Scaling From Lab To Pilot Demonstrations For H2O2 Electrolyzers
AUG 28, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
H2O2 Electrolyzer Technology Background and Objectives
Hydrogen peroxide (H2O2) has emerged as a versatile chemical with applications spanning across industries including pulp and paper bleaching, wastewater treatment, chemical synthesis, and medical disinfection. Traditionally produced through the anthraquinone auto-oxidation process, this method requires significant energy input and generates substantial waste. Electrochemical production of H2O2 represents a promising alternative that aligns with global sustainability goals by enabling on-site, on-demand production with potentially lower environmental impact.
The evolution of H2O2 electrolyzer technology can be traced back to fundamental electrochemical research in the early 20th century. However, significant advancements have occurred primarily in the last two decades, driven by improvements in electrode materials, membrane technology, and system design. Recent breakthroughs in selective catalysts and novel cell configurations have dramatically improved production efficiency and selectivity, making electrochemical H2O2 production increasingly viable for commercial applications.
Current technological trends indicate a shift toward more efficient oxygen reduction reaction (ORR) catalysts, innovative cell designs that maximize mass transport, and integrated systems that can operate using renewable electricity sources. The convergence of these developments with growing industrial demand for greener chemical production processes has created a fertile ground for H2O2 electrolyzer technology advancement.
The primary technical objective in scaling H2O2 electrolyzers from laboratory to pilot demonstrations involves addressing several critical challenges. These include enhancing catalyst stability under industrial operating conditions, improving current density while maintaining high faradaic efficiency, developing cost-effective components suitable for large-scale manufacturing, and designing systems capable of continuous operation with minimal maintenance requirements.
Additionally, scale-up objectives encompass the development of standardized testing protocols to accurately predict long-term performance, optimization of energy efficiency to ensure economic viability, and integration of control systems that can adapt to fluctuating input parameters. The ultimate goal is to demonstrate H2O2 production at rates exceeding 1 kg/hour with concentrations suitable for direct industrial application (typically >3 wt%) while maintaining competitive production costs.
Research institutions and industrial partners worldwide are collaborating to bridge the gap between promising laboratory results and commercially viable technology. These efforts are supported by increasing investment in clean technology development and growing regulatory pressure to reduce the environmental footprint of chemical manufacturing processes. The successful transition from laboratory to pilot scale demonstrations represents a critical milestone in establishing electrochemical H2O2 production as a sustainable alternative to conventional methods.
The evolution of H2O2 electrolyzer technology can be traced back to fundamental electrochemical research in the early 20th century. However, significant advancements have occurred primarily in the last two decades, driven by improvements in electrode materials, membrane technology, and system design. Recent breakthroughs in selective catalysts and novel cell configurations have dramatically improved production efficiency and selectivity, making electrochemical H2O2 production increasingly viable for commercial applications.
Current technological trends indicate a shift toward more efficient oxygen reduction reaction (ORR) catalysts, innovative cell designs that maximize mass transport, and integrated systems that can operate using renewable electricity sources. The convergence of these developments with growing industrial demand for greener chemical production processes has created a fertile ground for H2O2 electrolyzer technology advancement.
The primary technical objective in scaling H2O2 electrolyzers from laboratory to pilot demonstrations involves addressing several critical challenges. These include enhancing catalyst stability under industrial operating conditions, improving current density while maintaining high faradaic efficiency, developing cost-effective components suitable for large-scale manufacturing, and designing systems capable of continuous operation with minimal maintenance requirements.
Additionally, scale-up objectives encompass the development of standardized testing protocols to accurately predict long-term performance, optimization of energy efficiency to ensure economic viability, and integration of control systems that can adapt to fluctuating input parameters. The ultimate goal is to demonstrate H2O2 production at rates exceeding 1 kg/hour with concentrations suitable for direct industrial application (typically >3 wt%) while maintaining competitive production costs.
Research institutions and industrial partners worldwide are collaborating to bridge the gap between promising laboratory results and commercially viable technology. These efforts are supported by increasing investment in clean technology development and growing regulatory pressure to reduce the environmental footprint of chemical manufacturing processes. The successful transition from laboratory to pilot scale demonstrations represents a critical milestone in establishing electrochemical H2O2 production as a sustainable alternative to conventional methods.
