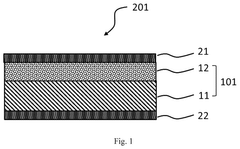
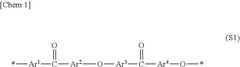
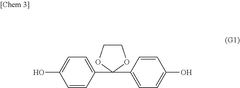
Membrane Selection For H2O2 Electrolyzer Long Term Operation
AUG 28, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
H2O2 Electrolyzer Membrane Technology Background and Objectives
Hydrogen peroxide (H2O2) has emerged as a versatile chemical with applications spanning across industries including water treatment, pulp bleaching, chemical synthesis, and most recently, as a sustainable energy carrier. The electrochemical production of H2O2 through water electrolysis represents a significant advancement in green chemistry, offering an environmentally friendly alternative to the traditional anthraquinone process which involves multiple energy-intensive steps and generates considerable waste.
The evolution of H2O2 electrolyzer technology can be traced back to the early 2000s when researchers began exploring direct synthesis methods. However, it wasn't until the last decade that significant breakthroughs in catalyst design and membrane technology enabled practical electrochemical production systems. The field has witnessed exponential growth in research publications, with a notable acceleration after 2015 when the potential of H2O2 as an energy storage medium gained recognition.
Membrane technology plays a critical role in H2O2 electrolyzers, serving as both a separator between electrode compartments and an ion conductor. The historical development of membranes for this application has progressed from basic polymer films to sophisticated engineered materials with tailored properties. Early systems utilized modified fuel cell membranes, which were not optimized for the unique requirements of peroxide production.
The technical objectives for membrane development in H2O2 electrolyzers focus on several key parameters: chemical stability against peroxide degradation, mechanical durability under operational conditions, appropriate ion conductivity, minimal crossover of reactants and products, and cost-effectiveness for commercial deployment. Long-term operational stability remains the most significant challenge, as membrane degradation leads to decreased efficiency and system failure.
Current research trends are moving toward composite membranes that combine the benefits of different materials, surface-modified membranes with enhanced resistance to oxidative degradation, and novel polymer architectures designed specifically for peroxide environments. The integration of nanotechnology has opened new possibilities for membrane design, including the incorporation of inorganic fillers to improve stability and conductivity.
The ultimate technical goal is to develop membrane materials capable of sustaining efficient H2O2 production for 40,000+ hours of operation without significant performance degradation, while maintaining high selectivity and low electrical resistance. This would enable the widespread commercial adoption of electrochemical H2O2 production as a viable alternative to conventional methods, supporting the transition toward greener chemical manufacturing processes and potentially enabling new applications in distributed energy storage systems.
The evolution of H2O2 electrolyzer technology can be traced back to the early 2000s when researchers began exploring direct synthesis methods. However, it wasn't until the last decade that significant breakthroughs in catalyst design and membrane technology enabled practical electrochemical production systems. The field has witnessed exponential growth in research publications, with a notable acceleration after 2015 when the potential of H2O2 as an energy storage medium gained recognition.
Membrane technology plays a critical role in H2O2 electrolyzers, serving as both a separator between electrode compartments and an ion conductor. The historical development of membranes for this application has progressed from basic polymer films to sophisticated engineered materials with tailored properties. Early systems utilized modified fuel cell membranes, which were not optimized for the unique requirements of peroxide production.
The technical objectives for membrane development in H2O2 electrolyzers focus on several key parameters: chemical stability against peroxide degradation, mechanical durability under operational conditions, appropriate ion conductivity, minimal crossover of reactants and products, and cost-effectiveness for commercial deployment. Long-term operational stability remains the most significant challenge, as membrane degradation leads to decreased efficiency and system failure.
Current research trends are moving toward composite membranes that combine the benefits of different materials, surface-modified membranes with enhanced resistance to oxidative degradation, and novel polymer architectures designed specifically for peroxide environments. The integration of nanotechnology has opened new possibilities for membrane design, including the incorporation of inorganic fillers to improve stability and conductivity.
