Coupling H2O2 Electrosynthesis With Renewable Electricity Sources
AUG 28, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
H2O2 Electrosynthesis Background and Objectives
Hydrogen peroxide (H2O2) has emerged as a versatile and environmentally benign oxidant with applications spanning across numerous industries including pulp and paper bleaching, wastewater treatment, chemical synthesis, and medical disinfection. Traditionally, H2O2 production has relied on the anthraquinone auto-oxidation process, an energy-intensive method that generates significant carbon emissions and requires centralized manufacturing facilities. This centralized production necessitates transportation and storage of concentrated H2O2 solutions, presenting safety hazards and logistical challenges.
The electrosynthesis of H2O2 represents a paradigm shift in production methodology, offering a direct, on-site generation pathway that eliminates many of the drawbacks associated with conventional methods. The electrochemical approach involves the two-electron oxygen reduction reaction (2e- ORR) or water oxidation, which can be performed under ambient conditions using water and oxygen as feedstocks. This process has gained significant attention over the past decade due to its potential for decentralized production and reduced environmental impact.
The coupling of H2O2 electrosynthesis with renewable electricity sources presents a particularly promising avenue for sustainable chemical production. As global renewable energy capacity continues to expand, with solar and wind power experiencing exponential growth, the opportunity to harness this clean electricity for chemical manufacturing becomes increasingly viable. This integration addresses two critical challenges simultaneously: the intermittent nature of renewable energy generation and the need for greener chemical production processes.
Historical development of H2O2 electrosynthesis can be traced back to the early 20th century, but significant advances in catalyst design, electrode materials, and system engineering have only emerged in the last two decades. The field has witnessed accelerated progress since 2015, with breakthrough developments in selective catalysts that favor the 2e- pathway over the competing 4e- oxygen reduction to water. These advances have pushed Faradaic efficiencies above 90% and increased production rates by orders of magnitude.
The primary technical objectives in this field now center on several key challenges: developing highly selective and stable catalysts that operate at industrially relevant current densities, designing scalable electrochemical reactors that maintain performance at larger scales, and creating integrated systems that can efficiently utilize variable renewable electricity inputs. Additionally, achieving economically viable concentrations of H2O2 directly from the electrochemical process remains a significant hurdle.
Looking forward, the technology aims to enable distributed, on-demand production of H2O2 powered by renewable electricity, potentially revolutionizing how this important chemical is produced and utilized across various applications. This approach aligns with broader sustainability goals by reducing carbon emissions, eliminating hazardous transportation requirements, and providing a valuable means of storing renewable energy in chemical form.
The electrosynthesis of H2O2 represents a paradigm shift in production methodology, offering a direct, on-site generation pathway that eliminates many of the drawbacks associated with conventional methods. The electrochemical approach involves the two-electron oxygen reduction reaction (2e- ORR) or water oxidation, which can be performed under ambient conditions using water and oxygen as feedstocks. This process has gained significant attention over the past decade due to its potential for decentralized production and reduced environmental impact.
The coupling of H2O2 electrosynthesis with renewable electricity sources presents a particularly promising avenue for sustainable chemical production. As global renewable energy capacity continues to expand, with solar and wind power experiencing exponential growth, the opportunity to harness this clean electricity for chemical manufacturing becomes increasingly viable. This integration addresses two critical challenges simultaneously: the intermittent nature of renewable energy generation and the need for greener chemical production processes.
Historical development of H2O2 electrosynthesis can be traced back to the early 20th century, but significant advances in catalyst design, electrode materials, and system engineering have only emerged in the last two decades. The field has witnessed accelerated progress since 2015, with breakthrough developments in selective catalysts that favor the 2e- pathway over the competing 4e- oxygen reduction to water. These advances have pushed Faradaic efficiencies above 90% and increased production rates by orders of magnitude.
The primary technical objectives in this field now center on several key challenges: developing highly selective and stable catalysts that operate at industrially relevant current densities, designing scalable electrochemical reactors that maintain performance at larger scales, and creating integrated systems that can efficiently utilize variable renewable electricity inputs. Additionally, achieving economically viable concentrations of H2O2 directly from the electrochemical process remains a significant hurdle.
Looking forward, the technology aims to enable distributed, on-demand production of H2O2 powered by renewable electricity, potentially revolutionizing how this important chemical is produced and utilized across various applications. This approach aligns with broader sustainability goals by reducing carbon emissions, eliminating hazardous transportation requirements, and providing a valuable means of storing renewable energy in chemical form.
