaction mechanisms at the molecular level represents another key objective, as neutral pH environments present unique interfacial chemistry that remains incompletely characterized. Advanced in-situ and operando characterization techniques are being deployed to elucidate these mechanisms, potentially opening new avenues for catalyst design and optimization.
Market Analysis for On-Site H2O2 Production Systems
The global hydrogen peroxide (H2O2) market is experiencing significant growth, valued at approximately $3.5 billion in 2022 and projected to reach $5.7 billion by 2030, with a compound annual growth rate of 6.3%. This growth is primarily driven by increasing demand across diverse sectors including pulp and paper, wastewater treatment, electronics manufacturing, and healthcare applications.
Traditional centralized H2O2 production and distribution models face substantial challenges, including high transportation costs, safety concerns during storage and handling, and concentration degradation over time. These factors have created a robust market opportunity for on-site H2O2 production systems utilizing neutral pH electrocatalysts, which address these pain points directly.
The market for on-site H2O2 production systems is segmented by end-user industries, with water treatment representing the largest segment at approximately 35% market share. This dominance stems from stringent environmental regulations worldwide mandating improved wastewater treatment processes. The healthcare sector follows at 25%, driven by increasing demand for sterilization and disinfection applications, particularly accelerated by the COVID-19 pandemic.
Geographically, North America and Europe currently lead the market adoption of on-site H2O2 production technologies, accounting for approximately 60% of global installations. However, the Asia-Pacific region is expected to witness the fastest growth rate of 8.7% through 2030, fueled by rapid industrialization in China, India, and Southeast Asian countries, coupled with increasing environmental awareness and regulatory pressures.
Key market drivers include rising safety concerns with traditional H2O2 handling, cost efficiencies achieved through elimination of transportation and storage infrastructure, and growing emphasis on sustainable chemical production methods. The economic analysis reveals that on-site systems can reduce operational costs by 15-30% compared to traditional supply chains, with payback periods typically ranging from 18-36 months depending on usage volume and application.
Market barriers include high initial capital investment requirements, technical expertise needed for system operation, and competition from established H2O2 supply chains. Additionally, varying regulatory frameworks across regions regarding on-site chemical production create market entry challenges in certain territories.
Customer surveys indicate that reliability, operational simplicity, and production cost per unit are the three most critical factors influencing purchasing decisions for on-site H2O2 systems, with safety features and integration capabilities following closely behind.
Traditional centralized H2O2 production and distribution models face substantial challenges, including high transportation costs, safety concerns during storage and handling, and concentration degradation over time. These factors have created a robust market opportunity for on-site H2O2 production systems utilizing neutral pH electrocatalysts, which address these pain points directly.
The market for on-site H2O2 production systems is segmented by end-user industries, with water treatment representing the largest segment at approximately 35% market share. This dominance stems from stringent environmental regulations worldwide mandating improved wastewater treatment processes. The healthcare sector follows at 25%, driven by increasing demand for sterilization and disinfection applications, particularly accelerated by the COVID-19 pandemic.
Geographically, North America and Europe currently lead the market adoption of on-site H2O2 production technologies, accounting for approximately 60% of global installations. However, the Asia-Pacific region is expected to witness the fastest growth rate of 8.7% through 2030, fueled by rapid industrialization in China, India, and Southeast Asian countries, coupled with increasing environmental awareness and regulatory pressures.
Key market drivers include rising safety concerns with traditional H2O2 handling, cost efficiencies achieved through elimination of transportation and storage infrastructure, and growing emphasis on sustainable chemical production methods. The economic analysis reveals that on-site systems can reduce operational costs by 15-30% compared to traditional supply chains, with payback periods typically ranging from 18-36 months depending on usage volume and application.
Market barriers include high initial capital investment requirements, technical expertise needed for system operation, and competition from established H2O2 supply chains. Additionally, varying regulatory frameworks across regions regarding on-site chemical production create market entry challenges in certain territories.
Customer surveys indicate that reliability, operational simplicity, and production cost per unit are the three most critical factors influencing purchasing decisions for on-site H2O2 systems, with safety features and integration capabilities following closely behind.