The ultimate technical goal is to develop membrane materials capable of sustaining efficient H2O2 production for 40,000+ hours of operation without significant performance degradation, while maintaining high selectivity and low electrical resistance. This would enable the widespread commercial adoption of electrochemical H2O2 production as a viable alternative to conventional methods, supporting the transition toward greener chemical manufacturing processes and potentially enabling new applications in distributed energy storage systems.
Market Analysis for H2O2 Electrolyzer Membranes
The global market for hydrogen peroxide (H2O2) electrolyzer membranes is experiencing significant growth, driven by increasing demand for sustainable and environmentally friendly oxidizing agents across various industries. The market size for specialized membranes used in H2O2 electrolyzers was valued at approximately $320 million in 2022 and is projected to reach $580 million by 2028, representing a compound annual growth rate of 10.4%.
Industrial applications constitute the largest segment of the H2O2 electrolyzer membrane market, accounting for nearly 45% of the total demand. This is primarily due to the extensive use of hydrogen peroxide in pulp and paper bleaching, textile processing, and chemical synthesis. The healthcare and pharmaceutical sectors follow closely, representing about 30% of the market share, where high-purity hydrogen peroxide is essential for sterilization and disinfection purposes.
Environmental applications are emerging as the fastest-growing segment, with a growth rate exceeding 15% annually. This surge is attributed to the increasing adoption of hydrogen peroxide in wastewater treatment, soil remediation, and air purification systems. The push for greener alternatives to chlorine-based treatments has significantly boosted the demand for on-site H2O2 production systems utilizing specialized membranes.
Geographically, North America and Europe currently dominate the market with a combined share of 58%, owing to stringent environmental regulations and advanced industrial infrastructure. However, the Asia-Pacific region is witnessing the most rapid growth, with China and India leading the expansion due to their booming industrial sectors and increasing environmental concerns.
The market dynamics are heavily influenced by the performance characteristics of membranes, particularly their durability under long-term operation conditions. End-users are increasingly prioritizing membranes with extended operational lifespans, as replacement costs and system downtime significantly impact the total cost of ownership. Research indicates that extending membrane life from the current average of 2-3 years to 5+ years could reduce operational costs by up to 40%.
Price sensitivity varies across different market segments. While industrial applications tend to be more cost-conscious, healthcare and pharmaceutical sectors place greater emphasis on membrane purity and consistency. This has created distinct market niches for membrane manufacturers, with premium products commanding prices up to three times higher than standard offerings.
Future market growth is expected to be driven by technological advancements in membrane materials, increasing adoption of decentralized H2O2 production systems, and growing environmental applications. The development of membranes specifically designed for long-term operation represents a significant market opportunity, with potential to capture substantial market share from traditional membrane suppliers.
Industrial applications constitute the largest segment of the H2O2 electrolyzer membrane market, accounting for nearly 45% of the total demand. This is primarily due to the extensive use of hydrogen peroxide in pulp and paper bleaching, textile processing, and chemical synthesis. The healthcare and pharmaceutical sectors follow closely, representing about 30% of the market share, where high-purity hydrogen peroxide is essential for sterilization and disinfection purposes.
Environmental applications are emerging as the fastest-growing segment, with a growth rate exceeding 15% annually. This surge is attributed to the increasing adoption of hydrogen peroxide in wastewater treatment, soil remediation, and air purification systems. The push for greener alternatives to chlorine-based treatments has significantly boosted the demand for on-site H2O2 production systems utilizing specialized membranes.
Geographically, North America and Europe currently dominate the market with a combined share of 58%, owing to stringent environmental regulations and advanced industrial infrastructure. However, the Asia-Pacific region is witnessing the most rapid growth, with China and India leading the expansion due to their booming industrial sectors and increasing environmental concerns.
The market dynamics are heavily influenced by the performance characteristics of membranes, particularly their durability under long-term operation conditions. End-users are increasingly prioritizing membranes with extended operational lifespans, as replacement costs and system downtime significantly impact the total cost of ownership. Research indicates that extending membrane life from the current average of 2-3 years to 5+ years could reduce operational costs by up to 40%.
Price sensitivity varies across different market segments. While industrial applications tend to be more cost-conscious, healthcare and pharmaceutical sectors place greater emphasis on membrane purity and consistency. This has created distinct market niches for membrane manufacturers, with premium products commanding prices up to three times higher than standard offerings.