Market Analysis for H2O2 Electrolyzer Applications
The global hydrogen peroxide (H2O2) market is experiencing significant growth, driven by increasing demand across multiple industries. Traditional H2O2 production methods, primarily the anthraquinone auto-oxidation process, are energy-intensive and environmentally problematic, creating a substantial market opportunity for electrochemical production technologies. The current market size for H2O2 is valued at approximately $3.5 billion, with projections indicating growth to $5.7 billion by 2027, representing a compound annual growth rate of 5.8%.
Electrochemical H2O2 production offers compelling advantages in terms of on-site generation capabilities, eliminating transportation hazards and costs associated with the highly concentrated commercial product. This decentralized production model is particularly attractive for water treatment facilities, pulp and paper industries, and healthcare applications where continuous, safe access to H2O2 is critical.
The water treatment sector represents the largest potential market for H2O2 electrolyzer technology, accounting for nearly 40% of potential applications. The growing global water scarcity issues and increasingly stringent wastewater regulations are driving demand for advanced oxidation processes where H2O2 plays a crucial role. Municipal water treatment facilities are showing particular interest in on-site generation systems that can be scaled to meet varying capacity requirements.
The pulp and paper industry constitutes another significant market segment, representing approximately 25% of potential applications. This sector's shift toward environmentally friendly bleaching processes has increased demand for H2O2 as a chlorine-free alternative. On-site electrochemical production aligns perfectly with the industry's continuous production requirements and sustainability goals.
Healthcare applications, including sterilization and disinfection, account for roughly 15% of the potential market. The COVID-19 pandemic has substantially increased demand for effective disinfection solutions, creating new market opportunities for H2O2 generation technologies that can produce appropriate concentrations safely and reliably.
Emerging applications in semiconductor manufacturing and advanced oxidation processes for environmental remediation represent smaller but rapidly growing market segments. The semiconductor industry's need for ultra-pure H2O2 creates a premium market niche where electrochemical production could offer significant quality advantages over traditional methods.
Geographically, North America and Europe currently lead in adoption potential due to stringent environmental regulations and higher technology acceptance rates. However, the Asia-Pacific region, particularly China and India, shows the highest growth potential driven by rapid industrialization, increasing water treatment needs, and growing manufacturing sectors.
Electrochemical H2O2 production offers compelling advantages in terms of on-site generation capabilities, eliminating transportation hazards and costs associated with the highly concentrated commercial product. This decentralized production model is particularly attractive for water treatment facilities, pulp and paper industries, and healthcare applications where continuous, safe access to H2O2 is critical.
The water treatment sector represents the largest potential market for H2O2 electrolyzer technology, accounting for nearly 40% of potential applications. The growing global water scarcity issues and increasingly stringent wastewater regulations are driving demand for advanced oxidation processes where H2O2 plays a crucial role. Municipal water treatment facilities are showing particular interest in on-site generation systems that can be scaled to meet varying capacity requirements.
The pulp and paper industry constitutes another significant market segment, representing approximately 25% of potential applications. This sector's shift toward environmentally friendly bleaching processes has increased demand for H2O2 as a chlorine-free alternative. On-site electrochemical production aligns perfectly with the industry's continuous production requirements and sustainability goals.
Healthcare applications, including sterilization and disinfection, account for roughly 15% of the potential market. The COVID-19 pandemic has substantially increased demand for effective disinfection solutions, creating new market opportunities for H2O2 generation technologies that can produce appropriate concentrations safely and reliably.
Emerging applications in semiconductor manufacturing and advanced oxidation processes for environmental remediation represent smaller but rapidly growing market segments. The semiconductor industry's need for ultra-pure H2O2 creates a premium market niche where electrochemical production could offer significant quality advantages over traditional methods.
Geographically, North America and Europe currently lead in adoption potential due to stringent environmental regulations and higher technology acceptance rates. However, the Asia-Pacific region, particularly China and India, shows the highest growth potential driven by rapid industrialization, increasing water treatment needs, and growing manufacturing sectors.
Technical Challenges in H2O2 Electrolyzer Scale-up
The scaling of hydrogen peroxide (H2O2) electrolyzers from laboratory to pilot scale presents numerous technical challenges that must be addressed to achieve commercial viability. One of the primary obstacles is maintaining electrochemical performance during scale-up. Laboratory-scale cells often demonstrate excellent selectivity and efficiency, but these metrics frequently deteriorate when increasing electrode surface area and cell dimensions. This performance drop is attributed to non-uniform current distribution across larger electrodes and increased mass transport limitations.