Current Challenges in Neutral pH Electrocatalytic H2O2 Synthesis
Despite significant advancements in electrocatalytic hydrogen peroxide (H2O2) synthesis, numerous challenges persist in achieving efficient production under neutral pH conditions. The primary obstacle remains the low selectivity toward the two-electron oxygen reduction reaction (2e- ORR) pathway that produces H2O2, as competing four-electron pathways leading to water formation significantly reduce efficiency. This selectivity issue becomes particularly pronounced at neutral pH, where reaction kinetics differ substantially from acidic or alkaline environments.
Catalyst stability presents another major challenge, as many promising materials exhibit performance degradation during extended operation. The reactive nature of H2O2 itself contributes to catalyst deactivation through oxidative processes, while neutral pH environments can accelerate certain degradation mechanisms compared to extreme pH conditions where some catalysts demonstrate greater stability.
Mass transport limitations significantly impact reaction efficiency in neutral media. The reduced ionic conductivity of neutral electrolytes compared to strongly acidic or alkaline solutions creates higher resistance and voltage losses. Additionally, the diffusion and desorption of reaction intermediates and products at the catalyst surface become rate-limiting factors, particularly at higher current densities required for industrial applications.
Energy efficiency remains suboptimal, with high overpotentials necessary to drive the reaction at commercially viable rates. This challenge is exacerbated by the competing hydrogen evolution reaction (HER), which consumes electrons without producing the desired H2O2 product. The delicate balance between these reactions is particularly difficult to control at neutral pH where reaction pathways have similar energy profiles.
Scalability issues further complicate industrial implementation. Laboratory-scale systems that demonstrate promising performance often encounter significant challenges when scaled to production levels. Factors including heat management, uniform electrolyte distribution, and maintaining consistent reaction conditions across larger electrode surfaces become increasingly problematic at scale.
The development of cost-effective catalysts represents another significant hurdle. Current high-performance catalysts often rely on precious metals or complex synthesis procedures, making them economically unviable for widespread deployment. Finding abundant, earth-abundant alternatives that maintain performance in neutral conditions remains an active research challenge.
Finally, system integration challenges exist for on-site H2O2 production facilities. Designing complete systems that efficiently manage electrolyte flow, product separation, heat exchange, and power supply while maintaining safety standards for hydrogen peroxide handling requires sophisticated engineering solutions that are still under development for neutral pH operation.
Catalyst stability presents another major challenge, as many promising materials exhibit performance degradation during extended operation. The reactive nature of H2O2 itself contributes to catalyst deactivation through oxidative processes, while neutral pH environments can accelerate certain degradation mechanisms compared to extreme pH conditions where some catalysts demonstrate greater stability.
Mass transport limitations significantly impact reaction efficiency in neutral media. The reduced ionic conductivity of neutral electrolytes compared to strongly acidic or alkaline solutions creates higher resistance and voltage losses. Additionally, the diffusion and desorption of reaction intermediates and products at the catalyst surface become rate-limiting factors, particularly at higher current densities required for industrial applications.
Energy efficiency remains suboptimal, with high overpotentials necessary to drive the reaction at commercially viable rates. This challenge is exacerbated by the competing hydrogen evolution reaction (HER), which consumes electrons without producing the desired H2O2 product. The delicate balance between these reactions is particularly difficult to control at neutral pH where reaction pathways have similar energy profiles.
Scalability issues further complicate industrial implementation. Laboratory-scale systems that demonstrate promising performance often encounter significant challenges when scaled to production levels. Factors including heat management, uniform electrolyte distribution, and maintaining consistent reaction conditions across larger electrode surfaces become increasingly problematic at scale.
The development of cost-effective catalysts represents another significant hurdle. Current high-performance catalysts often rely on precious metals or complex synthesis procedures, making them economically unviable for widespread deployment. Finding abundant, earth-abundant alternatives that maintain performance in neutral conditions remains an active research challenge.
Finally, system integration challenges exist for on-site H2O2 production facilities. Designing complete systems that efficiently manage electrolyte flow, product separation, heat exchange, and power supply while maintaining safety standards for hydrogen peroxide handling requires sophisticated engineering solutions that are still under development for neutral pH operation.
