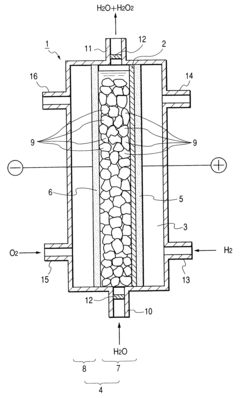
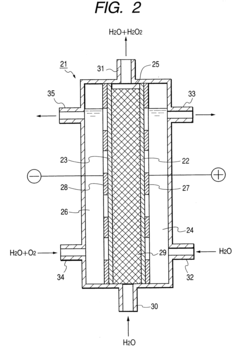
Cell Architectures For High Current Density H2O2 Electrosynthesis
AUG 28, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
H2O2 Electrosynthesis Background and Objectives
Hydrogen peroxide (H2O2) has emerged as a versatile and environmentally benign oxidant with applications spanning across multiple industries including pulp and paper bleaching, wastewater treatment, chemical synthesis, and medical disinfection. Traditionally, H2O2 production has been dominated by the anthraquinone auto-oxidation (AO) process, which despite its commercial success, presents significant environmental and economic challenges due to its energy-intensive nature and reliance on fossil fuels.
The electrochemical synthesis of H2O2 represents a promising alternative that aligns with global sustainability goals. This approach enables direct production from water and oxygen using renewable electricity, offering a pathway to decentralized, on-demand H2O2 generation with minimal environmental impact. The evolution of this technology has accelerated in recent years, driven by advances in electrocatalyst design, membrane technology, and electrochemical engineering.
Historical development of H2O2 electrosynthesis can be traced back to the early 20th century, but significant breakthroughs in achieving commercially viable current densities have only emerged in the past decade. The field has witnessed a transition from fundamental electrochemical studies to practical device engineering, with particular focus on overcoming the inherent limitations of traditional cell architectures.
Current research objectives in this domain center on developing cell architectures capable of sustaining high current densities (>500 mA/cm²) while maintaining high faradaic efficiency and H2O2 concentration. This represents a critical challenge as conventional designs face mass transport limitations, catalyst degradation, and product decomposition at elevated current densities.
The technological trajectory indicates a convergence toward innovative cell configurations that address these challenges through advanced flow field designs, optimized electrode structures, and novel membrane integration strategies. These developments aim to enhance oxygen mass transfer, mitigate parasitic reactions, and facilitate efficient product separation.
Looking forward, the field is poised for transformative advancements that could enable industrial-scale electrochemical H2O2 production with performance metrics surpassing those of the conventional AO process. The ultimate goal is to establish a technology platform that delivers high-concentration H2O2 (>5 wt%) at current densities exceeding 1 A/cm² with energy efficiencies above 70%, thereby creating a sustainable alternative to fossil fuel-dependent production methods.
This technological evolution aligns with broader industrial decarbonization efforts and represents a key opportunity for chemical manufacturing to embrace electrification as a pathway to sustainability. Success in this domain could catalyze similar transformations across other chemical production processes, contributing to the global transition toward carbon-neutral industrial operations.
The electrochemical synthesis of H2O2 represents a promising alternative that aligns with global sustainability goals. This approach enables direct production from water and oxygen using renewable electricity, offering a pathway to decentralized, on-demand H2O2 generation with minimal environmental impact. The evolution of this technology has accelerated in recent years, driven by advances in electrocatalyst design, membrane technology, and electrochemical engineering.
Historical development of H2O2 electrosynthesis can be traced back to the early 20th century, but significant breakthroughs in achieving commercially viable current densities have only emerged in the past decade. The field has witnessed a transition from fundamental electrochemical studies to practical device engineering, with particular focus on overcoming the inherent limitations of traditional cell architectures.