Heat management emerges as another critical challenge in larger systems. The electrochemical production of H2O2 generates significant heat, which in laboratory settings can be easily dissipated. However, pilot-scale systems require sophisticated cooling strategies to maintain optimal operating temperatures, as excessive heat can lead to H2O2 decomposition and reduced faradaic efficiency.
Material stability presents persistent difficulties when scaling up H2O2 electrolyzers. The highly oxidative environment created by H2O2 production accelerates corrosion of cell components, particularly at the higher current densities required for commercial production. Materials that perform adequately in short-duration laboratory tests often exhibit accelerated degradation during extended pilot operations, necessitating the development of more robust materials or protective coatings.
Electrode design optimization becomes increasingly complex at larger scales. The ideal catalyst loading, electrode thickness, and porosity that work effectively in laboratory cells may not translate directly to pilot systems due to differences in mass transport phenomena and electrical resistance. Engineers must recalibrate these parameters to maintain performance while considering manufacturing constraints.
System integration challenges also intensify during scale-up. The coordination of multiple cells into stacks requires careful consideration of fluid distribution, electrical connections, and pressure management. Ensuring uniform electrolyte flow across all cells in a stack is particularly challenging but essential for consistent H2O2 production and concentration.
Process control complexity increases exponentially with scale. Laboratory systems typically operate under well-controlled, steady-state conditions, while pilot demonstrations must handle variations in input parameters and respond to changing operating conditions. Developing robust control systems that can maintain optimal performance despite fluctuations becomes essential for reliable operation.
Finally, safety considerations become paramount at pilot scale. The handling of larger quantities of H2O2, particularly at higher concentrations, introduces significant hazards that require engineered safety systems, specialized materials, and rigorous operating procedures to mitigate risks of decomposition, pressure build-up, or uncontrolled reactions.
Heat management emerges as another critical challenge in larger systems. The electrochemical production of H2O2 generates significant heat, which in laboratory settings can be easily dissipated. However, pilot-scale systems require sophisticated cooling strategies to maintain optimal operating temperatures, as excessive heat can lead to H2O2 decomposition and reduced faradaic efficiency.
Material stability presents persistent difficulties when scaling up H2O2 electrolyzers. The highly oxidative environment created by H2O2 production accelerates corrosion of cell components, particularly at the higher current densities required for commercial production. Materials that perform adequately in short-duration laboratory tests often exhibit accelerated degradation during extended pilot operations, necessitating the development of more robust materials or protective coatings.
Electrode design optimization becomes increasingly complex at larger scales. The ideal catalyst loading, electrode thickness, and porosity that work effectively in laboratory cells may not translate directly to pilot systems due to differences in mass transport phenomena and electrical resistance. Engineers must recalibrate these parameters to maintain performance while considering manufacturing constraints.
System integration challenges also intensify during scale-up. The coordination of multiple cells into stacks requires careful consideration of fluid distribution, electrical connections, and pressure management. Ensuring uniform electrolyte flow across all cells in a stack is particularly challenging but essential for consistent H2O2 production and concentration.
Process control complexity increases exponentially with scale. Laboratory systems typically operate under well-controlled, steady-state conditions, while pilot demonstrations must handle variations in input parameters and respond to changing operating conditions. Developing robust control systems that can maintain optimal performance despite fluctuations becomes essential for reliable operation.
Finally, safety considerations become paramount at pilot scale. The handling of larger quantities of H2O2, particularly at higher concentrations, introduces significant hazards that require engineered safety systems, specialized materials, and rigorous operating procedures to mitigate risks of decomposition, pressure build-up, or uncontrolled reactions.
Current Scale-up Methodologies for H2O2 Electrolyzers
01 Electrode materials and coatings to prevent scaling
Specialized electrode materials and coatings can be used in H2O2 electrolyzers to prevent scaling and extend operational lifetime. These materials include noble metals, metal oxides, and composite coatings that resist mineral deposition and fouling. The surface properties of these materials help to minimize the adhesion of scale-forming compounds while maintaining high catalytic activity for hydrogen peroxide production.- Anti-scaling coatings for electrolyzer components: Various coating technologies can be applied to electrolyzer components to prevent scaling and extend operational lifetime. These coatings create a protective barrier that resists mineral deposition and scale formation on electrode surfaces and membranes. Advanced materials such as fluoropolymers, ceramic composites, and nanomaterials can be used to create hydrophobic or anti-fouling surfaces that minimize scale adhesion while maintaining electrochemical performance.