Market Analysis for Green H2O2 Production
The global hydrogen peroxide (H2O2) market is experiencing significant growth, with a market value estimated at $3.5 billion in 2022 and projected to reach $5.7 billion by 2030. This growth is primarily driven by increasing demand from various end-use industries including pulp and paper, textile bleaching, water treatment, electronics manufacturing, and healthcare applications. Traditional H2O2 production methods, particularly the anthraquinone auto-oxidation process, dominate the current market but face sustainability challenges due to high energy consumption and environmental impacts.
Green H2O2 production through electrochemical synthesis coupled with renewable electricity represents an emerging market segment with substantial growth potential. This approach aligns with global sustainability goals and carbon neutrality targets adopted by major economies. Market analysis indicates that the green H2O2 segment is growing at a compound annual growth rate (CAGR) of approximately 8.5%, outpacing the conventional H2O2 market growth rate of 5.2%.
Regional market distribution shows varying adoption rates for green H2O2 technologies. Europe leads the transition with supportive regulatory frameworks and ambitious decarbonization targets, particularly in Germany, Denmark, and the Netherlands. North America follows with significant investments in renewable energy integration and electrochemical technologies, while the Asia-Pacific region presents the largest growth opportunity due to expanding industrial bases in China, Japan, and South Korea.
Consumer industries are increasingly willing to pay premium prices for sustainably produced chemicals, creating favorable market conditions for green H2O2. The price differential between conventionally produced and electrochemically synthesized H2O2 is gradually narrowing as renewable electricity costs decrease and technology efficiencies improve. Current price premiums range from 15-30% but are expected to decrease to 5-10% by 2028 as technologies mature and scale.
Market barriers include high initial capital investment requirements, technological readiness levels, and competition from established production methods. However, these barriers are counterbalanced by strong policy support through carbon pricing mechanisms, renewable energy incentives, and green chemistry initiatives in major markets.
The competitive landscape features both established chemical manufacturers exploring green H2O2 production methods and innovative startups developing specialized electrochemical technologies. Strategic partnerships between technology developers, renewable energy providers, and end-users are emerging as a dominant market entry strategy, creating new value chain configurations and business models centered around localized, on-demand H2O2 production integrated with renewable energy sources.
Green H2O2 production through electrochemical synthesis coupled with renewable electricity represents an emerging market segment with substantial growth potential. This approach aligns with global sustainability goals and carbon neutrality targets adopted by major economies. Market analysis indicates that the green H2O2 segment is growing at a compound annual growth rate (CAGR) of approximately 8.5%, outpacing the conventional H2O2 market growth rate of 5.2%.
Regional market distribution shows varying adoption rates for green H2O2 technologies. Europe leads the transition with supportive regulatory frameworks and ambitious decarbonization targets, particularly in Germany, Denmark, and the Netherlands. North America follows with significant investments in renewable energy integration and electrochemical technologies, while the Asia-Pacific region presents the largest growth opportunity due to expanding industrial bases in China, Japan, and South Korea.
Consumer industries are increasingly willing to pay premium prices for sustainably produced chemicals, creating favorable market conditions for green H2O2. The price differential between conventionally produced and electrochemically synthesized H2O2 is gradually narrowing as renewable electricity costs decrease and technology efficiencies improve. Current price premiums range from 15-30% but are expected to decrease to 5-10% by 2028 as technologies mature and scale.
Market barriers include high initial capital investment requirements, technological readiness levels, and competition from established production methods. However, these barriers are counterbalanced by strong policy support through carbon pricing mechanisms, renewable energy incentives, and green chemistry initiatives in major markets.
The competitive landscape features both established chemical manufacturers exploring green H2O2 production methods and innovative startups developing specialized electrochemical technologies. Strategic partnerships between technology developers, renewable energy providers, and end-users are emerging as a dominant market entry strategy, creating new value chain configurations and business models centered around localized, on-demand H2O2 production integrated with renewable energy sources.
Current Challenges in H2O2 Electrosynthesis
Despite significant advancements in hydrogen peroxide (H2O2) electrosynthesis, several critical challenges persist that hinder its widespread coupling with renewable electricity sources. The intermittent nature of renewable energy sources such as solar and wind creates operational instability in electrosynthesis processes, resulting in fluctuating production rates and inconsistent H2O2 concentrations. These variations compromise product quality and system efficiency, particularly when scaling up for industrial applications.