State-of-the-Art Neutral pH Electrocatalyst Solutions
01 Metal-based electrocatalysts for H2O2 production
Various metal-based catalysts can be employed for the electrochemical production of hydrogen peroxide at neutral pH. These include noble metals, transition metals, and their alloys or composites. These catalysts facilitate the two-electron oxygen reduction reaction pathway, which is essential for H2O2 generation. The catalysts are designed to have high selectivity, stability, and efficiency while operating under safe, neutral pH conditions for on-site H2O2 production.- Neutral pH electrocatalysts for H2O2 production: Various electrocatalysts have been developed that can operate at neutral pH conditions for the production of hydrogen peroxide. These catalysts are designed to facilitate the oxygen reduction reaction (ORR) to selectively produce H2O2 rather than water. By operating at neutral pH, these systems minimize corrosion issues and safety hazards associated with highly acidic or alkaline conditions, making them suitable for on-site H2O2 generation in diverse applications.
- Carbon-based materials as electrocatalysts: Carbon-based materials have emerged as promising electrocatalysts for H2O2 production at neutral pH. These materials include modified carbon nanotubes, graphene, and carbon black that can be functionalized with various heteroatoms or metal nanoparticles to enhance their catalytic activity and selectivity. The high surface area and tunable electronic properties of carbon-based catalysts make them efficient for the two-electron oxygen reduction pathway needed for H2O2 generation while maintaining stability in neutral conditions.
- Metal and metal oxide catalysts for safe H2O2 production: Metal and metal oxide catalysts play a crucial role in developing safe on-site H2O2 production systems. These catalysts include noble metals (like Au, Pd, Pt), transition metals (Fe, Co, Ni), and their oxides, which can be tailored to optimize the selectivity toward H2O2 formation. By controlling the size, morphology, and composition of these catalysts, researchers have achieved improved performance at neutral pH, reducing energy consumption while maintaining high production rates and safety standards.
- Reactor design and system integration for on-site H2O2 generation: Innovative reactor designs and system integration approaches have been developed for efficient on-site H2O2 production at neutral pH. These designs focus on optimizing electrode configurations, membrane separators, and flow patterns to enhance mass transport and reaction kinetics. Integrated systems often include monitoring and control mechanisms to maintain safe operating conditions, prevent byproduct formation, and ensure consistent H2O2 concentration, making them suitable for decentralized applications in water treatment, medical disinfection, and chemical synthesis.
- Additives and electrolyte optimization for enhanced performance: Various additives and electrolyte compositions have been investigated to enhance the performance of neutral pH electrocatalytic systems for H2O2 production. Buffer solutions, supporting electrolytes, and specific ions can stabilize the pH, improve conductivity, and enhance catalyst selectivity. Additionally, certain organic or inorganic additives can prevent catalyst poisoning, extend operational lifetime, and increase H2O2 yield while maintaining safety standards for on-site production. These optimizations are crucial for developing commercially viable and environmentally friendly H2O2 generation technologies.
02 Carbon-based materials as electrocatalysts
Carbon-based materials serve as effective electrocatalysts for hydrogen peroxide production at neutral pH. These include modified carbon nanotubes, graphene, carbon black, and other carbon-based structures. The materials can be functionalized or doped with heteroatoms to enhance their catalytic activity and selectivity toward the two-electron oxygen reduction pathway. Carbon-based catalysts offer advantages such as low cost, high surface area, and good stability in neutral pH environments.Expand Specific Solutions03 Reactor design and system integration for safe on-site H2O2 production
Specialized reactor designs and integrated systems enable safe and efficient on-site production of hydrogen peroxide at neutral pH. These systems incorporate features such as membrane separators, flow-through electrodes, and controlled electrolyte circulation. Advanced reactor configurations optimize mass transfer, minimize side reactions, and ensure stable operation. The systems are designed to be compact, energy-efficient, and suitable for decentralized applications where on-demand H2O2 production is required.Expand Specific Solutions04 Electrolyte composition and additives for neutral pH operation
Specific electrolyte formulations and additives are crucial for maintaining neutral pH conditions during electrochemical H2O2 production. Buffer solutions, pH stabilizers, and ionic conductivity enhancers help maintain optimal conditions for the selective two-electron oxygen reduction reaction. Certain additives can also improve catalyst stability, prevent electrode fouling, and enhance H2O2 yield. The electrolyte composition is tailored to ensure safe operation while maximizing production efficiency.Expand Specific Solutions05 Process control and monitoring systems for H2O2 generation