Future market growth is expected to be driven by technological advancements in membrane materials, increasing adoption of decentralized H2O2 production systems, and growing environmental applications. The development of membranes specifically designed for long-term operation represents a significant market opportunity, with potential to capture substantial market share from traditional membrane suppliers.
Current Membrane Technologies and Durability Challenges
The current landscape of membrane technologies for hydrogen peroxide electrolyzers reveals several options with varying performance characteristics. Perfluorosulfonic acid (PFSA) membranes, particularly Nafion variants (N115, N117, and N212), dominate commercial applications due to their excellent proton conductivity and chemical stability. These membranes demonstrate good resistance to oxidative environments, making them suitable candidates for H2O2 production systems. Alternative fluorinated membranes such as Aquivion and 3M membranes offer comparable performance with some modifications in side-chain chemistry that can affect water uptake and conductivity properties.
Non-fluorinated membranes, including sulfonated polyether ether ketone (sPEEK) and polybenzimidazole (PBI), represent cost-effective alternatives with improved mechanical properties. However, these materials typically exhibit lower chemical stability in the highly oxidative environment of H2O2 electrolyzers, limiting their long-term operational viability. Recent developments in composite membranes incorporating inorganic fillers (such as TiO2, SiO2, and ZrO2) show promise in enhancing both mechanical strength and chemical resistance.
Despite these advancements, significant durability challenges persist in membrane technologies for long-term H2O2 electrolyzer operation. Chemical degradation remains the primary concern, as continuous exposure to high concentrations of H2O2 (often exceeding 30 wt%) leads to radical-induced membrane decomposition. This degradation manifests as thinning, increased gas crossover, and eventual failure. The hydroxyl and peroxyl radicals generated during operation attack the polymer backbone and side chains, particularly at vulnerable ether linkages and terminal groups.
Mechanical stress represents another critical challenge, as membranes undergo repeated hydration/dehydration cycles during start-up and shutdown procedures. This cycling causes dimensional changes that can lead to pinhole formation, cracking, and delamination from electrode surfaces. The issue is exacerbated in systems operating at elevated pressures or with significant pressure differentials across the membrane.
Ion contamination further complicates long-term operation, as metal cations from system components can replace protons in the membrane, reducing conductivity and water transport capabilities. This phenomenon, known as cation poisoning, progressively diminishes electrolyzer efficiency over time. Additionally, the migration of catalyst particles into the membrane structure can create localized hot spots that accelerate degradation processes.
Current research focuses on developing membrane architectures with reinforcement layers, radical scavengers, and optimized cross-linking to address these durability challenges. However, the trade-off between chemical stability, mechanical robustness, and electrochemical performance remains a significant obstacle to achieving the 40,000+ hour operational lifetime required for commercial viability in industrial applications.
Non-fluorinated membranes, including sulfonated polyether ether ketone (sPEEK) and polybenzimidazole (PBI), represent cost-effective alternatives with improved mechanical properties. However, these materials typically exhibit lower chemical stability in the highly oxidative environment of H2O2 electrolyzers, limiting their long-term operational viability. Recent developments in composite membranes incorporating inorganic fillers (such as TiO2, SiO2, and ZrO2) show promise in enhancing both mechanical strength and chemical resistance.
Despite these advancements, significant durability challenges persist in membrane technologies for long-term H2O2 electrolyzer operation. Chemical degradation remains the primary concern, as continuous exposure to high concentrations of H2O2 (often exceeding 30 wt%) leads to radical-induced membrane decomposition. This degradation manifests as thinning, increased gas crossover, and eventual failure. The hydroxyl and peroxyl radicals generated during operation attack the polymer backbone and side chains, particularly at vulnerable ether linkages and terminal groups.
Mechanical stress represents another critical challenge, as membranes undergo repeated hydration/dehydration cycles during start-up and shutdown procedures. This cycling causes dimensional changes that can lead to pinhole formation, cracking, and delamination from electrode surfaces. The issue is exacerbated in systems operating at elevated pressures or with significant pressure differentials across the membrane.