Energy efficiency remains a major obstacle, with current electrocatalytic systems exhibiting high overpotentials that reduce overall process efficiency. Most state-of-the-art catalysts require potentials significantly above the thermodynamic minimum, resulting in energy losses that make the process economically challenging when powered by renewable sources that may have higher levelized costs of electricity compared to conventional grid power.
Selectivity issues present another significant challenge, as competing reactions—particularly the four-electron oxygen reduction to water—often dominate over the desired two-electron pathway to H2O2. This selectivity problem is exacerbated under the variable load conditions typical of renewable energy sources, where optimal reaction parameters cannot be consistently maintained.
Catalyst stability under fluctuating power conditions represents a substantial hurdle. Most catalysts designed for H2O2 production demonstrate promising performance under laboratory conditions with constant power supply but degrade rapidly when subjected to the variable voltage and current profiles characteristic of renewable energy sources. This degradation leads to decreased catalytic activity, reduced selectivity, and shortened operational lifetimes.
The concentration limitation also poses significant challenges. Current electrochemical methods typically produce dilute H2O2 solutions (below 1 wt%), whereas commercial applications often require concentrations of 3-8 wt% or higher. Concentrating these solutions requires additional energy-intensive processes, diminishing the overall sustainability benefits of renewable-powered electrosynthesis.
System integration complexities further complicate implementation. Coupling electrolyzers with renewable energy sources requires sophisticated power electronics, energy storage systems, and control algorithms to manage power fluctuations. These components add significant capital costs and technical complexity to H2O2 production systems.
Scalability remains problematic as most successful demonstrations have occurred at laboratory scale. The transition to industrial-scale production introduces challenges related to heat management, mass transport limitations, and uniform current distribution that are not fully addressed by current technologies. These scaling issues are particularly pronounced when designing systems compatible with the distributed nature of many renewable energy installations.
Energy efficiency remains a major obstacle, with current electrocatalytic systems exhibiting high overpotentials that reduce overall process efficiency. Most state-of-the-art catalysts require potentials significantly above the thermodynamic minimum, resulting in energy losses that make the process economically challenging when powered by renewable sources that may have higher levelized costs of electricity compared to conventional grid power.
Selectivity issues present another significant challenge, as competing reactions—particularly the four-electron oxygen reduction to water—often dominate over the desired two-electron pathway to H2O2. This selectivity problem is exacerbated under the variable load conditions typical of renewable energy sources, where optimal reaction parameters cannot be consistently maintained.
Catalyst stability under fluctuating power conditions represents a substantial hurdle. Most catalysts designed for H2O2 production demonstrate promising performance under laboratory conditions with constant power supply but degrade rapidly when subjected to the variable voltage and current profiles characteristic of renewable energy sources. This degradation leads to decreased catalytic activity, reduced selectivity, and shortened operational lifetimes.
The concentration limitation also poses significant challenges. Current electrochemical methods typically produce dilute H2O2 solutions (below 1 wt%), whereas commercial applications often require concentrations of 3-8 wt% or higher. Concentrating these solutions requires additional energy-intensive processes, diminishing the overall sustainability benefits of renewable-powered electrosynthesis.
System integration complexities further complicate implementation. Coupling electrolyzers with renewable energy sources requires sophisticated power electronics, energy storage systems, and control algorithms to manage power fluctuations. These components add significant capital costs and technical complexity to H2O2 production systems.
Scalability remains problematic as most successful demonstrations have occurred at laboratory scale. The transition to industrial-scale production introduces challenges related to heat management, mass transport limitations, and uniform current distribution that are not fully addressed by current technologies. These scaling issues are particularly pronounced when designing systems compatible with the distributed nature of many renewable energy installations.
Existing Coupling Mechanisms for Renewable Integration
01 Electrochemical systems for H2O2 production
Various electrochemical systems have been developed for the efficient production of hydrogen peroxide (H2O2) through electrosynthesis. These systems typically involve specialized electrodes, catalysts, and cell configurations designed to optimize the oxygen reduction reaction pathway toward H2O2 formation. The electrochemical approach offers advantages such as mild reaction conditions, controllable production rates, and the ability to couple with renewable energy sources for sustainable manufacturing.- Electrocatalytic systems for H2O2 production: Various electrocatalytic systems can be employed for the efficient synthesis of hydrogen peroxide (H2O2). These systems typically involve specialized catalysts that facilitate the two-electron oxygen reduction reaction (ORR) or water oxidation. The catalysts can be designed with specific structures and compositions to enhance selectivity toward H2O2 formation while minimizing competing reactions. These electrocatalytic approaches offer advantages such as mild reaction conditions and tunable production rates.