- Electrolyte composition modifications to reduce scaling: Modifying the electrolyte composition can significantly reduce scaling in H2O2 electrolyzers. This includes adding scale inhibitors, chelating agents, or pH adjusters that prevent precipitation of calcium, magnesium, and other minerals. Controlled introduction of specific ions can disrupt crystal formation and growth, while maintaining optimal conductivity for efficient hydrogen peroxide production. These modifications can be implemented through continuous dosing systems or periodic treatment protocols.
- Flow design optimization to minimize scaling: Innovative flow field designs can reduce scaling in H2O2 electrolyzers by preventing stagnation zones where scale typically forms. Techniques include turbulent flow promotion, uniform fluid distribution across electrodes, and optimized channel geometries. These designs ensure continuous flushing of potential scale-forming particles and maintain consistent electrolyte concentration throughout the system, reducing localized scaling while improving overall system efficiency and hydrogen peroxide production rates.
- Automated cleaning and maintenance systems: Automated cleaning and maintenance systems can effectively manage scaling in H2O2 electrolyzers without requiring system shutdown. These systems incorporate periodic reverse polarity operations, ultrasonic cleaning mechanisms, or automated chemical cleaning cycles that dissolve and remove scale buildup. Integrated sensors monitor scale formation and trigger cleaning protocols when needed, while advanced control algorithms optimize cleaning frequency and intensity based on operating conditions and water quality parameters.
- Electrode material selection for scale resistance: Selecting appropriate electrode materials can significantly reduce scaling issues in H2O2 electrolyzers. Materials such as titanium alloys, platinum-coated substrates, and carbon-based composites offer superior resistance to scale formation while maintaining high catalytic activity. These materials can be engineered with specific surface properties that minimize nucleation sites for scale formation, while their inherent chemical stability prevents degradation in the presence of hydrogen peroxide and other reactive species generated during electrolysis.
02 Water treatment and pretreatment systems
Implementing water treatment and pretreatment systems can significantly reduce scaling in H2O2 electrolyzers. These systems may include ion exchange, reverse osmosis, or filtration technologies to remove scale-forming ions such as calcium, magnesium, and silica before water enters the electrolyzer. Continuous monitoring and adjustment of water quality parameters help maintain optimal operating conditions and prevent scale formation.Expand Specific Solutions03 Electrolyte composition and additives
Optimizing electrolyte composition and incorporating specific additives can inhibit scaling in H2O2 electrolyzers. Anti-scaling agents, chelating compounds, and pH modifiers can be added to the electrolyte solution to prevent precipitation of minerals on electrode surfaces. These additives work by either binding to scale-forming ions or altering the solution properties to keep potential scale-forming compounds in solution rather than depositing on surfaces.Expand Specific Solutions04 Operating parameters and control strategies
Implementing specific operating parameters and control strategies can minimize scaling in H2O2 electrolyzers. This includes optimizing current density, temperature, flow rates, and pressure to prevent conditions that promote scale formation. Periodic polarity reversal, pulsed operation, and automated cleaning cycles can also be employed to dislodge any scale that begins to form, maintaining electrolyzer efficiency and extending maintenance intervals.Expand Specific Solutions05 Mechanical and chemical descaling methods
Various mechanical and chemical descaling methods can be applied to remove scale from H2O2 electrolyzers when prevention measures are insufficient. These include periodic acid washing, ultrasonic cleaning, and mechanical scrubbing techniques that can be performed during scheduled maintenance. Some systems incorporate automated self-cleaning mechanisms that apply descaling agents or physical disruption to remove scale without requiring system disassembly.Expand Specific Solutions
Key Industry Players in H2O2 Electrolyzer Development
The hydrogen peroxide electrolyzer market is in an early growth phase, characterized by the transition from laboratory to pilot-scale demonstrations. This emerging technology sector shows promising market potential due to H2O2's applications in disinfection, chemical synthesis, and environmental remediation. The competitive landscape features a diverse mix of players, with academic institutions like Northwestern University, Fudan University, and Dalian Institute of Chemical Physics leading fundamental research, while industrial entities such as Siemens Energy, ABB Group, and ExxonMobil Chemical Patents focus on commercialization pathways. Specialized companies like H2Pro Ltd. are developing novel electrochemical technologies specifically for this application. The technology remains in early maturity stages, with key challenges in scaling production, improving efficiency, and reducing costs as operations move from laboratory to industrial implementation.