Current research objectives in this domain center on developing cell architectures capable of sustaining high current densities (>500 mA/cm²) while maintaining high faradaic efficiency and H2O2 concentration. This represents a critical challenge as conventional designs face mass transport limitations, catalyst degradation, and product decomposition at elevated current densities.
The technological trajectory indicates a convergence toward innovative cell configurations that address these challenges through advanced flow field designs, optimized electrode structures, and novel membrane integration strategies. These developments aim to enhance oxygen mass transfer, mitigate parasitic reactions, and facilitate efficient product separation.
Looking forward, the field is poised for transformative advancements that could enable industrial-scale electrochemical H2O2 production with performance metrics surpassing those of the conventional AO process. The ultimate goal is to establish a technology platform that delivers high-concentration H2O2 (>5 wt%) at current densities exceeding 1 A/cm² with energy efficiencies above 70%, thereby creating a sustainable alternative to fossil fuel-dependent production methods.
This technological evolution aligns with broader industrial decarbonization efforts and represents a key opportunity for chemical manufacturing to embrace electrification as a pathway to sustainability. Success in this domain could catalyze similar transformations across other chemical production processes, contributing to the global transition toward carbon-neutral industrial operations.
Market Analysis for High-Current Density H2O2 Production
The global hydrogen peroxide (H2O2) market is experiencing significant growth, driven by increasing demand across multiple industries. Currently valued at approximately 5.5 billion USD, the market is projected to reach 7.8 billion USD by 2028, representing a compound annual growth rate of 5.7%. This growth trajectory is particularly relevant for high-current density H2O2 production technologies, which offer more efficient and sustainable manufacturing methods compared to traditional approaches.
The pulp and paper industry remains the largest consumer of H2O2, accounting for roughly 45% of global consumption. However, emerging applications in wastewater treatment, electronics manufacturing, and healthcare are rapidly expanding market segments. The wastewater treatment sector, in particular, is showing accelerated growth at 7.2% annually, driven by stricter environmental regulations worldwide and increasing water scarcity concerns.
Regionally, Asia-Pacific dominates the H2O2 market with approximately 40% share, followed by North America and Europe. China and India represent the fastest-growing markets due to rapid industrialization and increasing environmental awareness. The demand for high-purity H2O2, especially in semiconductor manufacturing and healthcare applications, is growing at twice the rate of standard-grade peroxide.
The market for electrosynthesis technologies specifically is gaining momentum as industries seek greener alternatives to the traditional anthraquinone auto-oxidation process. Current estimates value the electrochemical H2O2 production equipment market at 320 million USD, with projections to reach 780 million USD by 2030. This represents a significant opportunity for high-current density cell architectures, which can reduce production costs by up to 30% compared to conventional methods.
Energy efficiency considerations are becoming increasingly important market drivers. With electricity costs representing 40-60% of operational expenses in electrochemical H2O2 production, technologies that can achieve higher current densities while maintaining faradaic efficiency have substantial economic advantages. Market analysis indicates that systems capable of operating above 500 mA/cm² while maintaining 90%+ selectivity could capture premium pricing of 15-20% above standard equipment.
Customer requirements are evolving toward modular, scalable systems that can be deployed on-site, reducing transportation costs and safety concerns associated with H2O2 handling. This decentralization trend is creating new market segments for smaller-scale, high-efficiency production units, particularly in remote locations and developing regions where infrastructure limitations exist.
The pulp and paper industry remains the largest consumer of H2O2, accounting for roughly 45% of global consumption. However, emerging applications in wastewater treatment, electronics manufacturing, and healthcare are rapidly expanding market segments. The wastewater treatment sector, in particular, is showing accelerated growth at 7.2% annually, driven by stricter environmental regulations worldwide and increasing water scarcity concerns.
Regionally, Asia-Pacific dominates the H2O2 market with approximately 40% share, followed by North America and Europe. China and India represent the fastest-growing markets due to rapid industrialization and increasing environmental awareness. The demand for high-purity H2O2, especially in semiconductor manufacturing and healthcare applications, is growing at twice the rate of standard-grade peroxide.