- Coupling H2O2 electrosynthesis with organic transformations: The in-situ generation of H2O2 through electrochemical methods can be directly coupled with organic synthesis reactions. This approach eliminates the need for storage and transportation of concentrated H2O2, reducing safety concerns. The electrogenerated H2O2 can serve as an oxidant for various transformations including hydroxylation, epoxidation, and oxidative coupling reactions. This integrated approach offers advantages in terms of atom economy, reduced waste, and potential for continuous flow processes.
- Reactor designs and process engineering for H2O2 electrosynthesis: Specialized reactor designs play a crucial role in optimizing H2O2 electrosynthesis and coupling reactions. These designs focus on parameters such as electrode configuration, membrane selection, electrolyte composition, and flow dynamics. Advanced reactor concepts include divided cells, flow-through electrodes, and microfluidic systems that enhance mass transfer and reaction control. Process engineering considerations also address scale-up challenges, energy efficiency, and integration with downstream applications.
- Materials innovation for selective H2O2 production: Novel materials development is critical for advancing H2O2 electrosynthesis. Research focuses on creating electrode materials with high selectivity for the two-electron oxygen reduction pathway over the competing four-electron pathway. These materials include modified carbon structures, metal-nitrogen-carbon composites, single-atom catalysts, and nanostructured metals or metal oxides. Material innovations aim to increase catalytic activity, stability, and selectivity while using earth-abundant elements to ensure economic viability.
- Integrated systems for sustainable H2O2 production: Integrated approaches combine H2O2 electrosynthesis with renewable energy sources or other value-added processes. These systems may couple H2O2 production with solar or wind power, wastewater treatment, or simultaneous generation of valuable chemicals. The integration enhances overall system efficiency and sustainability by utilizing otherwise wasted energy or creating multiple valuable products from a single process. Such approaches address challenges in green chemistry and circular economy principles by minimizing resource consumption and environmental impact.
02 Catalyst materials for selective H2O2 electrosynthesis
Advanced catalyst materials play a crucial role in the selective electrosynthesis of hydrogen peroxide. These catalysts are designed to promote the two-electron oxygen reduction reaction while suppressing the competing four-electron pathway that leads to water formation. Materials such as carbon-based catalysts, metal oxides, and specially modified noble metals have shown promising performance in terms of selectivity, activity, and stability for H2O2 production under various operating conditions.Expand Specific Solutions03 Coupling H2O2 electrosynthesis with organic transformations
The integration of hydrogen peroxide electrosynthesis with organic synthesis reactions represents an innovative approach in green chemistry. In these coupled processes, electrogenerated H2O2 serves as an in-situ oxidant for various transformations including hydroxylation, epoxidation, and oxidative coupling reactions. This approach eliminates the need for storage and transportation of concentrated H2O2, enhancing safety while enabling one-pot reaction sequences with improved atom economy and reduced waste generation.Expand Specific Solutions04 Flow reactor designs for continuous H2O2 production
Continuous flow reactor systems have been developed to address the challenges of batch production of hydrogen peroxide. These flow reactors feature optimized electrode arrangements, controlled electrolyte flow patterns, and integrated separation mechanisms to enable sustained H2O2 production. The flow configuration allows for better heat management, enhanced mass transfer, and improved process control, resulting in higher production rates and concentrations of hydrogen peroxide compared to conventional batch systems.Expand Specific Solutions05 Energy-efficient coupling strategies for H2O2 electrosynthesis
Energy efficiency is a critical consideration in hydrogen peroxide electrosynthesis. Various coupling strategies have been developed to reduce energy consumption, including the integration with renewable energy sources, utilization of waste heat, and implementation of energy recovery systems. Advanced electrode materials and cell designs that operate at lower overpotentials, coupled with optimized reaction conditions and electrolyte compositions, have demonstrated significant improvements in energy efficiency while maintaining high H2O2 production rates.Expand Specific Solutions
Key Industry Players and Research Institutions
The hydrogen peroxide electrosynthesis market is currently in an early growth phase, with increasing momentum driven by renewable electricity integration. Market size is expanding rapidly as industries seek sustainable chemical production methods, though still modest compared to traditional H2O2 manufacturing. Technologically, the field shows promising but varied maturity levels across different approaches. Leading academic institutions (Tianjin University, Tsinghua University, University of Delaware) are advancing fundamental research, while industrial players demonstrate different stages of commercial readiness. Companies like Siemens Energy and TotalEnergies are leveraging their energy expertise to develop scalable solutions, while specialized firms such as NGK Insulators and Shanghai Shen-Li High Tech are focusing on component optimization. The competitive landscape reflects a collaborative ecosystem where academic-industrial partnerships are accelerating technology transfer and commercialization pathways.