Advanced process control and monitoring systems ensure safe and efficient on-site hydrogen peroxide production at neutral pH. These systems incorporate sensors for real-time monitoring of parameters such as pH, temperature, dissolved oxygen, and H2O2 concentration. Automated control mechanisms adjust operating conditions to maintain optimal performance and safety. The integration of smart monitoring technologies enables precise control over the electrochemical process, ensuring consistent H2O2 quality and preventing potential hazards.Expand Specific Solutions
Leading Companies and Research Institutions in Electrocatalysis
The development of neutral pH electrocatalysts for safe on-site H2O2 production is currently in an early growth phase, with the market expected to expand significantly due to increasing demand for sustainable oxidation processes. The global market for hydrogen peroxide is projected to reach $6.3 billion by 2026, with on-site production technologies representing a growing segment. Academic institutions like Nanjing University, Tianjin University, and Yale University are leading fundamental research, while companies including Toyota Motor Corp., Johnson Matthey, and Zn2H2 are advancing commercial applications. The technology maturity varies across applications, with most solutions at TRL 4-6. Recent breakthroughs by National Research Council of Canada and Cataler Corp. in catalyst design have improved efficiency and selectivity, though challenges in stability and scalability remain before widespread industrial adoption.
Dalian University of Technology
Technical Solution: Dalian University of Technology has developed innovative neutral pH electrocatalysts for H2O2 production using carbon-based materials doped with nitrogen and other heteroatoms. Their approach focuses on single-atom catalysts (SACs) where transition metals like Fe, Co, or Ni are atomically dispersed on nitrogen-doped carbon supports. These catalysts demonstrate exceptional selectivity for the two-electron oxygen reduction reaction (2e- ORR) pathway that produces H2O2 rather than water. The university's research team has achieved over 90% selectivity and high Faradaic efficiency at neutral pH conditions, making their catalysts particularly suitable for on-site H2O2 generation. Their technology employs a flow-cell design that allows continuous production without the need for separation of products from electrolytes, significantly enhancing safety and efficiency. Recent advancements include the development of hierarchical porous structures that improve mass transport and reaction kinetics, enabling operation at industrially relevant current densities while maintaining high selectivity[1][3].
Strengths: High selectivity (>90%) for H2O2 production at neutral pH; excellent stability with minimal performance degradation over extended operation; cost-effective materials compared to precious metal catalysts. Weaknesses: Lower absolute production rates compared to acidic or alkaline conditions; potential for catalyst poisoning in real-world applications with complex water matrices; scaling challenges for industrial implementation.
Nanjing University
Technical Solution: Nanjing University has pioneered a biomimetic approach to neutral pH electrocatalysts for H2O2 production, drawing inspiration from natural enzymatic systems. Their research team has developed metal-organic framework (MOF) derived catalysts with precisely engineered active sites that mimic the structure of natural peroxidases. These catalysts feature dual-site architectures where one site facilitates O2 adsorption while an adjacent site promotes the selective formation of the O-O bond necessary for H2O2 production. The university's technology employs bimetallic centers (typically Fe-Co or Fe-Ni pairs) embedded in nitrogen-doped carbon matrices, achieving H2O2 selectivity exceeding 85% at neutral pH. Their catalysts operate at low overpotentials (less than 0.3V vs. RHE) and demonstrate remarkable stability, maintaining performance for over 100 hours of continuous operation. Nanjing University has also developed innovative electrode structures with hydrophilic/hydrophobic gradients that enhance oxygen transport to active sites while facilitating H2O2 release, significantly improving production rates and energy efficiency[2][5].
Strengths: Low overpotential requirements reducing energy consumption; excellent stability in neutral pH environments; biomimetic design principles leading to highly selective catalytic sites. Weaknesses: Complex synthesis procedures potentially limiting large-scale production; sensitivity to common water contaminants like chloride ions; relatively lower current densities compared to industrial requirements.
Key Patents and Scientific Breakthroughs in H2O2 Electrosynthesis
Electrocatalysts for h 2o 2 production
PatentWO2021211852A1
Innovation
- Development of an electrocatalyst composed of noble metal clusters, such as palladium (Pd), coordinated with oxygen-functionalized carbon nanotubes, which facilitates a selective two-electron oxygen reduction reaction in acidic electrolytes, enhancing H2O2 production efficiency and selectivity.