The market for electrosynthesis technologies specifically is gaining momentum as industries seek greener alternatives to the traditional anthraquinone auto-oxidation process. Current estimates value the electrochemical H2O2 production equipment market at 320 million USD, with projections to reach 780 million USD by 2030. This represents a significant opportunity for high-current density cell architectures, which can reduce production costs by up to 30% compared to conventional methods.
Energy efficiency considerations are becoming increasingly important market drivers. With electricity costs representing 40-60% of operational expenses in electrochemical H2O2 production, technologies that can achieve higher current densities while maintaining faradaic efficiency have substantial economic advantages. Market analysis indicates that systems capable of operating above 500 mA/cm² while maintaining 90%+ selectivity could capture premium pricing of 15-20% above standard equipment.
Customer requirements are evolving toward modular, scalable systems that can be deployed on-site, reducing transportation costs and safety concerns associated with H2O2 handling. This decentralization trend is creating new market segments for smaller-scale, high-efficiency production units, particularly in remote locations and developing regions where infrastructure limitations exist.
Current Technical Challenges in H2O2 Electrosynthesis
Despite significant advancements in hydrogen peroxide (H2O2) electrosynthesis, several critical technical challenges persist that limit the widespread industrial adoption of this environmentally friendly production method. The primary obstacle remains achieving high current density while maintaining acceptable energy efficiency and product concentration, creating a complex optimization problem for cell architecture design.
The selective two-electron oxygen reduction reaction (2e-ORR) pathway faces competition from the four-electron pathway that produces water instead of H2O2, significantly reducing faradaic efficiency. This selectivity challenge is exacerbated at higher current densities where reaction kinetics favor the complete reduction pathway. Current catalyst materials struggle to maintain selectivity under industrial-relevant operating conditions.
Mass transport limitations represent another major hurdle, particularly in high current density operations. Insufficient oxygen supply to the catalyst surface creates concentration polarization, while product removal becomes increasingly problematic as production rates rise. Conventional cell designs often fail to address these mass transport challenges effectively, resulting in performance plateaus at higher current densities.
Electrode flooding presents a persistent challenge in gas diffusion electrode (GDE) configurations. The hydrophobic-hydrophilic balance within porous electrodes must be precisely controlled to maintain the three-phase boundary necessary for efficient oxygen reduction while preventing electrolyte penetration that blocks gas transport pathways.
Stability issues plague many cell architectures, with performance degradation occurring over extended operation periods. This degradation stems from multiple sources including catalyst deactivation, electrode structure deterioration, and membrane fouling. The harsh oxidative environment created during H2O2 production accelerates component degradation, particularly at elevated current densities.
Scale-up challenges remain significant when transitioning from laboratory-scale demonstrations to industrially relevant systems. Many promising cell architectures that perform well in small-scale testing encounter unforeseen complications when scaled to larger dimensions, including uneven current distribution, thermal management issues, and mechanical stability concerns.
The integration of renewable energy sources introduces additional complexity, as intermittent power supply affects system stability and product quality. Cell architectures optimized for steady-state operation often perform poorly under dynamic operating conditions, necessitating novel designs specifically engineered for variable power input.
Material cost and availability constraints further complicate commercial implementation, with many high-performance catalysts and membranes relying on precious metals or specialized polymers. Developing cell architectures that can achieve high current density with earth-abundant materials remains an elusive goal for researchers in this field.
The selective two-electron oxygen reduction reaction (2e-ORR) pathway faces competition from the four-electron pathway that produces water instead of H2O2, significantly reducing faradaic efficiency. This selectivity challenge is exacerbated at higher current densities where reaction kinetics favor the complete reduction pathway. Current catalyst materials struggle to maintain selectivity under industrial-relevant operating conditions.