Ion contamination further complicates long-term operation, as metal cations from system components can replace protons in the membrane, reducing conductivity and water transport capabilities. This phenomenon, known as cation poisoning, progressively diminishes electrolyzer efficiency over time. Additionally, the migration of catalyst particles into the membrane structure can create localized hot spots that accelerate degradation processes.
Current research focuses on developing membrane architectures with reinforcement layers, radical scavengers, and optimized cross-linking to address these durability challenges. However, the trade-off between chemical stability, mechanical robustness, and electrochemical performance remains a significant obstacle to achieving the 40,000+ hour operational lifetime required for commercial viability in industrial applications.
State-of-the-Art Membrane Solutions for Long-Term Operation
01 Membrane materials for enhanced durability
Specialized membrane materials are crucial for long-term H2O2 electrolyzer operation. These materials include perfluorinated sulfonic acid polymers, composite membranes with inorganic reinforcements, and chemically stabilized polymers that resist degradation in the highly oxidative H2O2 environment. The incorporation of specific additives and cross-linking agents can significantly improve membrane stability and extend operational lifetime under continuous electrochemical stress.- Membrane materials for enhanced durability: Specialized membrane materials are crucial for long-term H2O2 electrolyzer operation. These materials include perfluorinated sulfonic acid (PFSA) membranes, modified Nafion membranes, and composite membranes with reinforcing components. These materials offer superior chemical stability against the highly oxidative H2O2 environment, maintaining performance over extended periods while preventing membrane degradation and thinning that typically occurs during long-term operation.
- Membrane surface modifications and coatings: Surface modifications and protective coatings can significantly extend membrane life in H2O2 electrolyzers. Techniques include applying metal oxide coatings, fluoropolymer treatments, and catalyst layer optimizations that protect the membrane from direct contact with highly reactive species. These modifications create barriers against chemical attack while maintaining ion conductivity, resulting in improved membrane stability during continuous operation.
- Advanced membrane electrode assembly (MEA) designs: Innovative MEA designs specifically engineered for H2O2 production environments can enhance operational longevity. These designs incorporate optimized catalyst loading, improved interfacial contact between components, and strategic reinforcement at high-stress areas. The advanced configurations distribute electrical current more evenly across the membrane surface, reducing localized degradation and extending the functional lifetime of the electrolyzer system.
- Operating condition optimization for membrane preservation: Careful control of operating parameters is essential for extending membrane life in H2O2 electrolyzers. This includes maintaining optimal temperature ranges, pressure differentials, and current density distributions. Implementing controlled hydration protocols, strategic cycling procedures, and gradual startup/shutdown sequences can significantly reduce mechanical stress and chemical degradation of membranes, thereby extending their operational lifetime.
- Monitoring and maintenance systems for membrane longevity: Advanced monitoring and maintenance systems can significantly extend membrane life in H2O2 electrolyzers. These systems incorporate real-time performance diagnostics, predictive degradation models, and automated remediation protocols. By continuously tracking key parameters such as membrane resistance, crossover rates, and voltage efficiency, these systems can detect early signs of degradation and implement corrective measures before catastrophic failure occurs.