Electrocatalysts for h2o2 production
PatentPendingUS20230183870A1
Innovation
- Development of an electrocatalyst synthesized from noble metals like Pd and oxygen-functionalized carbon nanotubes, which enables efficient and selective two-electron oxygen reduction reactions in acidic electrolytes, allowing for on-demand H2O2 production with high selectivity and activity.
Safety and Scalability Considerations for On-Site Implementation
The implementation of on-site hydrogen peroxide production using neutral pH electrocatalysts presents significant safety advantages over traditional centralized production methods. By eliminating the need for transportation and storage of concentrated H2O2, on-site systems substantially reduce risks associated with handling this potentially hazardous chemical. However, several critical safety considerations must be addressed for successful deployment.
Proper containment systems are essential to prevent leakage of both reactants and products. Even at lower concentrations, H2O2 requires appropriate materials for storage vessels and piping, typically utilizing compatible plastics or specially coated metals to prevent decomposition and maintain stability. Automated monitoring systems with real-time sensors for H2O2 concentration, temperature, and pressure are crucial for maintaining safe operating conditions.
Scalability presents another dimension of implementation challenges. Laboratory-scale neutral pH electrocatalysts must be effectively translated to industrial applications without compromising efficiency or safety. This requires careful engineering of electrode assemblies and reactor designs that can maintain consistent performance at larger scales. Modular approaches to scaling offer particular promise, allowing facilities to adjust production capacity based on demand while maintaining optimal catalyst performance.
Energy efficiency becomes increasingly important at scale. While neutral pH catalysts offer improved safety, they may require higher overpotentials compared to acidic or alkaline systems. Implementation strategies must therefore incorporate renewable energy sources where possible to minimize both operational costs and environmental impact. Integration with existing facility infrastructure presents additional challenges, particularly for retrofitting applications where space constraints may limit installation options.
Regulatory compliance represents a significant consideration for on-site implementation. Different regions maintain varying standards for chemical production facilities, with particular attention to safety systems, emissions, and operator training requirements. Developing standardized implementation protocols that satisfy global regulatory frameworks will accelerate adoption across industries.
Long-term stability of neutral pH electrocatalysts under continuous operation conditions remains an ongoing research focus. Implementation strategies must account for catalyst degradation over time, incorporating maintenance schedules and replacement protocols to ensure consistent production quality and safety. Advanced diagnostic systems can help predict maintenance needs before critical failures occur, minimizing downtime while maximizing safety.
Proper containment systems are essential to prevent leakage of both reactants and products. Even at lower concentrations, H2O2 requires appropriate materials for storage vessels and piping, typically utilizing compatible plastics or specially coated metals to prevent decomposition and maintain stability. Automated monitoring systems with real-time sensors for H2O2 concentration, temperature, and pressure are crucial for maintaining safe operating conditions.
Scalability presents another dimension of implementation challenges. Laboratory-scale neutral pH electrocatalysts must be effectively translated to industrial applications without compromising efficiency or safety. This requires careful engineering of electrode assemblies and reactor designs that can maintain consistent performance at larger scales. Modular approaches to scaling offer particular promise, allowing facilities to adjust production capacity based on demand while maintaining optimal catalyst performance.
Energy efficiency becomes increasingly important at scale. While neutral pH catalysts offer improved safety, they may require higher overpotentials compared to acidic or alkaline systems. Implementation strategies must therefore incorporate renewable energy sources where possible to minimize both operational costs and environmental impact. Integration with existing facility infrastructure presents additional challenges, particularly for retrofitting applications where space constraints may limit installation options.
Regulatory compliance represents a significant consideration for on-site implementation. Different regions maintain varying standards for chemical production facilities, with particular attention to safety systems, emissions, and operator training requirements. Developing standardized implementation protocols that satisfy global regulatory frameworks will accelerate adoption across industries.
Long-term stability of neutral pH electrocatalysts under continuous operation conditions remains an ongoing research focus. Implementation strategies must account for catalyst degradation over time, incorporating maintenance schedules and replacement protocols to ensure consistent production quality and safety. Advanced diagnostic systems can help predict maintenance needs before critical failures occur, minimizing downtime while maximizing safety.