Tianjin University
Technical Solution: Tianjin University has developed an advanced electrochemical system for H2O2 production using renewable electricity sources. Their approach employs specially designed carbon-based catalysts with optimized oxygen reduction reaction (ORR) pathways to achieve selective 2-electron reduction to H2O2. The system incorporates a novel flow-cell design that maximizes mass transport and reaction efficiency while minimizing energy losses. Their technology achieves H2O2 production rates exceeding 50 mmol/h·cm² with Faradaic efficiencies above 90% when coupled with solar or wind power sources. The system includes intelligent power management modules that can handle the intermittent nature of renewable inputs while maintaining stable H2O2 production parameters. This integrated approach enables decentralized H2O2 production at point-of-use facilities, eliminating transportation and storage concerns associated with traditional H2O2 manufacturing.
Strengths: High selectivity and efficiency in H2O2 production; excellent integration with intermittent renewable sources; modular design suitable for distributed applications. Weaknesses: Catalyst degradation over extended operation periods; requires further scale-up validation for industrial implementation; higher capital costs compared to centralized production methods.
Siemens Energy Global GmbH & Co. KG
Technical Solution: Siemens Energy has engineered a comprehensive H2O2 electrosynthesis platform designed specifically for integration with renewable energy sources. Their system employs proprietary PEM (Proton Exchange Membrane) electrolyzer technology modified for selective oxygen reduction to hydrogen peroxide. The platform features advanced power electronics that enable dynamic response to fluctuating renewable inputs from wind and solar sources, maintaining optimal production parameters despite variability. Siemens' solution incorporates a grid-balancing capability that allows the H2O2 production system to serve as a flexible load, absorbing excess renewable generation during peak production periods. The technology achieves energy efficiency of approximately 65-70% with production concentrations of 2-5 wt% H2O2 directly from the electrochemical cell. Their integrated control system optimizes production based on electricity price signals, renewable availability, and H2O2 demand forecasts, creating a smart manufacturing approach that maximizes economic and environmental benefits.
Strengths: Exceptional grid integration capabilities; robust system engineering with industrial reliability standards; comprehensive digital twin for optimization and predictive maintenance. Weaknesses: Higher initial capital investment compared to conventional methods; requires specific water quality parameters; limited to moderate H2O2 concentrations without additional concentration steps.
Critical Catalyst and Electrode Technologies
Synthesis of h 2o 2 using a rhenium metal-based electrocatalyst and rhenium electrocatalyst
PatentWO2025083671A1
Innovation
- A green synthesis process using an electrocatalyst based on metallic Renio nanoparticles supported on carbon nanotubes for the electrochemical production of hydrogen peroxide, which reduces production costs and environmental impact.
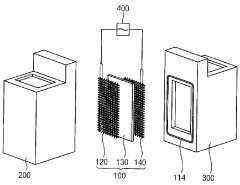
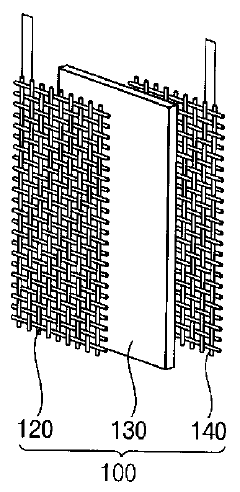
Electrode assembly for generating hydrogen peroxide and electrolysis system for generating hydrogen peroxide including same
PatentActiveKR1020140073180A
Innovation
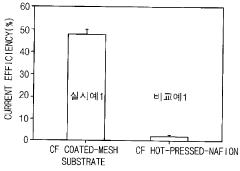
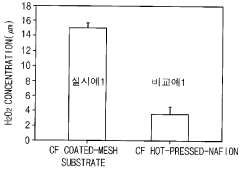
- An electrode assembly using mesh-shaped electrodes coated with an electroactive material, comprising carbon-containing conductive materials and a cation exchange membrane, generates hydrogen peroxide from water without electrolytes, enhancing electrical conductivity and reaction efficiency.