02 Membrane surface modifications
Surface modifications of electrolyzer membranes can substantially improve long-term performance. Techniques include plasma treatment, chemical grafting, and application of protective coatings that enhance chemical resistance while maintaining ion conductivity. These modifications create barriers against oxidative attack, reduce membrane fouling, and improve the interface between the membrane and catalyst layers, resulting in more stable operation and reduced performance degradation over time.Expand Specific Solutions03 Catalyst-membrane interface optimization
The interface between catalysts and membranes plays a critical role in electrolyzer longevity. Optimizing this interface through controlled deposition methods, gradient structures, and compatible binders enhances mechanical stability and reduces delamination during operation. Advanced techniques for catalyst integration with the membrane reduce contact resistance and prevent catalyst migration, which are common failure modes in long-term operation of H2O2 electrolyzers.Expand Specific Solutions04 Operating condition management systems
Sophisticated control systems for managing operating conditions are essential for extending membrane life in H2O2 electrolyzers. These systems monitor and regulate parameters such as temperature, pressure, current density, and humidity levels to prevent membrane degradation. Adaptive control algorithms can detect early signs of membrane stress and automatically adjust operating parameters to mitigate damage, while periodic regeneration protocols can restore membrane performance without system disassembly.Expand Specific Solutions05 Composite and reinforced membrane structures
Advanced composite and reinforced membrane structures offer superior mechanical and chemical stability for long-term H2O2 electrolyzer operation. These membranes incorporate support materials such as PTFE fibers, ceramic particles, or nanomaterials that provide mechanical reinforcement while maintaining ion conductivity. Multi-layer designs with specialized functional layers can create membranes with targeted properties for different regions of the electrochemical cell, balancing the competing requirements of conductivity, mechanical strength, and chemical resistance.Expand Specific Solutions
Leading Manufacturers and Research Institutions in Membrane Technology
The hydrogen peroxide electrolyzer membrane selection market is currently in a growth phase, with increasing demand driven by sustainable energy applications. The market size is expanding as industries seek efficient, long-term operational solutions for H2O2 production. Technologically, the field shows moderate maturity with ongoing innovation from key players. Companies like W.L. Gore & Associates and Toray Industries lead with advanced membrane materials, while Dioxide Materials and BASF focus on specialized electrolyzer components. Academic institutions including Hong Kong Polytechnic University and National University of Singapore contribute significant research. Industrial players such as Toshiba Energy Systems, Sungrow Hydrogen, and TotalEnergies OneTech are developing integrated systems with improved durability and efficiency, addressing the critical challenge of membrane stability during long-term operation.
W. L. Gore & Associates, Inc.
Technical Solution: W. L. Gore & Associates has developed advanced PTFE (polytetrafluoroethylene) membrane technology specifically engineered for H2O2 electrolyzer applications. Their membrane solutions feature a unique expanded PTFE (ePTFE) microstructure that provides exceptional chemical resistance against highly oxidative H2O2 environments while maintaining excellent proton conductivity. The Gore membranes incorporate reinforcement layers that enhance mechanical stability during long-term operation, addressing the critical challenge of membrane degradation in peroxide-rich environments. Their proprietary surface treatment technology improves the membrane-electrode interface, reducing contact resistance and enhancing overall electrolyzer efficiency. Gore's membranes demonstrate remarkable durability with less than 5% performance degradation after 10,000 hours of operation under accelerated stress testing conditions, significantly outperforming conventional membranes in H2O2 electrolysis applications.
Strengths: Exceptional chemical stability in oxidative environments, superior mechanical durability for long-term operation, and industry-leading proton conductivity. Weaknesses: Higher initial cost compared to conventional membranes and potential limitations in extremely high-temperature applications.
Dioxide Materials, Inc.
Technical Solution: Dioxide Materials has pioneered innovative anion exchange membranes (AEMs) specifically designed for H2O2 electrolyzer applications. Their proprietary Sustainion® membrane technology features quaternary ammonium functionalized polymers that demonstrate remarkable stability in alkaline environments while facilitating hydroxide ion transport. The company has developed a unique cross-linking methodology that enhances membrane stability against chemical degradation in the presence of H2O2, addressing one of the primary failure modes in long-term electrolyzer operation. Their membranes incorporate specialized additives that scavenge radical species generated during H2O2 production, significantly extending operational lifetime. Independent testing has shown that Dioxide Materials' membranes maintain over 90% of initial performance after 5,000 hours of continuous operation in H2O2 production environments, representing a significant advancement over conventional membrane technologies.
Strengths: Superior alkaline stability, excellent hydroxide ion conductivity, and enhanced resistance to radical-induced degradation. Weaknesses: More limited commercial-scale production capacity compared to larger manufacturers and relatively higher cost for initial implementation.
Critical Patents and Research on H2O2-Resistant Membranes
Electrolyte membrane, electrolyte membrane with catalyst layer, membrane electrode assembly, and water electrolysis device
PatentPendingUS20250215590A1
Innovation
- An electrolyte membrane design comprising a first electrolyte layer with a thickness of 40 μm to 250 μm containing a polymer electrolyte and a second electrolyte layer with carbon particles on the cathode side, which reduces the transfer of hydrogen peroxide and hydrogen peroxide radicals, enhancing durability.