Siemens Energy Global GmbH & Co. KG
Technical Solution: Siemens Energy has developed a scalable electrochemical platform for H2O2 production called "HydrOx" that utilizes specialized polymer electrolyte membranes and advanced electrode materials. Their approach focuses on modular stack design with individual cells of 0.5m² that can be combined to create systems of varying capacities. The company has successfully demonstrated scale-up from laboratory units (100cm²) to pilot plants with 10m² total active area, producing H2O2 at concentrations of 12-15% with current densities exceeding 300mA/cm². A key innovation in their scale-up strategy is the implementation of computational fluid dynamics modeling to optimize flow distribution across larger cell areas, addressing one of the critical challenges in electrolyzer scaling. Their pilot demonstrations incorporate advanced manufacturing techniques including automated stack assembly and quality control processes that ensure consistency across multiple cells. Siemens' technology operates at moderate pressures (5-10 bar) and temperatures (40-60°C), balancing production efficiency with operational safety requirements.
Strengths: Modular design enables flexible scaling to match production requirements; advanced manufacturing processes ensure consistency across multiple cells; established global supply chain and service network supports commercial deployment. Weaknesses: Polymer membrane components may require periodic replacement, increasing operational costs; moderate operating pressure increases system complexity compared to ambient-pressure alternatives; current H2O2 concentrations may require additional processing for some applications.
ABB Group
Technical Solution: ABB has developed an integrated electrochemical system for H2O2 production called "PeroxiGen" that combines advanced electrode materials with sophisticated process control. Their technology utilizes specialized carbon-supported catalysts with tailored selectivity for the oxygen reduction pathway, achieving faradaic efficiencies exceeding 90% for H2O2 production. The company has successfully scaled their approach from laboratory cells (50cm²) to pilot demonstrations with 5m² active area, producing H2O2 at concentrations of 10-12% with production rates of up to 100kg per day. A distinctive feature of ABB's scale-up strategy is their implementation of digital twin technology that enables virtual testing of different operating conditions and scale-up scenarios before physical implementation. Their pilot systems incorporate advanced automation with self-diagnostic capabilities that identify and address performance issues in real-time, maintaining efficiency during scale-up. The technology operates at near-ambient conditions and incorporates safety systems designed for industrial environments, including hydrogen detection and emergency shutdown protocols.
Strengths: High faradaic efficiency (>90%) reduces energy consumption; advanced digital twin and automation technologies optimize performance during scale-up; comprehensive safety systems facilitate industrial deployment. Weaknesses: Carbon-supported catalysts may experience degradation over time, requiring periodic replacement; current H2O2 concentrations lower than some competing technologies; system complexity may increase maintenance requirements.
Critical Patents and Research in H2O2 Electrolyzer Design
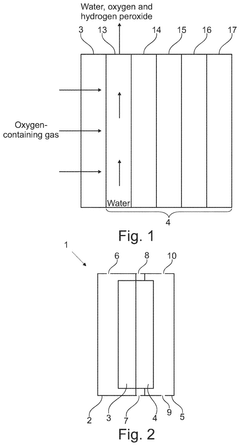
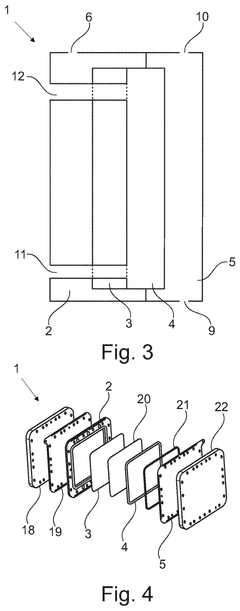
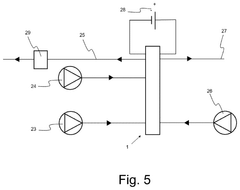
Apparatus and method for producing hydrogen peroxide
PatentPendingUS20250034729A1
Innovation
- The proposed solution involves an electrochemical cell design that incorporates a porous electrically conductive gas transport layer to facilitate uniform oxygen delivery and current collection, while water flows through the cathode gas diffusion layer to aid in hydrogen peroxide extraction, thereby optimizing the utilization of the electrode area.