Techno-economic Assessment of Electrosynthesis Systems
The techno-economic assessment of hydrogen peroxide (H2O2) electrosynthesis systems coupled with renewable electricity sources reveals several critical economic factors that influence commercial viability. Capital expenditure (CAPEX) for these systems primarily consists of electrolyzer costs, renewable energy infrastructure, and balance-of-plant components. Current electrolyzer costs range from $500-1,500/kW depending on technology maturity and scale, with significant cost reduction potential through economies of scale and technological improvements.
Operational expenditure (OPEX) includes electricity costs, maintenance, water treatment, and catalyst replacement. Electricity represents 60-75% of total production costs, making renewable integration particularly attractive for long-term economic sustainability. Maintenance costs typically account for 2-5% of CAPEX annually, while catalyst degradation rates significantly impact replacement frequency and associated costs.
Levelized cost of H2O2 production via electrosynthesis currently ranges from $1.2-2.5/kg, compared to traditional anthraquinone process costs of $0.8-1.2/kg. However, this gap is narrowing as renewable electricity prices continue to decline and electrolyzer efficiencies improve. Sensitivity analysis indicates that electricity price and system capacity factor are the most influential parameters affecting production economics.
Renewable integration introduces additional considerations including intermittency management, grid connection costs, and potential revenue from grid services. Solar PV-coupled systems show production costs of $1.5-2.8/kg depending on location, while wind-coupled systems achieve $1.3-2.3/kg with higher capacity factors. Hybrid renewable systems with appropriate storage can achieve optimal economics with capacity factors exceeding 70%.
Return on investment (ROI) analysis demonstrates payback periods of 5-8 years for optimized systems in regions with favorable renewable resources and supportive policy frameworks. Carbon pricing mechanisms significantly improve economic viability, with each $50/ton CO2 price reducing effective production costs by approximately $0.15-0.25/kg H2O2 compared to fossil-based production methods.
Scale-up economics indicate substantial cost reductions at larger production volumes, with systems exceeding 10 tons/day approaching cost parity with conventional methods. Distributed production models show particular promise for reducing transportation costs in remote applications, potentially improving overall economics by 15-25% compared to centralized production and distribution models.
Operational expenditure (OPEX) includes electricity costs, maintenance, water treatment, and catalyst replacement. Electricity represents 60-75% of total production costs, making renewable integration particularly attractive for long-term economic sustainability. Maintenance costs typically account for 2-5% of CAPEX annually, while catalyst degradation rates significantly impact replacement frequency and associated costs.
Levelized cost of H2O2 production via electrosynthesis currently ranges from $1.2-2.5/kg, compared to traditional anthraquinone process costs of $0.8-1.2/kg. However, this gap is narrowing as renewable electricity prices continue to decline and electrolyzer efficiencies improve. Sensitivity analysis indicates that electricity price and system capacity factor are the most influential parameters affecting production economics.
Renewable integration introduces additional considerations including intermittency management, grid connection costs, and potential revenue from grid services. Solar PV-coupled systems show production costs of $1.5-2.8/kg depending on location, while wind-coupled systems achieve $1.3-2.3/kg with higher capacity factors. Hybrid renewable systems with appropriate storage can achieve optimal economics with capacity factors exceeding 70%.
Return on investment (ROI) analysis demonstrates payback periods of 5-8 years for optimized systems in regions with favorable renewable resources and supportive policy frameworks. Carbon pricing mechanisms significantly improve economic viability, with each $50/ton CO2 price reducing effective production costs by approximately $0.15-0.25/kg H2O2 compared to fossil-based production methods.
Scale-up economics indicate substantial cost reductions at larger production volumes, with systems exceeding 10 tons/day approaching cost parity with conventional methods. Distributed production models show particular promise for reducing transportation costs in remote applications, potentially improving overall economics by 15-25% compared to centralized production and distribution models.