Mass transport limitations represent another major hurdle, particularly in high current density operations. Insufficient oxygen supply to the catalyst surface creates concentration polarization, while product removal becomes increasingly problematic as production rates rise. Conventional cell designs often fail to address these mass transport challenges effectively, resulting in performance plateaus at higher current densities.
Electrode flooding presents a persistent challenge in gas diffusion electrode (GDE) configurations. The hydrophobic-hydrophilic balance within porous electrodes must be precisely controlled to maintain the three-phase boundary necessary for efficient oxygen reduction while preventing electrolyte penetration that blocks gas transport pathways.
Stability issues plague many cell architectures, with performance degradation occurring over extended operation periods. This degradation stems from multiple sources including catalyst deactivation, electrode structure deterioration, and membrane fouling. The harsh oxidative environment created during H2O2 production accelerates component degradation, particularly at elevated current densities.
Scale-up challenges remain significant when transitioning from laboratory-scale demonstrations to industrially relevant systems. Many promising cell architectures that perform well in small-scale testing encounter unforeseen complications when scaled to larger dimensions, including uneven current distribution, thermal management issues, and mechanical stability concerns.
The integration of renewable energy sources introduces additional complexity, as intermittent power supply affects system stability and product quality. Cell architectures optimized for steady-state operation often perform poorly under dynamic operating conditions, necessitating novel designs specifically engineered for variable power input.
Material cost and availability constraints further complicate commercial implementation, with many high-performance catalysts and membranes relying on precious metals or specialized polymers. Developing cell architectures that can achieve high current density with earth-abundant materials remains an elusive goal for researchers in this field.
State-of-the-Art Cell Design Solutions for High Current Density
01 Electrode materials for H2O2 electrosynthesis
Various electrode materials can significantly impact the efficiency of H2O2 electrosynthesis and achievable current densities. Carbon-based electrodes, noble metals, and metal oxides have been developed with specific surface modifications to enhance selectivity and activity. These materials are designed to facilitate the two-electron oxygen reduction reaction pathway while suppressing the competing four-electron pathway, thereby improving H2O2 yield at higher current densities.- Electrode materials for H2O2 electrosynthesis: Various electrode materials can significantly impact the efficiency of H2O2 electrosynthesis and achievable current densities. Carbon-based electrodes, noble metals, and metal oxides have been developed with specific surface modifications to enhance selectivity and activity. These materials are designed to facilitate the two-electron oxygen reduction reaction pathway while suppressing the competing four-electron pathway, allowing for higher current densities and improved H2O2 production rates.
- Flow cell configurations for enhanced current density: Advanced flow cell architectures have been developed to optimize mass transport and reaction kinetics in H2O2 electrosynthesis. These designs include microfluidic cells, flow-through electrodes, and gas diffusion electrode systems that facilitate efficient oxygen delivery to the electrode surface. By minimizing transport limitations and enhancing reactant accessibility, these configurations can achieve significantly higher current densities compared to conventional cell designs.
- Electrolyte composition and pH effects on current density: The composition and pH of the electrolyte solution play crucial roles in determining the current density and selectivity of H2O2 electrosynthesis. Acidic, neutral, and alkaline conditions each offer different advantages in terms of reaction pathways and stability of the produced H2O2. Electrolyte additives and buffers can be incorporated to maintain optimal pH conditions and enhance conductivity, thereby improving current density while maintaining high faradaic efficiency.
- Membrane and separator technologies: Advanced membrane and separator technologies are essential components in cell architectures for H2O2 electrosynthesis. Ion-exchange membranes, porous separators, and composite membrane systems can effectively separate the cathode and anode compartments while allowing selective ion transport. These materials help prevent H2O2 decomposition at the counter electrode and reduce ohmic resistance, enabling operation at higher current densities without compromising efficiency or product stability.