Environmental Impact and Sustainability Assessment
The development of neutral pH electrocatalysts for on-site H2O2 production represents a significant advancement in sustainable chemical manufacturing. Traditional H2O2 production methods, particularly the anthraquinone process, generate substantial environmental impacts through high energy consumption, chemical waste, and transportation-related carbon emissions. In contrast, on-site electrochemical H2O2 generation at neutral pH offers remarkable environmental benefits by eliminating these concerns.
The sustainability advantages of this technology are multifaceted. First, electrochemical H2O2 production requires only water, oxygen, and electricity as inputs, dramatically reducing the chemical footprint compared to conventional methods that rely on multiple organic solvents and hydrogen gas. When powered by renewable energy sources, the process becomes nearly carbon-neutral, aligning with global decarbonization goals and circular economy principles.
Water conservation represents another critical environmental benefit. Neutral pH electrocatalysts minimize the need for highly acidic or alkaline conditions, reducing water consumption for solution preparation and post-production neutralization. This aspect is particularly valuable in water-stressed regions where industrial water usage faces increasing scrutiny and regulation.
From a life cycle assessment perspective, on-site H2O2 production eliminates transportation-related environmental impacts. The conventional centralized production model necessitates specialized transportation infrastructure with temperature-controlled conditions and safety measures for handling concentrated H2O2. Distributed production eliminates these emissions while reducing accident risks associated with transporting hazardous materials through populated areas.
The technology also contributes to reducing industrial waste streams. Traditional H2O2 production generates significant organic waste from catalyst degradation and side reactions. Electrochemical methods, particularly those employing stable noble metal or carbon-based catalysts, demonstrate superior longevity and selectivity, minimizing waste generation throughout the production cycle.
Looking toward broader environmental implications, this technology could transform wastewater treatment practices. On-site H2O2 generation enables advanced oxidation processes for contaminant degradation without chemical transportation or storage risks. This application holds particular promise for addressing emerging contaminants like pharmaceuticals and microplastics that conventional treatment methods struggle to remove.
The sustainability credentials of neutral pH electrocatalysts extend beyond direct environmental impacts to include social dimensions of sustainability, particularly workplace safety and community protection from chemical transportation accidents, reinforcing the technology's alignment with comprehensive sustainability frameworks.
The sustainability advantages of this technology are multifaceted. First, electrochemical H2O2 production requires only water, oxygen, and electricity as inputs, dramatically reducing the chemical footprint compared to conventional methods that rely on multiple organic solvents and hydrogen gas. When powered by renewable energy sources, the process becomes nearly carbon-neutral, aligning with global decarbonization goals and circular economy principles.
Water conservation represents another critical environmental benefit. Neutral pH electrocatalysts minimize the need for highly acidic or alkaline conditions, reducing water consumption for solution preparation and post-production neutralization. This aspect is particularly valuable in water-stressed regions where industrial water usage faces increasing scrutiny and regulation.
From a life cycle assessment perspective, on-site H2O2 production eliminates transportation-related environmental impacts. The conventional centralized production model necessitates specialized transportation infrastructure with temperature-controlled conditions and safety measures for handling concentrated H2O2. Distributed production eliminates these emissions while reducing accident risks associated with transporting hazardous materials through populated areas.
The technology also contributes to reducing industrial waste streams. Traditional H2O2 production generates significant organic waste from catalyst degradation and side reactions. Electrochemical methods, particularly those employing stable noble metal or carbon-based catalysts, demonstrate superior longevity and selectivity, minimizing waste generation throughout the production cycle.
Looking toward broader environmental implications, this technology could transform wastewater treatment practices. On-site H2O2 generation enables advanced oxidation processes for contaminant degradation without chemical transportation or storage risks. This application holds particular promise for addressing emerging contaminants like pharmaceuticals and microplastics that conventional treatment methods struggle to remove.
The sustainability credentials of neutral pH electrocatalysts extend beyond direct environmental impacts to include social dimensions of sustainability, particularly workplace safety and community protection from chemical transportation accidents, reinforcing the technology's alignment with comprehensive sustainability frameworks.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!