Environmental Impact and Sustainability Metrics
The integration of hydrogen peroxide (H2O2) electrosynthesis with renewable electricity sources represents a significant advancement in sustainable chemical production. When evaluating this coupling from an environmental and sustainability perspective, several key metrics emerge that provide a comprehensive assessment framework.
Life Cycle Assessment (LCA) studies indicate that renewable-powered H2O2 electrosynthesis can reduce carbon emissions by 60-80% compared to traditional anthraquinone process methods. This substantial reduction stems primarily from eliminating fossil fuel dependencies in both the energy input and chemical feedstock requirements. The carbon footprint of electrochemically produced H2O2 using solar or wind power has been calculated at approximately 0.3-0.5 kg CO2-eq per kg H2O2, compared to 1.2-1.8 kg CO2-eq for conventional methods.
Water utilization metrics also demonstrate significant improvements, with electrosynthesis requiring approximately 40% less water consumption across the production lifecycle. This reduction becomes particularly valuable in water-stressed regions where chemical manufacturing facilities operate.
Resource efficiency indicators show that renewable-coupled electrosynthesis eliminates the need for multiple chemical precursors required in traditional methods. The direct synthesis pathway reduces material intensity by approximately 35%, minimizing extraction impacts associated with catalyst materials and chemical feedstocks.
Waste generation metrics reveal another advantage, as electrochemical routes produce minimal by-products compared to conventional processes that generate various organic waste streams requiring treatment. This translates to approximately 70% reduction in hazardous waste management requirements.
Energy return on investment (EROI) calculations demonstrate that while initial energy inputs for electrosynthesis may be higher, the elimination of multiple energy-intensive purification steps results in comparable or improved overall energy efficiency when system boundaries are properly defined. The intermittent nature of renewable sources presents challenges, but integration with energy storage systems can achieve steady production with 85-95% renewable fraction.
Land use impact assessments indicate that decentralized production enabled by this technology can reduce transportation emissions by 30-50% through localized manufacturing closer to end-use points. This distributed production model aligns with circular economy principles, particularly when implemented within industrial symbiosis frameworks where H2O2 serves multiple applications.
Biodiversity impact metrics suggest reduced ecosystem disruption compared to conventional chemical manufacturing, primarily through decreased air and water pollutant emissions associated with fossil fuel combustion and chemical synthesis pathways.
Life Cycle Assessment (LCA) studies indicate that renewable-powered H2O2 electrosynthesis can reduce carbon emissions by 60-80% compared to traditional anthraquinone process methods. This substantial reduction stems primarily from eliminating fossil fuel dependencies in both the energy input and chemical feedstock requirements. The carbon footprint of electrochemically produced H2O2 using solar or wind power has been calculated at approximately 0.3-0.5 kg CO2-eq per kg H2O2, compared to 1.2-1.8 kg CO2-eq for conventional methods.
Water utilization metrics also demonstrate significant improvements, with electrosynthesis requiring approximately 40% less water consumption across the production lifecycle. This reduction becomes particularly valuable in water-stressed regions where chemical manufacturing facilities operate.
Resource efficiency indicators show that renewable-coupled electrosynthesis eliminates the need for multiple chemical precursors required in traditional methods. The direct synthesis pathway reduces material intensity by approximately 35%, minimizing extraction impacts associated with catalyst materials and chemical feedstocks.
Waste generation metrics reveal another advantage, as electrochemical routes produce minimal by-products compared to conventional processes that generate various organic waste streams requiring treatment. This translates to approximately 70% reduction in hazardous waste management requirements.
Energy return on investment (EROI) calculations demonstrate that while initial energy inputs for electrosynthesis may be higher, the elimination of multiple energy-intensive purification steps results in comparable or improved overall energy efficiency when system boundaries are properly defined. The intermittent nature of renewable sources presents challenges, but integration with energy storage systems can achieve steady production with 85-95% renewable fraction.
Land use impact assessments indicate that decentralized production enabled by this technology can reduce transportation emissions by 30-50% through localized manufacturing closer to end-use points. This distributed production model aligns with circular economy principles, particularly when implemented within industrial symbiosis frameworks where H2O2 serves multiple applications.
Biodiversity impact metrics suggest reduced ecosystem disruption compared to conventional chemical manufacturing, primarily through decreased air and water pollutant emissions associated with fossil fuel combustion and chemical synthesis pathways.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!