- Process parameters optimization for high current density operation: Optimizing process parameters such as temperature, pressure, and applied potential is critical for achieving high current densities in H2O2 electrosynthesis. Advanced control systems can dynamically adjust these parameters to maintain optimal reaction conditions. Additionally, pulsed electrolysis techniques and pressure-modulated systems have been developed to overcome mass transport limitations and enhance reaction kinetics, allowing for sustained operation at elevated current densities without sacrificing selectivity or efficiency.
02 Flow cell designs for improved current density
Advanced flow cell architectures have been developed to enhance mass transport and reaction kinetics in H2O2 electrosynthesis. These designs include microfluidic cells, flow-through electrodes, and gas diffusion electrode systems that optimize the three-phase interface. By reducing mass transport limitations and improving oxygen availability at the electrode surface, these cell designs can achieve significantly higher current densities while maintaining selectivity for H2O2 production.Expand Specific Solutions03 Electrolyte composition and pH effects
The composition and pH of the electrolyte solution play crucial roles in determining the efficiency and current density of H2O2 electrosynthesis. Acidic, neutral, and alkaline conditions each offer different advantages in terms of reaction pathways and stability of the produced H2O2. Buffered electrolytes and specific ionic additives can enhance conductivity, stabilize the produced H2O2, and allow operation at higher current densities without compromising selectivity.Expand Specific Solutions04 Membrane and separator technologies
Advanced membrane and separator technologies are essential for high-performance H2O2 electrosynthesis cells. Ion-exchange membranes, porous separators, and composite membrane systems help maintain separation between electrode compartments while facilitating ion transport. These components prevent product crossover and degradation, allowing for sustained operation at higher current densities and improving overall energy efficiency of the electrosynthesis process.Expand Specific Solutions05 Pressure and temperature optimization
Operating conditions such as pressure and temperature significantly affect the current density and efficiency of H2O2 electrosynthesis. Elevated oxygen pressure can increase the concentration of dissolved oxygen at the electrode surface, enabling higher current densities. Temperature control is equally important, as it affects reaction kinetics, mass transport, and the stability of produced H2O2. Optimized combinations of pressure and temperature can substantially improve production rates while maintaining high faradaic efficiency.Expand Specific Solutions
Leading Companies and Research Institutions in H2O2 Electrosynthesis
The hydrogen peroxide electrosynthesis market is currently in an early growth phase, characterized by increasing research activity and emerging commercial applications. The global market size for electrochemically produced H2O2 is projected to expand significantly as industries seek greener alternatives to traditional anthraquinone processes. Technologically, the field remains in development with varying levels of maturity across different cell architectures. Leading players include Siemens Energy and Panasonic Energy focusing on industrial-scale implementations, while academic institutions like Delft University and City University of Hong Kong drive fundamental research innovations. Companies such as Utility Global and EDAC Labs are developing specialized electrochemical systems for H2O2 production, while established materials companies like Samsung Electronics and ITOCHU are investing in component technologies to enhance current density capabilities and system efficiency.
Forschungszentrum Jülich GmbH
Technical Solution: Forschungszentrum Jülich has developed advanced electrochemical cell architectures specifically optimized for high current density H2O2 electrosynthesis. Their approach utilizes gas diffusion electrodes (GDEs) with tailored porosity and hydrophobicity to facilitate efficient oxygen reduction to H2O2. The cell design incorporates a three-dimensional carbon-based cathode with optimized mass transport properties, allowing for current densities exceeding 500 mA/cm² while maintaining H2O2 selectivity above 90%. Their proprietary catalyst formulation features carbon materials doped with nitrogen and transition metals that suppress the competing 4-electron oxygen reduction pathway. The cell architecture also includes innovative membrane electrode assembly configurations that minimize ohmic losses and prevent H2O2 decomposition through careful management of local pH and electrode potential distributions.
Strengths: Exceptional selectivity for H2O2 production even at high current densities; sophisticated mass transport optimization; advanced catalyst formulations. Weaknesses: Complex manufacturing process; potential durability issues under industrial operating conditions; higher capital costs compared to conventional systems.