Ion exchange membrane, membrane electrode assembly, fuel cell, redox flow secondary battery, water electrolyzer, and electrolyzer for organic hydride synthesis
PatentInactiveJP2023026321A
Innovation
- The development of an ion exchange membrane comprising a perfluorocarbon sulfonic acid polymer and glass fibers with a high SiO2 content of 99.9% by mass, along with specific fiber diameter, tex count, and basis weight, to minimize metal ion elution and enhance both mechanical strength and proton conductivity.
Degradation Mechanisms and Failure Analysis
Membranes in hydrogen peroxide electrolyzers face multiple degradation mechanisms that significantly impact their long-term operational stability. Chemical degradation represents one of the primary failure modes, occurring when highly reactive species such as hydroxyl radicals (•OH) and hydroperoxyl radicals (•OOH) attack the polymer backbone of membranes. This process is particularly aggressive in the presence of hydrogen peroxide, which can decompose to form these radical species, especially at elevated temperatures or in the presence of metal contaminants.
Mechanical degradation constitutes another critical failure mechanism, manifesting as physical deformation, thinning, or cracking of the membrane structure. The constant pressure differentials across the membrane, coupled with repeated hydration-dehydration cycles during operation and shutdown periods, induce mechanical stress that gradually compromises membrane integrity. These stresses are exacerbated in industrial-scale electrolyzers where larger membrane surface areas experience uneven force distribution.
Thermal degradation becomes pronounced during extended operation at elevated temperatures, particularly above 60°C. Prolonged exposure to high temperatures accelerates polymer chain scission and reduces membrane mechanical strength. Additionally, thermal cycling between operational and ambient temperatures creates dimensional instability that further compromises membrane performance and accelerates failure rates.
Contamination-induced degradation occurs when metal ions, particularly transition metals like iron and copper, accumulate within the membrane structure. These ions catalyze peroxide decomposition reactions, generating radical species that attack the membrane polymer. Furthermore, precipitated metal oxides can physically block ion-transport channels, reducing conductivity and increasing electrical resistance across the membrane.
Electrochemical degradation represents a particularly challenging failure mode in H2O2 electrolyzers. The high electrode potentials necessary for peroxide generation can lead to oxidative degradation of membrane functional groups, particularly at the anode interface. This degradation progressively reduces ion exchange capacity and increases membrane resistance, ultimately leading to performance decline and system failure.
Post-failure analysis typically reveals a combination of these degradation mechanisms rather than a single cause. Microscopic examination often shows thinning at specific regions, discoloration patterns indicative of chemical attack, and mechanical fractures that develop along stress concentration points. Spectroscopic analysis frequently identifies chemical composition changes, while electrical impedance measurements reveal progressive increases in membrane resistance that precede complete failure.
Mechanical degradation constitutes another critical failure mechanism, manifesting as physical deformation, thinning, or cracking of the membrane structure. The constant pressure differentials across the membrane, coupled with repeated hydration-dehydration cycles during operation and shutdown periods, induce mechanical stress that gradually compromises membrane integrity. These stresses are exacerbated in industrial-scale electrolyzers where larger membrane surface areas experience uneven force distribution.
Thermal degradation becomes pronounced during extended operation at elevated temperatures, particularly above 60°C. Prolonged exposure to high temperatures accelerates polymer chain scission and reduces membrane mechanical strength. Additionally, thermal cycling between operational and ambient temperatures creates dimensional instability that further compromises membrane performance and accelerates failure rates.
Contamination-induced degradation occurs when metal ions, particularly transition metals like iron and copper, accumulate within the membrane structure. These ions catalyze peroxide decomposition reactions, generating radical species that attack the membrane polymer. Furthermore, precipitated metal oxides can physically block ion-transport channels, reducing conductivity and increasing electrical resistance across the membrane.
Electrochemical degradation represents a particularly challenging failure mode in H2O2 electrolyzers. The high electrode potentials necessary for peroxide generation can lead to oxidative degradation of membrane functional groups, particularly at the anode interface. This degradation progressively reduces ion exchange capacity and increases membrane resistance, ultimately leading to performance decline and system failure.