Electrolysis cell for hydrogen peroxide production and method of use
PatentInactiveEP2064367A1
Innovation
- An electrolytic cell with a high hydraulic permeability separator dividing two compartments, using deionised water or acidic/aqueous solutions in the anodic compartment and oxygen in the cathodic compartment, with catalytic porous films and a bimodal gas-diffusion cathode structure to achieve high faradic efficiency and pure hydrogen peroxide production.
Environmental Impact Assessment of H2O2 Production Technologies
The environmental impact assessment of hydrogen peroxide (H2O2) production technologies reveals significant differences between conventional anthraquinone auto-oxidation (AO) processes and emerging electrochemical methods. Traditional AO processes consume substantial energy and generate considerable carbon emissions, with estimates suggesting 1.5-2.0 kg CO2 equivalent per kg of H2O2 produced. These processes also utilize organic solvents and palladium catalysts, creating additional environmental concerns related to waste management and resource depletion.
In contrast, electrochemical H2O2 production demonstrates promising environmental advantages. Life cycle assessments indicate potential carbon emission reductions of 30-60% compared to conventional methods when powered by renewable electricity sources. This significant improvement stems from the direct synthesis pathway that eliminates the need for hydrogen gas transportation and storage infrastructure, reducing associated risks and environmental impacts.
Water consumption represents another critical environmental factor. Conventional H2O2 production requires approximately 10-15 liters of water per kg of product for process cooling and purification. Electrolyzer systems potentially reduce this requirement by 40-50% through more efficient heat management and simplified purification processes, contributing to water conservation efforts in water-stressed regions.
Land use impacts also differ substantially between technologies. Traditional centralized H2O2 production facilities occupy significant industrial footprints, whereas electrochemical systems enable distributed production models that can be integrated into existing infrastructure with minimal additional land requirements. This decentralized approach reduces transportation emissions associated with H2O2 distribution, which can account for up to 15% of the product's total carbon footprint.
Waste generation profiles reveal further environmental distinctions. The AO process produces hazardous organic waste streams requiring specialized treatment, while electrochemical methods generate primarily inorganic by-products with lower environmental toxicity. Studies indicate waste reduction potential of 70-80% with electrochemical systems, significantly decreasing end-of-life environmental impacts.
Scaling electrochemical H2O2 production from laboratory to pilot demonstrations introduces additional environmental considerations. Materials selection for electrodes, membranes, and system components significantly influences the overall environmental profile. Recent research indicates that replacing platinum-group metals with earth-abundant catalysts could reduce embodied carbon by up to 40% while maintaining performance standards. Additionally, optimizing system durability extends operational lifetimes, distributing manufacturing impacts over longer service periods and improving sustainability metrics.
In contrast, electrochemical H2O2 production demonstrates promising environmental advantages. Life cycle assessments indicate potential carbon emission reductions of 30-60% compared to conventional methods when powered by renewable electricity sources. This significant improvement stems from the direct synthesis pathway that eliminates the need for hydrogen gas transportation and storage infrastructure, reducing associated risks and environmental impacts.
Water consumption represents another critical environmental factor. Conventional H2O2 production requires approximately 10-15 liters of water per kg of product for process cooling and purification. Electrolyzer systems potentially reduce this requirement by 40-50% through more efficient heat management and simplified purification processes, contributing to water conservation efforts in water-stressed regions.
Land use impacts also differ substantially between technologies. Traditional centralized H2O2 production facilities occupy significant industrial footprints, whereas electrochemical systems enable distributed production models that can be integrated into existing infrastructure with minimal additional land requirements. This decentralized approach reduces transportation emissions associated with H2O2 distribution, which can account for up to 15% of the product's total carbon footprint.
Waste generation profiles reveal further environmental distinctions. The AO process produces hazardous organic waste streams requiring specialized treatment, while electrochemical methods generate primarily inorganic by-products with lower environmental toxicity. Studies indicate waste reduction potential of 70-80% with electrochemical systems, significantly decreasing end-of-life environmental impacts.