The Regents of the University of California
Technical Solution: The University of California has pioneered a revolutionary cell architecture for H2O2 electrosynthesis featuring hierarchically structured electrodes that enable unprecedented current densities while maintaining high faradaic efficiency. Their system employs a flow-through electrode design with precisely engineered pore structures that optimize reactant transport and product removal. The cathode incorporates carbon-based materials with tailored surface chemistry to promote the 2-electron oxygen reduction pathway, achieving H2O2 selectivity exceeding 95% at current densities up to 700 mA/cm². A key innovation is their bipolar membrane configuration that creates separate pH environments for the cathode and anode, preventing H2O2 decomposition while enabling water oxidation at the anode. The cell architecture also features integrated cooling channels to manage heat generation at high current densities, maintaining optimal temperature profiles throughout the reactor.
Strengths: Exceptional current density capabilities; outstanding selectivity; innovative bipolar membrane design for pH management. Weaknesses: Complex system integration requirements; potential scaling challenges for industrial implementation; higher energy consumption at maximum current densities.
Critical Patents and Breakthroughs in H2O2 Electrocatalysis
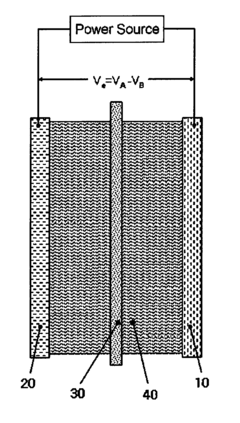
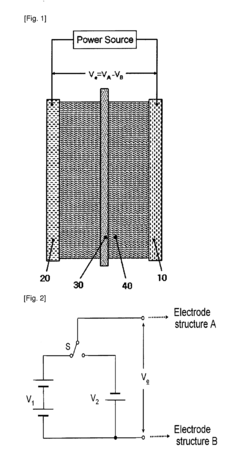
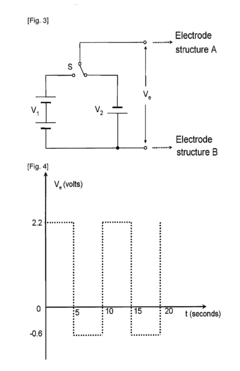
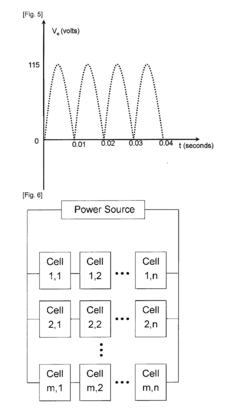
Electrolytic Synthesis of Hydrogen Peroxide Directly from Water and Application Thereof
PatentInactiveUS20120048744A1
Innovation
- An electrochemical cell with time-dependent polarity voltage applied across two electrode structures in an aqueous solution, allowing hydrogen peroxide generation directly from water without external oxygen or air, using a water-soluble electrolyte and porous conducting materials with catalysts, and optionally a separator membrane.
Process and electrolytic cell for producing hydrogen peroxide
PatentInactiveUS6254762B1
Innovation
- An electrolytic cell with ion-conductive materials, such as ion-exchange resin, electrically connecting the electrodes to facilitate high current density production of hydrogen peroxide, using a two- or three-chamber configuration with diaphragms to manage electrolysis reactions and minimize electrical resistance.
Scalability and Industrial Implementation Considerations
The scalability of hydrogen peroxide electrosynthesis systems represents a critical challenge for industrial implementation. Current laboratory-scale cell architectures demonstrating high current densities often face significant hurdles when scaled to commercial production levels. The primary scaling considerations include electrode surface area requirements, system pressure management, and heat dissipation mechanisms that become increasingly complex at larger scales.