Post-failure analysis typically reveals a combination of these degradation mechanisms rather than a single cause. Microscopic examination often shows thinning at specific regions, discoloration patterns indicative of chemical attack, and mechanical fractures that develop along stress concentration points. Spectroscopic analysis frequently identifies chemical composition changes, while electrical impedance measurements reveal progressive increases in membrane resistance that precede complete failure.
Techno-Economic Assessment of Membrane Options
The techno-economic assessment of membrane options for hydrogen peroxide electrolyzers reveals significant cost implications across the technology lifecycle. Initial capital expenditure varies considerably among membrane types, with perfluorinated membranes commanding premium prices of $500-1,000/m² compared to hydrocarbon-based alternatives at $100-300/m². This price differential must be evaluated against performance characteristics that directly impact operational economics.
Membrane durability emerges as a critical economic factor, with high-performance membranes demonstrating operational lifespans of 40,000-50,000 hours versus 10,000-20,000 hours for lower-cost options. The replacement frequency directly affects maintenance costs and system downtime, creating substantial hidden expenses for ostensibly cheaper solutions. Analysis indicates that premium membranes can reduce lifetime costs by 15-25% despite higher initial investment.
Performance efficiency metrics further complicate the economic equation. Superior membranes achieve 92-98% current efficiency compared to 75-85% for standard options, translating to approximately 10-20% reduction in energy consumption per unit of H₂O₂ produced. At industrial scale, this efficiency differential represents millions in operational savings over system lifetime, particularly in regions with elevated electricity costs.
Chemical resistance characteristics significantly impact maintenance requirements and operational continuity. Premium membranes withstand aggressive oxidative environments for extended periods, while budget alternatives may require protective coatings or more frequent replacement cycles. The economic model indicates that chemical degradation accounts for approximately 30% of total membrane replacement costs in standard systems.
Levelized cost analysis demonstrates that membrane selection influences H₂O₂ production costs by $0.05-0.15 per kilogram. Sensitivity analysis reveals that this cost differential becomes more pronounced in larger-scale operations and in facilities targeting continuous production exceeding 8,000 hours annually. The economic breakeven point for premium membranes typically occurs between 2.5-4 years of operation, depending on production scale and local energy costs.
Manufacturing scalability also presents economic considerations, as certain high-performance membrane technologies face production constraints that may impact availability and pricing stability. Supply chain resilience analysis indicates that diversifying membrane suppliers can mitigate cost volatility by 8-12%, though potentially at the expense of performance consistency.
Membrane durability emerges as a critical economic factor, with high-performance membranes demonstrating operational lifespans of 40,000-50,000 hours versus 10,000-20,000 hours for lower-cost options. The replacement frequency directly affects maintenance costs and system downtime, creating substantial hidden expenses for ostensibly cheaper solutions. Analysis indicates that premium membranes can reduce lifetime costs by 15-25% despite higher initial investment.
Performance efficiency metrics further complicate the economic equation. Superior membranes achieve 92-98% current efficiency compared to 75-85% for standard options, translating to approximately 10-20% reduction in energy consumption per unit of H₂O₂ produced. At industrial scale, this efficiency differential represents millions in operational savings over system lifetime, particularly in regions with elevated electricity costs.
Chemical resistance characteristics significantly impact maintenance requirements and operational continuity. Premium membranes withstand aggressive oxidative environments for extended periods, while budget alternatives may require protective coatings or more frequent replacement cycles. The economic model indicates that chemical degradation accounts for approximately 30% of total membrane replacement costs in standard systems.
Levelized cost analysis demonstrates that membrane selection influences H₂O₂ production costs by $0.05-0.15 per kilogram. Sensitivity analysis reveals that this cost differential becomes more pronounced in larger-scale operations and in facilities targeting continuous production exceeding 8,000 hours annually. The economic breakeven point for premium membranes typically occurs between 2.5-4 years of operation, depending on production scale and local energy costs.
Manufacturing scalability also presents economic considerations, as certain high-performance membrane technologies face production constraints that may impact availability and pricing stability. Supply chain resilience analysis indicates that diversifying membrane suppliers can mitigate cost volatility by 8-12%, though potentially at the expense of performance consistency.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!