Scaling electrochemical H2O2 production from laboratory to pilot demonstrations introduces additional environmental considerations. Materials selection for electrodes, membranes, and system components significantly influences the overall environmental profile. Recent research indicates that replacing platinum-group metals with earth-abundant catalysts could reduce embodied carbon by up to 40% while maintaining performance standards. Additionally, optimizing system durability extends operational lifetimes, distributing manufacturing impacts over longer service periods and improving sustainability metrics.
Economic Feasibility of Industrial-Scale H2O2 Electrolyzers
The economic viability of industrial-scale hydrogen peroxide (H2O2) electrolyzers represents a critical factor in determining their potential for widespread adoption. Current market analysis indicates that electrochemical H2O2 production could achieve cost competitiveness with traditional anthraquinone auto-oxidation (AO) processes when scaled appropriately. Preliminary economic models suggest production costs between $0.85-1.30 per kilogram of H2O2 at industrial scale, compared to the $0.70-1.10 range for conventional methods.
Capital expenditure requirements for industrial-scale H2O2 electrolyzers are estimated at $500-800 per kilowatt of installed capacity, with economies of scale significantly reducing unit costs as production volumes increase. The decentralized production model enabled by electrochemical methods offers substantial savings in transportation and storage costs, which typically account for 25-40% of total H2O2 expenses in the traditional centralized production paradigm.
Energy consumption represents the most significant operational cost factor, with current electrolyzer designs requiring 5-7 kWh per kilogram of H2O2 produced. Sensitivity analysis reveals that electricity prices below $0.06/kWh are necessary to achieve cost parity with conventional methods in most markets. Renewable energy integration presents an opportunity to further reduce operational costs while enhancing sustainability credentials.
Catalyst longevity and replacement schedules significantly impact long-term economic performance. Current noble metal catalysts demonstrate degradation rates of 0.5-2% per 1,000 operating hours, necessitating replacement every 2-3 years. Development of more durable and less expensive catalyst materials could reduce lifetime costs by an estimated 15-20%.
Return on investment calculations indicate payback periods of 3-5 years for industrial installations, assuming continuous operation and stable market conditions. This timeframe aligns favorably with industry expectations for capital-intensive chemical production equipment.
Market segmentation analysis reveals that on-site production for water treatment, pulp bleaching, and semiconductor manufacturing offers the most promising initial commercialization pathways, with potential premium pricing of 10-15% over bulk market rates due to enhanced purity and elimination of stabilizers required in transported H2O2.
Regulatory compliance costs for safety systems and environmental controls add approximately 8-12% to total capital expenditure but remain essential for operational approval. These investments yield long-term benefits through reduced insurance premiums and minimized liability exposure.
Capital expenditure requirements for industrial-scale H2O2 electrolyzers are estimated at $500-800 per kilowatt of installed capacity, with economies of scale significantly reducing unit costs as production volumes increase. The decentralized production model enabled by electrochemical methods offers substantial savings in transportation and storage costs, which typically account for 25-40% of total H2O2 expenses in the traditional centralized production paradigm.
Energy consumption represents the most significant operational cost factor, with current electrolyzer designs requiring 5-7 kWh per kilogram of H2O2 produced. Sensitivity analysis reveals that electricity prices below $0.06/kWh are necessary to achieve cost parity with conventional methods in most markets. Renewable energy integration presents an opportunity to further reduce operational costs while enhancing sustainability credentials.
Catalyst longevity and replacement schedules significantly impact long-term economic performance. Current noble metal catalysts demonstrate degradation rates of 0.5-2% per 1,000 operating hours, necessitating replacement every 2-3 years. Development of more durable and less expensive catalyst materials could reduce lifetime costs by an estimated 15-20%.
Return on investment calculations indicate payback periods of 3-5 years for industrial installations, assuming continuous operation and stable market conditions. This timeframe aligns favorably with industry expectations for capital-intensive chemical production equipment.
Market segmentation analysis reveals that on-site production for water treatment, pulp bleaching, and semiconductor manufacturing offers the most promising initial commercialization pathways, with potential premium pricing of 10-15% over bulk market rates due to enhanced purity and elimination of stabilizers required in transported H2O2.
Regulatory compliance costs for safety systems and environmental controls add approximately 8-12% to total capital expenditure but remain essential for operational approval. These investments yield long-term benefits through reduced insurance premiums and minimized liability exposure.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!