Transitioning from laboratory prototypes to industrial-scale production necessitates careful engineering of cell components. Materials selection becomes particularly crucial, as components must withstand both the corrosive nature of H2O2 and maintain performance integrity over extended operational periods. Industrial implementation typically requires electrode materials that balance catalytic activity with long-term stability, often necessitating trade-offs between performance metrics and durability.
Energy efficiency at scale presents another significant consideration. While laboratory demonstrations may achieve impressive current densities, industrial systems must optimize for overall energy consumption per unit of H2O2 produced. This optimization frequently involves redesigning cell architectures to minimize ohmic losses and maximize mass transport efficiency across larger electrode surfaces.
Process integration represents a fundamental aspect of industrial implementation. H2O2 electrosynthesis cells must be effectively incorporated into broader production systems, including feedstock preparation, product separation, and concentration processes. The integration complexity increases with production scale, requiring sophisticated control systems to maintain optimal operating conditions across multiple cell units.
Capital expenditure and operational costs significantly influence industrial viability. High current density cell architectures often require advanced materials and precise manufacturing techniques, potentially increasing initial investment requirements. However, these costs must be balanced against operational benefits such as reduced footprint, higher production rates, and potentially lower energy consumption per unit product.
Safety considerations become paramount at industrial scales. The simultaneous presence of hydrogen, oxygen, and concentrated hydrogen peroxide creates potential hazards that must be systematically addressed through cell design, process controls, and operational protocols. Compartmentalization of reaction zones, pressure management systems, and robust monitoring capabilities are essential components of industrially viable cell architectures.
Regulatory compliance adds another layer of complexity to industrial implementation. Cell architectures must be designed to meet relevant safety standards, environmental regulations, and quality specifications for the intended application markets. This often necessitates additional engineering features beyond those required for basic electrochemical performance.
Transitioning from laboratory prototypes to industrial-scale production necessitates careful engineering of cell components. Materials selection becomes particularly crucial, as components must withstand both the corrosive nature of H2O2 and maintain performance integrity over extended operational periods. Industrial implementation typically requires electrode materials that balance catalytic activity with long-term stability, often necessitating trade-offs between performance metrics and durability.
Energy efficiency at scale presents another significant consideration. While laboratory demonstrations may achieve impressive current densities, industrial systems must optimize for overall energy consumption per unit of H2O2 produced. This optimization frequently involves redesigning cell architectures to minimize ohmic losses and maximize mass transport efficiency across larger electrode surfaces.
Process integration represents a fundamental aspect of industrial implementation. H2O2 electrosynthesis cells must be effectively incorporated into broader production systems, including feedstock preparation, product separation, and concentration processes. The integration complexity increases with production scale, requiring sophisticated control systems to maintain optimal operating conditions across multiple cell units.
Capital expenditure and operational costs significantly influence industrial viability. High current density cell architectures often require advanced materials and precise manufacturing techniques, potentially increasing initial investment requirements. However, these costs must be balanced against operational benefits such as reduced footprint, higher production rates, and potentially lower energy consumption per unit product.
Safety considerations become paramount at industrial scales. The simultaneous presence of hydrogen, oxygen, and concentrated hydrogen peroxide creates potential hazards that must be systematically addressed through cell design, process controls, and operational protocols. Compartmentalization of reaction zones, pressure management systems, and robust monitoring capabilities are essential components of industrially viable cell architectures.
Regulatory compliance adds another layer of complexity to industrial implementation. Cell architectures must be designed to meet relevant safety standards, environmental regulations, and quality specifications for the intended application markets. This often necessitates additional engineering features beyond those required for basic electrochemical performance.
Environmental Impact and Sustainability Assessment
The electrosynthesis of hydrogen peroxide (H2O2) represents a significant advancement in green chemistry, offering a more sustainable alternative to the traditional anthraquinone process. When evaluating cell architectures for high current density H2O2 production, environmental impact and sustainability considerations are paramount to ensuring this technology delivers on its promise of eco-friendly chemical manufacturing.
Electrochemical H2O2 production demonstrates substantial environmental advantages through the elimination of harmful chemical intermediates and reduction of energy consumption. Compared to the conventional anthraquinone process, which requires multiple reaction steps and organic solvents, direct electrosynthesis utilizes only water, oxygen, and electricity as inputs. This simplification results in an estimated 30-40% reduction in carbon footprint when renewable electricity sources are employed.
Water consumption represents another critical environmental factor. High current density cell architectures typically require continuous water flow for both reactant supply and cooling. Advanced cell designs incorporating water recirculation systems have demonstrated up to 80% reduction in freshwater requirements compared to first-generation cells. This improvement is particularly significant in water-stressed regions where industrial water usage faces increasing scrutiny.
The materials used in cell construction also warrant sustainability assessment. Current high-performance electrodes often incorporate precious metals like platinum or palladium, raising concerns about resource depletion and mining impacts. Recent innovations in carbon-based catalysts and earth-abundant metal alternatives show promise for reducing dependence on scarce materials while maintaining performance metrics. Life cycle assessments indicate that catalyst longevity significantly influences overall environmental impact, with extended catalyst lifespans of >5,000 hours necessary to achieve net environmental benefits.
Waste generation and management constitute additional sustainability considerations. Cell architectures employing membrane separators generate spent membranes requiring disposal, while membraneless designs may produce more dilute H2O2 solutions necessitating energy-intensive concentration steps. Closed-loop systems that recover and reuse components show potential to minimize waste streams by up to 90% compared to conventional approaches.
Energy efficiency remains the dominant factor in environmental performance. State-of-the-art cell architectures achieving current densities above 500 mA/cm² with Faradaic efficiencies exceeding 80% represent the sustainability benchmark. When powered by renewable electricity, these systems can achieve carbon intensity reductions of 70-95% compared to conventional H2O2 production methods, positioning electrochemical synthesis as a key technology for decarbonizing the chemical industry.
Electrochemical H2O2 production demonstrates substantial environmental advantages through the elimination of harmful chemical intermediates and reduction of energy consumption. Compared to the conventional anthraquinone process, which requires multiple reaction steps and organic solvents, direct electrosynthesis utilizes only water, oxygen, and electricity as inputs. This simplification results in an estimated 30-40% reduction in carbon footprint when renewable electricity sources are employed.
Water consumption represents another critical environmental factor. High current density cell architectures typically require continuous water flow for both reactant supply and cooling. Advanced cell designs incorporating water recirculation systems have demonstrated up to 80% reduction in freshwater requirements compared to first-generation cells. This improvement is particularly significant in water-stressed regions where industrial water usage faces increasing scrutiny.
The materials used in cell construction also warrant sustainability assessment. Current high-performance electrodes often incorporate precious metals like platinum or palladium, raising concerns about resource depletion and mining impacts. Recent innovations in carbon-based catalysts and earth-abundant metal alternatives show promise for reducing dependence on scarce materials while maintaining performance metrics. Life cycle assessments indicate that catalyst longevity significantly influences overall environmental impact, with extended catalyst lifespans of >5,000 hours necessary to achieve net environmental benefits.
Waste generation and management constitute additional sustainability considerations. Cell architectures employing membrane separators generate spent membranes requiring disposal, while membraneless designs may produce more dilute H2O2 solutions necessitating energy-intensive concentration steps. Closed-loop systems that recover and reuse components show potential to minimize waste streams by up to 90% compared to conventional approaches.
Energy efficiency remains the dominant factor in environmental performance. State-of-the-art cell architectures achieving current densities above 500 mA/cm² with Faradaic efficiencies exceeding 80% represent the sustainability benchmark. When powered by renewable electricity, these systems can achieve carbon intensity reductions of 70-95% compared to conventional H2O2 production methods, positioning electrochemical synthesis as a key technology for decarbonizing the chemical industry.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!